Research on Drug-Patent Linkage System under the New Situation
2019-05-27HuangYufengLuoSupingYuanHongmei
Huang Yufeng, Luo Suping, Yuan Hongmei*
(School of Business Administration, Shenyang Pharmaceutical University, Shenyang 110016, China)
Abstract Objective To explore the extent to which the drug-patent linkage system should be implemented in China.Methods The present situation of drug-patent linkage system internationally was analyzed. Then the practices of drug-patent linkage system were discussed through such methods as literature reviews and quantitative scoring methods in combination of selecting the representative countries. Finally the extent to which the drugpatent linkage system should be implemented in China was explored through comparative analysis. Results and Conclusion Compared with the United States, China should establish a drug-patent linkage system that will weaken the protection of original patented drugs and promote the timely marketing of high-quality generic drugs and appropriately prevent the patent abuse.
Keywords: drug-patent linkage system; original patented drug; generic drug; patent abuse
On October 8, 2017, the relevant documents of the State Council clearly stated that “exploring the establishment of a drug-patent linkage system”,it means that China will establish a real drug-patent linkage system. Prior to this, China has established a patent declaration system for drug registration applications, but has not yet established a drug-patent linkage system. Establishing a substantive drugpatent linkage system can avoid the risk of generic patent infringement to a certain extent, safeguard the legitimate rights and interests of original patented drug, and achieve a balance between original patented drug and generic drugs.
At present, domestic research on the system can be divided into three categories: The fi rst category is to analyze the feasibility of the system. For example,in 2016, Ding Jinxi and others concluded that China should implement the system based on the stakeholder analysis[1]. The second category is to analyze the hidden dangers of the system. For example, in 2017,Liang Zhiwen analyzed the Trips-Plus rules and suggested that China should carefully transplant the US-style drug-patent linkage system[2]. The third category is to analyze the similarities and differences of the system between China and the United States and propose perfect suggestions. For example, in 2018, Li Wei made a comparative analysis of the Chinese and American systems and proposed some reasonable suggestions according to China's national conditions[3]. It can be seen that few scholars have studied the degree of drug-patent linkage system in China under the new policy situation, and this paper aims to make up for this research gap.
1 Introduction of the drug-patent linkage system
1.1 The history of drug-patent linkage system
The drug-patent linkage system refers to the“link” between the approval of generic drugs and the expiration of original patented drug patents, that is, the generic drug registration application should consider the patent status of previously listed drugs, thus avoiding possible patent infringement. The system originated in the United States and it is a product of a specif i c historical background in the United States. In the 1950s and 1960s, the “reaction stop” incident that swept the entire Western world sounded the alarm for drug regulation in the United States, which eventually promoted the emergence of “Kefauver Harris Amendment”. The relevant provisions of the Act improved the safety and effectiveness of listed drugs,thereby it ensured the safety of public drug use, but at the same time, it brought some negative effects to the US pharmaceutical industry. Not only has the research and development enthusiasm of the original research drug manufacturers been greatly reduced, but also the time-to-market of generic drugs has been postponed,which has restricted the development of the generic drug industry, resulting in a decline in the overall development level of the pharmaceutical industry in the United States, which damaged the public interest.
In order to stimulate the original drug innovation so that the generic drugs can be listed as soon as possible, the US Congress passed the Hath-Waxman Act in 1984, laying the foundation for the drug-patent linkage system.
1.2 Contents of drug-patent linkage system
After more than 30 years of development, the US drug-patent linkage system has become perfect.Its main contents include the orange book system,the simplified application system for generic drugs,the patent declaration system, the link system for regulatory approval agencies, and the system for exclusive protection of data.
1.2.1 Orange book system
The original drug applicant should submit the relevant patent information while submitting the New Drug Application (NDA) to the US Food and Drug Administration (FDA), and it will be registered on the orange book after the NDA is approved. If the patent is obtained, the patent registration should be fi led with the FDA within 30 days to get the patent.
The scope of patents that can be registered in the orange book includes pharmaceutical active ingredients, pharmaceutical products and pharmaceutical use patents.
1.2.2 Simplif i ed application system for generic drugs
A generic drug applicant needn't submit evidence of safety and efficacy when submitting Abbreviated New Drug Application (ANDA) to the FDA, but only submits evidence of bioequivalence.
