Identification and Control of HLB Disease in Citrus grandis
2019-04-25,*,,,,,,
, *, ,, , , ,
1.Yunnan Yuntianhua Co., Ltd., Kunming 650228, China; 2.Yunnan Chemical Research Institute, Kunming 650228, China; 3.Yunnan Agricultural University, Kunming 650201, China
Abstract HLB disease has been endangering citrus production, and it is an important task in Citrus grandis production to identify and control HLB disease. Diaphorina citri and Trioza erytreae are the main vectors for the spreading of HLB disease. Scientific and proper release of predatory natural enemies such as ladybugs, combined with chemical control can effectively control psyllid. For suspected HLB disease strains, a simple "HLB disease detection reagent" can be used for detection. This method is simple, cheap and accurate, and it is an economical and feasible identification method for ordinary growers.
Key words Citrus grandis, HLB disease, Identification, Control measures
1 Introduction
Citrus HLB disease was first discovered in China, and Reinking described a yellow leaf mottle on citrus in South China in 1919. Chen Qibao first reported that citrus HLB disease occurred seriously in Chaoshan area of Guangdong Province from 1938 to 1941. Because tip is called "Long" in Chaoshan area of Guangdong Province, it is named HLB (Huanglongbing) disease[1]. Professor Lin Kongxiang, a plant pathologist in China, confirmed for the first time that the disease was an infectious disease[2]. In 1995, the 13th International Organization of Citrus Virologists (IOCV), held in Fuzhou, China, officially named such disease "Huanglongbing", abbreviated as HLB, and took "citrus greening disease", which had been used abroad, as the local name[3].
Experts found that sweet orange, orange and so on are varieties highly susceptible to HLB disease, andCitrusgrandisis a variety moderately susceptible to this disease[1]. Nevertheless, the harm of HLB disease toCitrusgrandisindustry is still very great. Through the investigation of majorCitrusgrandisgrowers in Ruili City, Yunnan Province, it was found that HLB disease plants or suspected HLB disease plants appeared inCitrusgrandisplots above a certain scale. After theCitrusgrandistree was infected with HLB disease, it could show symptoms in the whole year, and the incidence of HLB disease inCitrusgrandisshoot was the highest in summer and autumn in pomelo orchard, followed by that in spring. At the beginning of the disease, the new shoots ofCitrusgrandistree stopped turning green, and there was an obvious "yellow shoot" phenomenon at the top of the crown. The leaves showed mottled or mosaic yellowing, among which mottled yellowing was the most typical. Yellowing symptoms appeared from the base of the vein and near the lateral vein, and then gradually expanded to form irregular, different sizes, different shapes of mottled yellow spots. Once theCitrusgrandistree is infected with HLB disease, it will affect the yield and quality, and even cause "tree death and garden destruction". In field farming, the symptoms of HLB disease are often similar to those caused by poor management of pomelo orchards, and it is difficult for ordinary farmers to identify them effectively. Moreover, due to the limitations and influence of many factors, such as time and cost, it is almost impossible to send samples to colleges and research institutions for identification. It can be seen that the identification and prevention of HLB disease is very important. Based on the actual production, this paper discusses a method of identification and control of HLB disease suitable for ordinary growers ofCitrusgrandis, hoping to be helpful toCitrusgrandisgrowers to a certain extent.
2 Pathogen of HLB disease and its host
2.1PathogenicbacteriaofHLBdiseaseHuman cognition of pathogenic bacteria of citrus HLB disease has gone through "pathogenic physiological factors, virus stage", "pathogenic mycoplasma, Rickettsia stage" and "pathogenic bacterial stage". Until 1984, Garnieretal. found peptidoglycan between the outer wall and the inner wall of HLB disease pathogen by electron microscope, which was similar to the cell wall structure of Gram-negative bacteria, therefore, it was considered that HLB disease pathogen was a kind of Gram-negative bacteria[4]. In 1995, the pathogen of HLB disease was classified as a candidate genus ofCandidatusliberibactersp, belonging to the subclass α ofProteobacteriacea. According to the sensitivity of HLB disease pathogen to heat, insect vector and geographical distribution, it was divided into three species:Candidatusliberibacterasiaticus(Las), withDiaphorinacitrias insect vector;Candidatusliberibacterafricanus(Laf), withTriozaerytreaeas insect vector;Candidatusliberibacteramericanus(Lam), withDiaphorinacitrias insect vector. Las belongs to heat-resistant type, and the suitable temperature for the disease is 27-32℃, which often exists in low altitude and hot areas. Laf belongs to heat-sensitive type, and the suitable temperature for the disease is 22-25℃, it can only be found on citrus planted 700 m above sea level. Lam also belongs to heat-resistant type, and the suitable temperature for the disease is 27-32℃, which is closer to Las[5]in symptom manifestation.
