在纯水体系和活细胞中高选择检测Fe3+的香豆素荧光探针
2019-05-07刘琪梦郭昊冉
刘琪梦 汪 欢 郭昊冉 郭 媛
(西北大学化学与材料科学学院,国家级化学实验教育示范中心,西安 710127)
Many heavy metal ions have attracted great attention owing to their broader influence on the environment and human health[1-2].In particular,iron(Fe3+)is one of the most plentiful transition elements,and it performs crucial roles in many physiologic processes including cell metabolism,hemoglobin formation and so on[3-7].However,abnormal Fe3+content may cause many serious diseases to people,for instance,abdominal pain,hyperferremic disorders,Parkinson′s disease and Alzheimer′s disease[8-10].Thus,it is of considerable importance to develop an efficient method for highly selective detection of Fe3+.
Compared with conventional analytical methods like colorimetry and voltammetry[11-14],fluorescent probes have been regarded as one of the most valid tools to detect Fe3+due to the advantages of simple manipulation,high sensitivity and low cost[15-21].Hence,an increasing number of Fe3+fluorescent probes based on the different mechanisms have been developed[22-31].Unfortunately,among these developed Fe3+fluorescent probes,many fluorescent probes are easily interfered with Al3+,Cu2+and Cr3+,and few of probes possess ideal water-solubility.To the best of our knowledge,ideal water-solubility is conducive to the biological application of probes.Therefore,it is highly desired to develop a high selective fluorescent probe for detecting Fe3+in 100%aqueous medium.We herein designed and synthesized a novel fluorescent probe CF470 for sensing Fe3+in deionized water.CF470 was designed by using coumarin derivative as a fluorophore,and the trihydroxyl group as both the response site and hydrophilic group,as shown in Scheme 1.The intense blue fluorescence of CF470 was quenched within 2 min after the addition of Fe3+(100 equiv.),which was attributed to the paramagnetic quenching effect.As expected,the probe CF470 not only possessed good selectivity,ideal water-solubility owing to existence of the exceedingly hydrophilic trihydroxyl group,but also had a rapid response to Fe3+.In especial,the probe,with low toxicity and good biocompatibility,was successfully employed to image Fe3+in A549 cells.
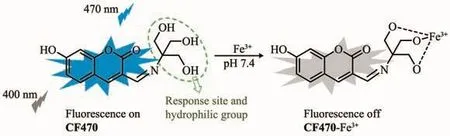
Scheme 1 Sensing mechanism of CF470 for Fe3+
1 Experimental
1.1 Materials and instruments
All materials, unless special stated, were obtained from commercial sources and used without further purification.Fluorescence spectra and absorption spectra were performed on a Hitachi F-2700 fluorescence spectrophotometer and Shimadzu UV-2550 spectrometer,respectively.Forallspectral measurements,the excitation wavelength was set at 400 nm,and the slit widths of excitation and emission were 2.5 nm and 5 nm,respectively.NMR spectra were obtained on a Varian Unity INOVA-400 spectrometer with tetramethylsilane (TMS)as an internal standard.A Mettler Toledo pH meter was used for the all pH measurements.High-resolution mass spectra were measured on a Bruker micrOTOF-Q II mass spectrometer.Infrared spectra were acquired with a Bruker Vertex 70 FI-IR spectrometer.The cell experiments were carried out with an Olympus FV1000 confocal microscope.
1.2 Preparation of sample solutions
For all spectrometric studies, CF470 was prepared in analytical grade DMSO to obtain the stock solution of the probe (1 mmol·L-1).Stock solutions of various metal ions,such as Fe3+,Fe2+,K+,Mn2+,Ni2+,Cd2+,Al3+,Co2+,Cr3+,Mg2+,Hg2+,Ba2+,Zn2+,Cu2+,Ca2+,Pb2+,Na+,Sn2+,and Ag+were prepared in doubly distilled water(10 mmol·L-1,respectively).
1.3 Synthesis of probe CF470
Synthesis of CF470 was presented in Scheme 2.The characterization details were displayed in the supporting information(Fig.S3~S10).