1.2.3 Patent declaration system
A generic drug applicant is required to submit one of the following four statements when submitting an ANDA: (1) Paragraph I: No relevant patents are registered in the orange book; (2) Paragraph II: The relevant patent in the orange book has expired; (3)Paragraph III: Not to be listed before the expiration of the relevant patent period; (4) Paragraph IV: The relevant patent is invalid or the generic drug does not infringe a patent.
The applicant who submits the statement in paragraph IV must notify the patentee that the patentee can file a lawsuit within 45 days after receiving the notice and then initiate a 30-month containment period. There will be only a containment period.
Moreover, the fi rst generic drug that challenges successfully can get a 180-day market exclusivity.
1.2.4 Linkage system for regulatory approval agencies
When FDA accepts the ANDA, it submits the relevant materials to the USPTO. The USPTO conducts a substantive examination of the patent information contained in the ANDA and returns the actual patent status information of the drug to the FDA.
1.2.5 Data exclusive protection system
New drugs containing new chemicals enjoy a 5-year data protection period, rare drugs having a 7-year data protection period, and children's drugs enjoy a data protection period of 6 months plus the original patent term.
Under the interaction of these systems, the United States has achieved the balance between the original drug manufacturers and generic drug manufacturers. Therefore, it can safeguard the interests of the public, and then achieve the balance of interests of the three parties, which well ref l ects the spirit of Hath-Waxman Act in pursuit of the balance of interests.
1.3 Promotion of drug-patent linkage system
The United States not only implements the drugpatent linkage system in its own country, but also promotes the system indirectly or directly through the signing of free trade agreements with other countries in the world. The purpose is to improve the standards of intellectual property protection and better protect the interests of the original drug manufacturers in the United States.
1.3.1 Indirect promotion of drug-patent linkage system
Less than a decade after the establishment of the drug-patent linkage system in the United States,Canada established this system as well, and the establishment of this system is related to Canada's long-standing compulsory licensing system. Prior to 1987, in order to reduce the high drug prices in the country, the Canadian government required all prescription drugs should be compulsory, but the compulsory licensing system conflicted with the high standards of intellectual property protection in the United States. Therefore, the United States exerted pressure on the free trade agreement with Canada. In order to reach a trade agreement that can generate huge economic benef i ts, Canada canceled the compulsory licensing system in 1993, and in the same year it issued the “NOC”, replacing the provisions of the Patent Law on compulsory licensing, to achieve the balance between the timely entry of generic drugs into the market and the patent protection of original drugs through patent linkage.
1.3.2 Direct promotion of drug-patent linkage system
After the conclusion of the Trips agreement,many countries regarded it as the “ceiling” of intellectual property protection. However, they found that the negotiations of the Trips agreement were only the “floor” of intellectual property protection standards. In the Post-Trips era (the era after the signing of the Trips agreement), pharmaceutical industry actively promoted the internationalization of higher-level intellectual property protection standards,one of the main means is the free trade agreement.In bilateral free trade agreements, the United States directly introduced a series of “Trips-Plus” rules in exchange for high-level intellectual property protection standards of various countries on the condition of opening up the U.S. trade market. With a view to suppressing multilateral negotiations after the break-up of various countries and seeking international high-level intellectual property protection standards to safeguard their interests, the drug-patent linkage system becomes an important part of the “Trips-Plus”rule.
The core approach of the system in free trade agreements is: linking the approval of the generic drug to the patent status of the original drug, that is, when the generic drug is submitted for listing, the drug regulatory department shall review the patent status of the corresponding original drug. If it is within the patent term of the original drug, the listing application will not be approved unless it is agreed or acquiesced by the patentee.
The 12 bilateral free trade agreements (involving 17 countries) signed by the United States after the Trips agreement are shown in Table 1. Jordan is the only that did not have a core approach to the system,so we excluded it in the follow-up analysis.