2.2OccurrenceandepidemicregularityofHLBdiseaseThe transmission route of HLB disease is mainly through infected scions, seedlings and citrus psyllid insect vectors. HLB pathogen can also be transmitted from citrus to herbaceous plantCatharanthusroseusthroughCuscutachinensis. The pathogens overwinter mainly in phloem tissue in the field, the initial infection sources are diseased plants, infected seedlings and Diaphorina citri.
The main citrus psyllids that transmit HLB disease in the field areDiaphorinacitri andTriozaerytreae. Both like to eat buds, the eggs are mostly laid on the edge of the tender leaf, and the nymphs live on the back of the edge of the leaf or in the veins and petioles. The nymphs of theTriozaerytreaeare implanted in the back of the leaves to form a concave nest with protuberances. When it grows into an adult, the nest is empty and it is still protruding. However, theDiaphorinacitrigenerally does not produce protuberance, but in severe cases, the leaves are twisted[6].
2.3HLBdiseasehostThe plant hosts of citrus HLB Disease are mainly citrus and related plants of citrus subfamily of Rutaceae. In addition to Rutaceae, citrus HLB disease bacteria can also be transmitted to non-Rutaceae plants such asCatharanthusroseus, tobacco and tomato throughCuscutachinensis. The symptoms ofCatharanthusroseusare more obvious than those of citrus, and the density of germs is higher, so it is often used as an indicator plant of HLB disease[1,5].
3 Identification method of Citrus grandis HLB disease
3.1FieldidentificationmethodThe field identification method is mainly based on the maturity symptoms of the new shoot to identify HLB disease. If yellowing and mottled symptoms appear in the new shoots, many growers will basically determine theCitrusgrandistree as a suspected HLB disease tree. However, because the symptoms of HLB disease are easily confused with other symptoms caused by mismanagement, malnutrition or diseases and pests, especially plants that have been infected with HLB disease but have not yet shown obvious symptoms are difficult to be accurately judged on the basis of symptoms.
3.2LaboratoryidentificationmethodLaboratory determination method is the main method to study and quarantine HLB disease. After many years of research by citrus scholars, electron microscopic observation, serological diagnosis, nucleic acid probe hybridization and PCR amplification have been reported[3,6]. However, due to time, cost and other factors, in the actual production,Citrusgrandisgrowers almost do not send samples to colleges and universities and scientific research institutes for laboratory determination.
3.2.1Electron microscopic observation. By electron microscopic observation, we can clearly observe the morphology, size and wall membrane structure of HLB pathogen, and the diagnosis is very accurate, which is an important identification and diagnosis method.
3.2.2Serological diagnosis. Because HLB pathogen can not be cultured artificially and pure culture can not be obtained, it is difficult to prepare efficient monoclonal antibody against HLB pathogen. Even if it has been obtained, because its monoclonal antibody specificity is too strong, it is difficult to adapt to the variation of HLB pathogen strains in different regions, and there may be missed detection, so its application and promotion is also greatly limited.
3.2.3Nucleic acid probe hybridization. The principles of nucleic acid molecular hybridization are: The nucleotide bonds between two nucleic acid single strands with certain homology have affinity. Under certain conditions, the two single strands are annealed according to the principle of base complementarity to form double strands. The two sides of hybridization are nucleic acids to be detected and labeled probes, and radioisotopes or non-radioactive substances such as biotin are often used as markers. After hybridization, the detection can be achieved by labeled probe autoradiography or nylon membrane banding. The advantages of nucleic acid molecular hybridization are: the detection is fast and specific, and the sensitivity is as high as ng level. However, due to the complexity of molecular probe hybridization process and the need for a large number of DNAs, the wide application of this technique is limited.
3.2.4PCR amplification. At present, polymerase chain reaction (PCR) has become the most reliable and widely used detection method in the world because of its rapid, simple features, high sensitivity and strong specificity. On the basis of conventional PCR, nested PCR with higher sensitivity and specificity has been developed. In order to overcome the problems that conventional PCR and nested-PCR are unable to quantify accurately, easy to cross-pollute and prone to false positive errors, real-time fluorescence quantitative PCR has been gradually developed. This technique combines PCR and DNA probe hybridization. It is a method of including fluorescent groups in PCR reaction system, using fluorescence signal accumulation to monitor the whole PCR process in real time. Finally, the unknown template is quantitatively analyzed by standard curve. As a quantitative method of nucleic acid, this technique has the advantages of high sensitivity, high specificity, high accuracy, real-time, less pollution and so on. It has realized the leap from qualitative to quantitative PCR, and has been widely used in the detection of citrus HLB disease.