1.3.1 Synthesis of compound 1
A mixture of 2,4-dihydroxybenzaldehyde(0.69 g,5.0 mmol),three drops of triethylamine and anhydrous sodium acetate (1.05 g,11.0 mmol)in propionic anhydride(15 mL)was stirred at 140℃for 6 h.After 6 h,100 mL ice-water was poured onto the mixture solution.Then the solution was removed by filtration,and the residue was recrystallized from methanol to obtain white solid.Yield:56%.1H NMR (400 MHz,CDCl3)δ:7.54(s,1H),7.45(d,J=8.0 Hz,1H),7.12(s,1H),7.05(d,J=8.0 Hz,1H),2.70~2.64(m,2H),2.24(s,3H),1.31(t,J=6.0 Hz,3H).HRMS:m/z Calcd.for C13H13O4+[M+H]+:233.079 5,Found:233.080 8.

Scheme 2 Synthetic route of CF470
1.3.2 Synthesis of compound 2
A mixture of compound 1 (1.16 g,5.0 mmol),NBS(2.23 g,12.5 mmol)and a trace amount of AIBN was dissolved in CCl4(150 mL).After the mixture was stirred at 80 C for 12 h,the solvent was evaporated under reduced pressure to give the resulting residue.The mixture of the resulting residue,sodium acetate(2.42 g,29.5 mmol)and acetic acid (80 mL)was unceasingly stirred at 100 ℃ for 12 h.HCl(2 mol·L-1,80 mL)was poured in the mixture solution and unceasingly stirred for 30 min.The solution then was cooled down to room temperature and was discarded by filtration.The resulting crude solid was washed with water(10 mL)and methanol(10 mL)to afford brown powder.(870 mg,87%).1H NMR (400 MHz,DMSO-d6)δ:11.30(s,1H),9.96(s,1H),8.58(s,1H),7.82(d,J=12.0 Hz,1H),6.87(d,J=8.0 Hz,1H),6.77(s,1H).HRMS:m/z Calcd.for C10H7O4+[M+H]+:191.019 8,Found:191.018 6.
1.3.3 Synthesis of probe CF470
A mixture of compound 2(190 mg,1.0 mmol)and tris(hydroxymethyl)aminomethane(145.2 mg,1.2 mmol)in ethanol(5 mL)was refluxed at 80℃under the argon atmosphere.After the reaction completed,the solution was cooled to room temperature,the precipitate was acquired through filtration,washed with anhydrous ethanol to obtain orange product(100 mg,75%).1H NMR(400 MHz,DMSO-d6)δ:10.60(s,1H),8.05(s,1H),7.60(d,J=8.0 Hz,1H),6.79(d,J=8.0 Hz,1H),6.72(s,1H),5.35(s,1H),4.75(s,1H),3.70(d,J=8.0 Hz,1H),3.62(s,J=8.0 Hz,1H),3.41(s,4H).13C NMR (100 MHz,DMSO-d6)δ:162.03,160.27,155.43,140.69,130.58,122.38,113.83,111.40,102.31,87.70,69.27,67.48,63.11,62.69.HRMS:m/z Calcd.for C14H16NO6+[M+H]+:294.095 9,Found:294.097 2.IR(KBr,cm-1)ν:3296,2801,1698,1616,1361,1250,1191,1114,864,764,655,505.
1.4 Detection limit
The detection limit of CF470 for detecting Fe3+was calculated by means of following equation:
Detection limit=3σ/k
σ is the standard deviation of eleven blank measurements and k is the slope of standard curve.
1.5 Cytotoxicity assay
Cytotoxicity studies of CF470 to A549 cells were evaluated using standard MTT assay.Cells were incubated in 96-well microplates at a density of 1×105cells per well in Dulbecco′s modified Eagle′s medium(DMEM)containing 10%fetal calf serum for 24 h.After that,different concentrations of CF470 (0,5,10,15,20 and 25 μmol·L-1)were added the culture dish and treated for 24 h.MTT reagent(10 μL)was added and sequentially incubated for 4 h.Then,the culture medium was washed with PBS three times and DMSO (200 μL)was cultured with cells to dissolve formazan.The absorbance at 490 nm was recorded using the microplate reader.
2 Results and discussion
2.1 Spectral properties of probe CF470
In consideration of the effect of pH on the propertiesofthe fluorescentprobe,we initially examined the fluorescence intensities of the probe and reaction product within various pH values(3.0~11.0).Fig.1 showed that the probe CF470 exhibited a maximum fluorescence emission intensity at 470 nm and the fluorescence intensity was almost stable with pH ranging from 7.0 to 8.0.After adding hundred equivalences of Fe3+,fluorescence intensity at 470 nm of CF470 was drastically weakened and kept fine stability within a wide pH region of 3.0~11.0.Given the cell imaging and spectral response of CF470 to Fe3+,all experiments selected pH 7.4.