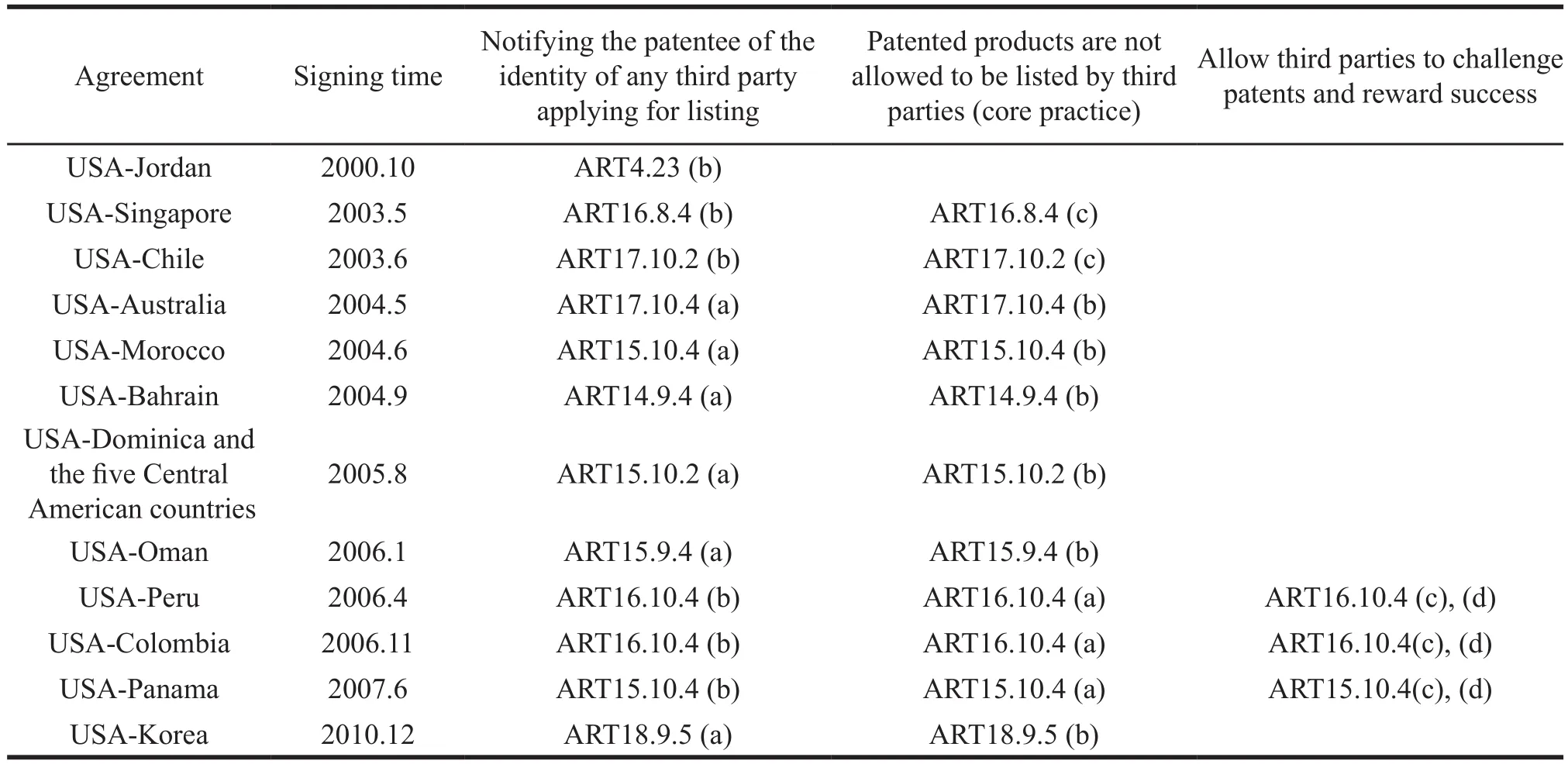
Table 1 Drug-patent linkage system in free trade agreement
2 Analysis and enlightenment of drug-patent linkage system
2.1 International distribution of drug-patent linkage system
At present, a total of 17 countries have introduced the drug-patent linkage system, one indirectly, and the other 16 directly. However, even though so many countries have introduced the system, the EU and India are against the system. The international distribution of the drug-patent linkage system is shown in Figure 1, where “1” represents the country that introduced the system and “0” represents the country that opposes the system.