3.3Indicatoridentification[1,3]The identification of indicator plants is to graft suspicious branches onto susceptible indicator plants and observe the incidence of the disease 3-4 months after inoculation. Although the indicator plant grafting identification is more accurate than the field diagnosis method, but the cycle is too long, which is not conducive to rapid detection.
3.3.1Identification of woody plant indicators. The indicating hosts used in different regions are also different, such asCitrussinensisOsb.cv.Valenciaused in South Africa and Citrus aurantifolia used in India as the indicator plants for HLB disease. Ponkan is often used as an indicator of HLB disease in China. After grafting, the leaves of Ponkan turn mottled yellowing at 25℃ to 32℃ for about 3 months.
3.3.2Identification of herbaceous plant indicators. The herbaceous indicator plantCatharanthusroseuswas planted in the insect-proof screen. When the plant grew to 4-6 leaves, it was inoculated withCuscutareflexaorCuscutacampestris.CatharanthusroseusandCuscutachinensisinoculation began to show obvious symptoms about three months. At the beginning, the lower leaves began to yellow from the veins, and the upper leaves still showed healthy green. After that, the whole leaf gradually yellowed uniformly, and the yellowing leaves gradually increased from bottom to top, and finally the yellowing leaves gradually fell off.
3.3.3Identification of virus transmission by psyllid. From the suspected diseased plants in the field, 50-100 psyllids were collected and put onto the indicating plants. Or the 4th and 5th instar nymphs or adults were used to take virus on the diseased plants for 30 min, lasting 8-12 d. The infectivity was maintained throughout the life cycle, and finally the changes in indicators were observed.
3.4ReagentidentificationIn this paper, "HLB disease detection reagent (patent No.201820411023.5)" was used inCitrusgrandisproduction, and its accuracy was verified. In this paper, it is considered that the reagent can be used in combination with field identification method, which increases the accuracy of the judgment ofCitrusgrandisHLB disease, and can achieve the purpose of rapid detection and low cost at the same time.
3.4.1Verification of accuracy. In the project of "Science and Technology Demonstration Manor of Plateau Characteristic Crop (Ruili pomelo)", four samples ofCitrusgrandisbranches were collected in Ruili crystal pomelo orchard when 3002 Guanxi honey pomelo trees were used for top grafting (replaced by Ruili crystal pomelo). They were sent to Huazhong Agricultural University for laboratory identification of HLB disease (HLB), bark cracking disease (CEVd), decline disease (CTV) and tatter leaf virus (CTLV) (samples sent on December 5, 2017, report available on December 26, 2017).
In order to verify the accuracy of "HLB disease detection reagent", we took samples from the same tree in the same pomelo orchard and used "HLB disease detection reagent" for rapid detection of HLB disease. The test results and the original identification results of Huazhong Agricultural University are summarized in Table 1.
Table1ComparisonofdetectionresultsofHLBdiseasedetectionreagentandlaboratoryidentificationmethod
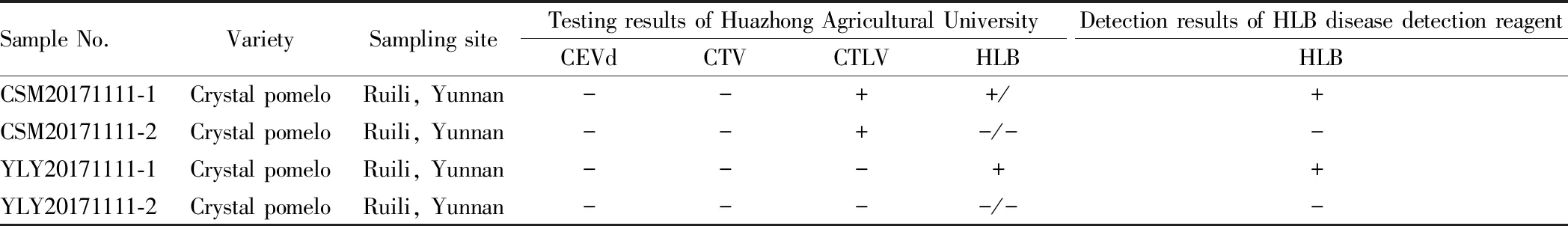
Sample No.VarietySampling siteTesting results of Huazhong Agricultural UniversityCEVdCTVCTLVHLBDetection results of HLB disease detection reagentHLBCSM20171111-1Crystal pomeloRuili, Yunnan--++/+CSM20171111-2Crystal pomeloRuili, Yunnan--+-/--YLY20171111-1Crystal pomeloRuili, Yunnan---++YLY20171111-2Crystal pomeloRuili, Yunnan----/--
Note: +for positive, -for negative; nested-PCR method was used to identify HLB disease in Huazhong Agricultural University; the "HLB disease detection reagent" was judged to be negative when the color of the reagent was yellow or brown, and positive when it was black or dark black.