UV-Vis absorption spectra of CF470 toward Fe3+was firstly studied in phosphate buffer(pH=7.4)solution (Fig.S1).The free CF470 exhibited a main absorption peak at 410 nm.With successive addition of Fe3+(0~100 equiv.)into the buffer solution of CF470,the absorption peak at 410 nm gradually enhanced.We also estimated the fluorescence spectra of CF470 to Fe3+(Fig.2a).The probe CF470 displayed a strong blue fluorescence.However,upon the addition of Fe3+(0~100 equiv.)to CF470 solution,a significant fluorescence attenuation was observed,which was attributed to the paramagnetic quenching effect.After the reaction between probe and Fe3+(100 equiv.),the strong fluorescence almost entirely disappeared (Fig.2b).And the fluorescence intensity at 470 nm demonstrated good linear relationship with the concentrations of Fe3+(0~35 equiv.)with a detection limit(1.16 μmol·L-1)(S/N=3)(Fig.2b inset).The results showed that CF470 could realize quantitative detection of Fe3+.
To study the selectivity of CF470 toward Fe3+,different metal ions were examined under same conditions (Fig.3a).CF470 demonstrated obvious fluorescence quenching in the presence of Fe3+in phosphate buffer(pH=7.4)solution,while other metal ions did not cause significant fluorescence attenuation and color changes on CF470 than Fe3+(Fig.3a inset).Simultaneously,competition experiments were also conducted(Fig.3b).When Fe3+(100 equiv.)was added to the mixture solution of CF470 and other metal ions,a clear fluorescence attenuation was discerned,indicating that the presence of other metal ions could not interfere CF470 for detecting Fe3+.Thus,the above experimental results demonstrated that CF470 had high specificity for Fe3+.

Fig.4 (a)Plot of fluorescence intensity at 470 nm versus time for the reaction between CF470(10 μmol·L-1)and Fe3+(100 equiv.);(b)Job′s plot of CF470 in phosphate buffer(pH=7.4)solution
To assess the response ability of CF470 for Fe3+,the kinetic studies were carried out(Fig.4a).The addition of Fe3+(100 equiv.)to the solution of CF470 led to a remarkable attenuation in the fluorescence intensity at 470 nm,and the fluorescence intensity attained stable within 2 min,with a 25-fold decrease.
2.2 Proposed sensing mechanism
The possible sensing mechanism of CF470 to Fe3+was depicted in Scheme 1.The complexation of CF470 with Fe3+resulted in the fluorescence quenching,which was possibly ascribed to the paramagnetic property of Fe3+.HRMS analysis and Job′s plot were carried out to verify this mechanism.As depicted in Fig.4b,a strong fluorescence intensity at mole fraction 0.5 suggested 1:1 stoichiometry for the CF470-Fe3+complex.Similarly,the binding mode of CF470 with Fe3+was also testified by HRMS.After the probe reacted with Fe3+,a new peak at m/z=345.998 7 corresponding to[CF470-Fe3+]appeared(Fig.S2).As a result,the experimental results fully confirmed the response mechanism of CF470 toward Fe3+.
2.3 Computational sudies
To furtherprove the sensing mechanism of CF470 toward Fe3+,theoretical calculation was carried out by the Gaussian 09 program.The optimum structure and electron distributions of CF470-Fe3+were obtained using B3LYP/6-31G(d,p)(Fig.5).The electrons on the HOMO of CF470-Fe3+were mostly situated on coumarin moiety,whereas the electrons on the LUMO were mainly located around Fe3+,suggesting that the fluorescence quenching was due to the coordination of CF470 with Fe3+.

Fig.5 (a)Optimum structure and(b)HOMO-LUMO energy level of CF470-Fe3+
2.4 Cellular imaging
To assess the toxicity of CF470(0,5,10,15,20 and 25 μmol·L-1)towards A549 cells,MTT assay was carried out.As shown in Fig.6,even after cells were cultured with high concentration of CF470(25 μmol·L-1)for 24 h,cell survival rate was still more than 80%,showing the low cytotoxicity and good biocompatibility of CF470 to living cells.