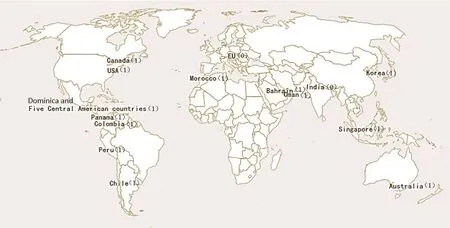
Figure 1 International distribution of drug-patent linkage system
2.2 Research objects of international analysis of drug-patent linkage system
The situations of the system in these countries with patent linking system are studied to provide reference for China's government. Therefore the representative countries with the indirect or direct promotion were selected to analyze. Since Canada is the only country with the indirect promotion, it is analyzed. South Korea is selected among the countries with direct promotion as the representative country for the following reasons. First of all, in terms of geographical location, South Korea and Singapore are Asian countries, and South Korea is closest to China.Second, in terms of traditional genetic resources,both Korea and China have rich traditional genetic resources. Korean medicines are similar to Chinese medicines and Korea's practices are more useful.Finally, in terms of introduction time, South Korea is the last country that introduced this system, and its approach can better ref l ect the attitude of US towards the establishment of the system in other countries.
Among the countries that reject the drug-patent linkage system, the most typical ones are the EU and India. Therefore, the reasons for their opposition to the system are also explored, which helps us to think about the advantages and disadvantages of the system from the opposite side.
2.3 Implementation level of drug-patent linkage system in Canada and South Korea
The main research method used in this section is quantitative scoring method[4].
Since the drug-patent linkage system of Canada and South Korea originated from the United States,they take the US drug-patent linkage system as the main reference, and then combine with the actual situation of their countries to form their own specif i c system. Therefore, when their drug-patent linkage system is discussed, the US comparative analysis is inseparable.
By analyzing the main contents of the US drugpatent linkage system, we can find that the goals of each regulation are not the same, but they have three goals: protecting original drugs, promoting the timely listing of generic drugs and preventing the abuse of patent rights. Canada and South Korea also have the same goals. However, the target levels embodied in these three countries are different. For example, as to protecting the original drugs, some countries may want strong protection, while others may want weak protection. Therefore, the relevant regulations of the United States, Canada, and South Korea are sorted out and different scores to each rule are assigned. The scores represent the level of the target embodied in the regulation. They are 5, 4, 3, 2 and 1 that represent the degree of very strong, strong, moderate, weak, and very weak ( If a country does not mention a regulation,no score will be given). Then the statistical processing is carried out to understand the different target levels of the United States, Canada and South Korea.
The basic process of scoring includes three steps. Firstly, all the relevant laws and regulations for the drug-patent linkage system promulgated by these countries are classified into three categories such as protecting original drugs, promoting the timely listing of generic drugs and preventing the abuse of patent rights according to the objectives of the law. Secondly,the contents of the corresponding laws and regulations of the three countries are carefully compared,combining with the opinions of professional scholars in the fi eld to judge the target level ref l ected in each law. Finally, the law score is assigned. The specific regulatory assignment table is shown in Table 2.
Through Table 2, we can learn that in terms of the protection of original drugs, the timely promotion of generic drugs, and the prevention of patent abuse,the average score of USA is 4.25, 4.25 and 3.5 respectively. The average score of Canada is 3.75, 3 and 3.5. And the average score of Korea is 3.75, 4.25 and 3.25.
From this we can learn that Canada is weakerthan the United States in protecting original drugs and promoting the timely listing of generic drugs. The prevention of patent abuse is the same as that in the United States, and it relatively more focuses on the protection of original drugs. South Korea is weaker than the United States in protecting original drugs and preventing the abuse of patent rights. The timely promotion of generic drugs to market is as much as the United States and it focuses on promoting the timely listing of generic drugs more.