From the comparison in Table 1, it is found that the detection results are consistent. However, there are many problems in the use of "HLB disease detection reagent". The results of new shoots collected from CSM20171111-1 and YLY20171111-1 samples were negative, but the results of old leaves were positive. Therefore, the correct way to use the "HLB disease detection reagent" is: for eachCitrusgrandistree, more than ten old leaves or symptomatic leaves should be collected for detection respectively, as long as one leaf is positive (+), it is determined that theCitrusgrandistree is a HLB disease tree. If there are too few leaves detected, the guiding significance of the detection of "HLB disease detection reagent" is not great.
3.4.2Application of HLB disease detection reagent. In the pomelo orchard of Buyi Farm in Ruili City (97°44-32″ E, 23°57-39″ N), 12Citrusgrandistrees suspected of HLB disease were sampled and detected. ten old leaves or mottled leaves were collected from each tree and it was detected ten times per tree. It was found that four trees were identified as positive (+), belonging to HLB disease tree. The remaining eightCitrusgrandistrees suspected of HLB disease were determined to be non-HLB disease trees. By examining the root system, the root system of eight non-HLB disease trees started to rot, and it should be yellowing caused by root rot.
4 Methods of prevention and treatment of HLB disease
4.1"Three-stepmethod" Up to now, citrus HLB pathogen can not be purely cultured in vitro, so the study of the disease is restricted[1]. There is also no drug on the market that directly kills the pathogen of HLB disease (Candidatusliberibactersp). Therefore, for the control of HLB disease, we have always used the "three-step method" put forward by Chinese plant pathologist Professor Lin Kongxiang[2]. That is, non-toxic reproductive materials are used to eradicate pathogens (trees) and control psyllids. This method is still the only method for the prevention and control of HLB disease, and has been widely used in the world.
4.2Strictimplementationofplantquarantineandestablishmentofdisease-freenurseriestocultivatedisease-freeseedlingsIn order to prevent HLB disease from spreading from the epidemic area to the new area, strengthening plant quarantine is the most important means. Ruili municipal government is guiding and building Nongdao pomelo manor. The intervention of the government and the strict implementation of the plant quarantine system is an important means to ensure the green and sustainable development of Nongdao pomelo manor.
The cultivation of disease-free seedlings is the basic guarantee to prevent citrus HLB disease. Therefore, agricultural scientific research institutes and seedling cultivation companies must establish disease-free nurseries, and the disease-free nurseries should be far away from or isolated from HLB epidemic areas. In addition to the strict selection of disease-free mother plants, reproductive materials should also be sterilized. Scions or seedlings were treated with hot and humid air at 49℃ for 50 min. Or scion was treated intermittently with 47-50℃ hot water for 6-12 min. They were treated 3 times every 24 h. Or the scion was sterilized with a mixture of tetracycline and dimethoate and soaked for 1-2 h. Then it was rinsed with clean water and dried for later use[7], which can effectively remove HLB pathogen.
4.3ResolutecontrolovercitruspsyllidCitrus psyllid is a small plant-feeding insect in the faimily of Psyllidae, mainly harming Rutaceae plants. The host plants of citrus psyllid are citrus,Clausenalansium,Murrayaexotica,Lyciumbarbarumand orange,etc. Among them, psyllid is the most harmful to citrus and is the main natural vector to transmit citrus HLB disease[8]. The main psyllids transmitting HLB disease areDiaphorinacitriandTriozaerytreae. However,CacopsyllacitrisugaYang&Li, which was found in Dehong Prefecture of Yunnan Province in recent years, is also a vector of HLB disease[9]. The morphological characteristics of the adults of three species of psyllid are shown in Fig.1.

Fig.1AdultsofDiaphorinacitri,TriozaerytreaeandCacopsyllacitrisugaYang&Li
The control of these three species of psyllids transmitting HLB disease is an important link in the effective control ofCitrusgrandisHLB disease.