Then,the cellular imaging experiments of CF470 toward Fe3+were conducted on A549 cells.The A549 cells were cultured with CF470 (10 μmol·L-1)in DMEM for 20 min.After that,cells were washed repeatedly with PBS for three times,and finally cultured with Fe3+(100 equiv.)for 10 min.As seen in Fig.7,strong blue fluorescence was observed after A549 cells was pretreated with CF470.In contrast,cells displayed slight fluorescence in the presence of Fe3+.These experimental results manifested that CF470 possessed good cell membrane permeable as well as could be used for the detection of Fe3+in living cells.
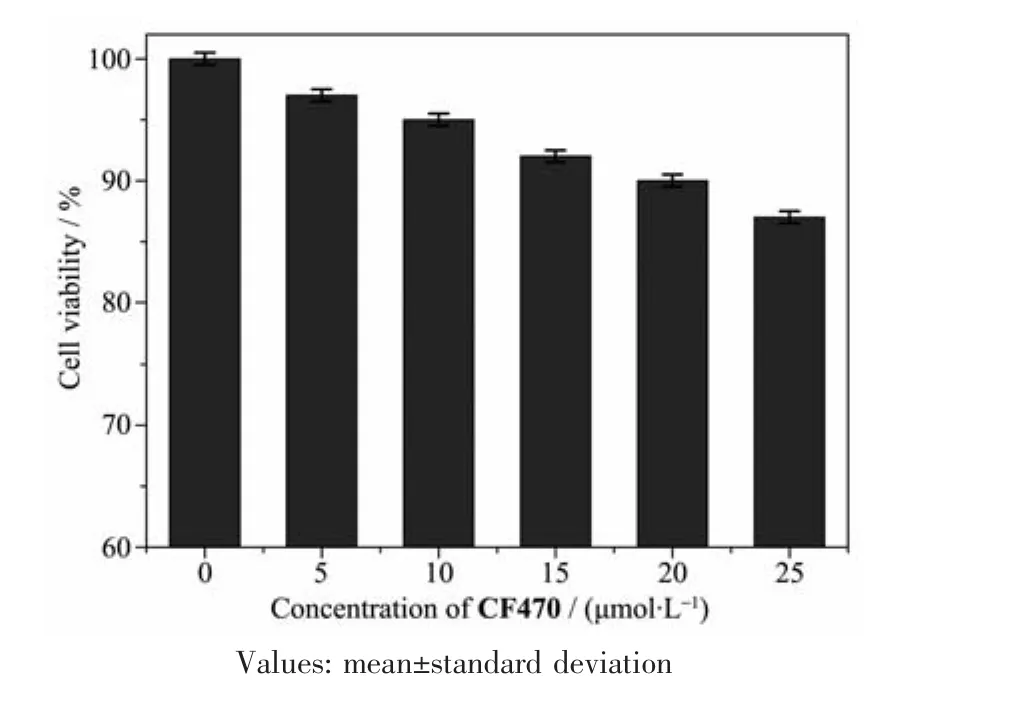
Fig.6 Cytotoxicity of CF470 in A549 cells estimated by the MTT method

Fig.7 Cellular imaging of Fe3+in A549 cells cultured with CF470(10 μmol·L-1,20 min)and further incubated with Fe3+(1 000 μmol·L-1,10 min);(b)Fluorescence intensity per one blue channel
3 Conclusions
In summary,we have developed a novel fluorescent probe CF470,for detecting Fe3+in 100%aqueous solution through complexation reaction on the basis of fluorescence quenching,comprised of coumarin derivative as a fluorophore,and the trihydroxyl group as both the Fe3+-sensing site and hydrophilic group.Remarkably,the sensing mechanism of CF470 to Fe3+was proved by HRMS analysis and theoretical calculation.A palpable fluorescence quenching was observed after the addition of Fe3+into the CF470 solution.The probe CF470,with ideal water-solubility on account of existence of the exceedingly hydrophilic trihydroxyl group,can realize a highly selective and rapid detection of Fe3+.Furthermore,cell imaging results demonstrated that CF470 with low cytotoxicityhad admirable membrane permeability and could detect Fe3+in living cells.
Supporting information is available at http://www.wjhxxb.cn