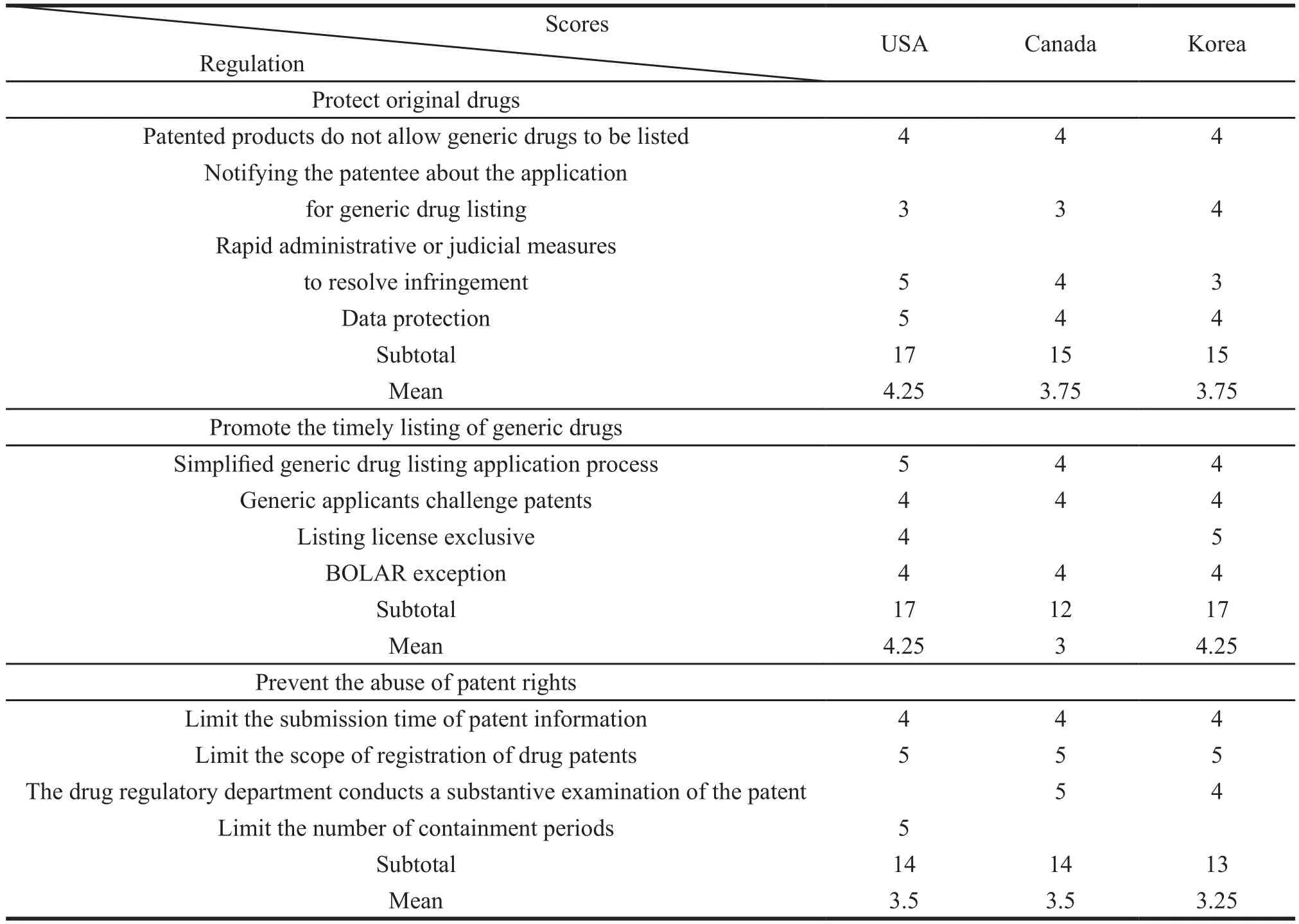
Table 2 Regulatory assignment table
2.4 EU and India on the practice of drug-patent linkage system
2.4.1 EU denies drug-patent linkage system
The generic drug industry in the EU is operating well with the support of policies. Generic drugs account for half of the European drug prescriptions and can save a large amount of health expenditure every year. Therefore, it has become an indispensable part of the European health care system[5].
For the drug-patent linkage system, the European Commission believes that the original drug manufacturers can use this system to threaten generic drug manufacturers by infringement of patent rights and prevent the listing of generic drugs. Therefore, the EU stipulates in the EC Directive that the marketing license for drugs cannot be refused, suspended or revoked, except for the reasons stipulated by the regulations and directives[6]. This clearly denies the drug-patent linkage system and removes the factors that hinder the timely listing of generic drugs.
2.4.2 India does not have a drug-patent linkage system
India's well-developed generics industry is world-famous and it produces one-f i fth of the world's generic drugs. More than 200 countries around the world import generic drugs from India. The generics industry plays a key role in its domestic economic development[7].
On October 31, 2008, Bayer filed a lawsuit against the Indian Sipura Company and the Indian Food and Drug Administration to the Delhi High Court, arguing that the Indian Food and Drug Administration should not approve the Sipura Company's license to list generic medicines for Bayer's patented medicines. Thus it triggered a huge discussion in India on the drug-patent linkage system. In the end, Bayer lost the lawsuit, and the most important reason is that the existing laws in India do not have the relevant provisions to interpret drug-patent linkage system. This also shows that the drug-patent linkage system has not been explicitly recognized by the Indian legislature[8]. The attitude of the legislature just proves that India wants to protect and motivate its domestic generics industry. But the drug-patent linkage system will undoubtedly have an impact on India's generic drug industry, so India opposes the system.