4.3.1Control of citrus psyllid by predatory natural enemy ladybug. In Florida,HyperaspisgyotokuiKamiya,Coelophorabiplagiata,Cyclonedasanguinea, andHarmoniaaxyridis,etc. are reported to be the main predatory natural enemies of citrus psyllid. Under natural conditions, their cumulative predation on citrus psyllid eggs and nymphs can reach 80%-100%[10]. Pang Hong, from Guangdong Insect Research institute, found thatMenochilussexmaculata,PhrynocaeiacongenerandPropylaeajaponicacan prey not only on nymphs with weak crawling ability, but also on adults of psyllid with strong activity. The predation ofLemnia(artemis)circumstaon citrus psyllid nymphs in individual orchards can exceed 80%. These ladybugs play an important role in controlling citrus psyllid in nature[11].
Therefore, the scientific and proper release of predatory natural enemy ladybug in pomelo orchard can reduce the population base of citrus psyllid, which is beneficial to the control of citrus psyllid in pomelo orchard, but it must be combined with chemical control in order to achieve twice the result with half the effort.
4.3.2Chemical control of citrus psyllid. Chemical control of citrus psyllid is still the main means ofCitrusgrandisproduction. The technical essentials are: selecting effective pesticides, scientific preparation of pesticides, reducing or delaying the resistance of psyllid to pesticides; selecting a good control period to avoid the spread of HLB disease.
The following pesticides recommended for the control of citrus psyllid. (i) Urea insecticides: Lufenuron; (ii) Pyrethroid insecticides: Deltamethrin, Bifenthrin, Fenpropathrin, Lambda-cyhalothrin,etc.; (iii) The second generation nicotine insecticides: Thiamethoxam, Clothianidin,etc.; (iv) Macrolides insecticides: Abamectin, Spinosad,etc.; (v) Thiadiazine insecticides: Buprofezinetc.; (vi) Organophosphorus insecticides: Chlorpyrifos,etc.; (vii) Pyridine insecticidal or acaricidal agents: Imidacloprid, Acetamiprid,etc.; (viii) Benzamide insecticides: Chlorantraniliprole,etc.; (ix) Biological insecticidal or acaricidal agents: Emamectin benzoate,etc.; (x) Insect growth regulator: Pyriproxyfen.
Chemical control period. 6-12 generations of citrus psyllid occur annually. The adults overwinter on the back of theCitrusgrandisleaves and lay eggs on the spring shoots in early to mid-March of the following year. The first generation occurs from mid-March to early May, and the last generation occurs from early October to early December. As long as there are young shoots, adults will lay eggs, and the number of young shoots determines the number of generations. With the peak of spring shoot, summer shoot and autumn shoot, there are three peaks of nymphs. Therefore, the period of chemical control is the appearing period of spring shoot, summer shoot and autumn shoot.
4.4RemovalofdiseasedtreesThe practice at home and abroad has proved that timely removal of diseased plants is the key means to effectively prevent and control HLB disease. Only cutting off the diseased branches is ineffective in the prevention and treatment of HLB disease. After digging up the diseased tree, the seedlings can be replenished after spreading appropriate amount of quicklime in the hole and exposure to the sun for 10-20 d.
5 Conclusions and discussions
Because of the long incubation period of HLB disease, there are no symptoms or no obvious symptoms in the early infection stage ofCitrusgrandis. Therefore, how to detect the diseased tree early is challenging. At present, laboratory identification method is mainly used by experts and scholars in the research. It is difficult forCitrusgrandisgrowers, even major growers, to detectCitrusgrandisHLB disease by laboratory identification. Because of the long time, the indicator identification method has not been found to be used by growers in our investigation, and has also become a method used by experts and scholars. In this paper, it is recommended that the field identification method should be combined with the detection method of "HLB disease detection reagent", which has practical significance inCitrusgrandisproduction.
杂志排行
Asian Agricultural Research的其它文章
- Establishment and Optimization of Two-dimensional Electrophoresis System for Spleen Proteome of Sillago sihama Forsskål
- Breeding of a New Tussah Variety "Gaoyou 1"
- Study of the Discount on Private Placements and Risk of Stock Market Crash in Listed Companies
- Investigation and Analysis on Diversity of Lucanidae spp. in Fanjing Mountain National Nature Reserve
- Spatio-temporal Variability of Disastrous Convective Weather in China from 1961 to 2016
- Impact of Sino-US Trade Friction on Import and Export Trade Pattern of Soybean in Heilongjiang