2.5 The enlightenment of international drug-patent linkage system to China
Canada established a drug-patent linkage system under the circumstances that the domestic generic drug industry developed fast but the original drug research was slow and it faced external pressure. Therefore, the degree of implementation of protecting original drugs and promoting the timely listing of generic drugs was weaker than that of the United States, but it was the same as the United States in preventing the abuse of patent rights. It can be seen that under the influence of the United States, Canada focuses on the patent protection of the original research drug and weakens the protection of its dominant industries. At the same time, however, it has also increased the supervision of the original drug and prevented its abuse to balance the development of generic drugs and original drugs.
Like many other Asian countries, South Korea's domestic pharmaceutical industry focuses on the generics industry. Therefore, South Korea established the drug-patent linkage system to fulfill the obligations of the US-South Korea Free Trade Agreement when the generic drug industry was more developed than the original drug industry. However,only the core practices were mentioned in the free trade agreement. Therefore, Korea's autonomy in implementing the system was greatly enhanced, and it established a favorable drug-patent linkage system for the development of its generic drug industry.
The generics industry plays a vital role in the EU's health care system and India's national economy,and its development is at a mature and stable stage.Therefore, the drug-patent linkage system cannot promote its development, but it will hinder the role. That is why the EU and India have adopted a unanimous attitude towards the system.
In terms of establishing a suitable drug-patent linkage system in China, we can refer to the practices of Canada and South Korea and take the development of the domestic pharmaceutical industry and the external pressures into consideration. As to the advantages and disadvantages, although the system does more harm than good for the EU and India,China should firmly establish a drug-patent linkage system because the generic drug industry is still in the growth stage, and the promotion effect of the system will be greater than the hindrance effect.
3 Suggestions on establishing a good drugpatent linkage system in China
China often faces infringement lawsuits from foreign original research drug manufacturers because we have copied foreign original research drugs. As there is no effective judicial procedure to solve the lawsuits in China, foreign original research drug manufacturers are dissatisf i ed with this situation. On April 27, 2018, in the annual report “Special 301 Report of 2018” issued by the Office of the United States Trade Representative on the protection of foreign intellectual property rights, China once again appeared on the “Priority Watch List”. China has been on the list for 14 consecutive years[9]. In the last year's report, it accused China of not establishing an effective system for “notifying patentees of generic drug listing applications” to solve the patent disputes as soon as possible.
Due to the pressure from the United States,China's current establishment of a drug-patent linkage system cannot ignore the expectations of the United States for the system. But fortunately, the United States only emphasizes that China should establish an effective system of notifying the patentee. By observing the United States attitude towards South Korea's establishment of a drug-patent linkage system,we can see that the United States emphasizes the stipulation that products protected by patents are not allowed to be listed by a third party. Therefore, even the factors of the United States are considered, China still has a large choice in system design. On the basis of satisfying the expectations of the United States appropriately, we can establish a drug-patent linkage system according to the actual situation in China.
In terms of protecting the original research drugs, since the research and development capability of the original research drugs in China is still weak,we need weak protection instead of strong protection.The degree of protection should correspond to the gradual research and development capability so that we can effectively promote the development of the original research drugs. In terms of promoting the timely listing of generic drugs, due to the uneven quality of generic drugs in China, we should focus on encouraging the timely listing of good quality generic drugs, rather than all generic drugs. As to preventing the abuse of patent rights, because of the weak protection of the original drug, the abuse of patent rights will not be serious, so this function can be weakened.
To sum up, compared with the United States,China should establish a drug-patent linkage system that weakens the protection of original drugs, promotes the timely listing of high-quality generic drugs, and prevents the abuse of patent rights appropriately. The specific details should be finalized by the relevant departments according to the actual situation, and they must be operable so that a good drug-patent linkage system suitable for China can be truly set up.
杂志排行
亚洲社会药学杂志的其它文章
- Information for Authors
- Biopharmaceutical Industrial Zone in the United States:Development Experience and Its Enlightenment
- lnf l uence of Psychological Contract on Customer Loyalty to Drugstores
- Import of Health care Products in China: Current Status and Countermeasures
- Research on Protection of the Interests of Generic Drug Companies in the Drug-Patent Linkage System Based on Cournot Model
