Establishment and Optimization of Two-dimensional Electrophoresis System for Spleen Proteome of Sillago sihama Forsskål
2019-04-25ANG
, , , , , ANG
Fisheries College, Guangdong Ocean University, Zhanjiang 524088, China; Guangdong Provincial Key Laboratory of Pathogenic Biology and Epidemiology for Aquatic Economic Animals, Zhanjiang 524088, China; Key Laboratory of Diseases Controlling for Aquatic Economic Animals of Guangdong Higher Education Institutions, Zhanjiang 524088, China; National Demonstration Center for Experimental Aquaculture Science Education, Zhanjiang 524088, China
Abstract Taking Sillago sihama Forsskål as the research object, through the optimization of the extraction method, hydration and isoelectric focusing, a two-dimensional electrophoresis system was established for spleen proteome of S. sihama Forsskål. The results showed that the twp-step hydration and isoelectric focusing method is better than the hydration and isoelectric focusing integrated method. The protein spots of the spleen of S. sihama Forsskål were detected by two-dimensional electrophoresis mainly at pH 4.0-7.0. The establishment of this technical system will lay a foundation for further research on proteomics of S. sihama Forsskål in the future.
Key words Sillago sihama Forsskål, Spleen, Proteomics, Two-dimensional electrophoresis
1 Introduction
SillagosihamaForsskål (Perciformes: Sillaginidae:Sillago) is long tapered in head and elongated, slightly flattened and slightly cylindrical in body[1]. Adult fish ofS.sihamaForsskål are mostly scattered in the waters of sandy coastal areas. Due to timidity, it often get into sand, commonly known as sand driller or sardine.S.sihamaForsskål has delicious meat, and it is the main edible economic fish in China, and was once the main target fish in China’s sea fishing operations[2]. At present, large-scale farming of this species is emerging. China’s coastal areas, especially western Guangdong, have higher yields.
Proteomics is an important research method in the post-genomics era. It is also a hot spot in current biological research. Proteome was first proposed by Wilkinsetal[3]. in 1994 and was defined as "all proteins expressed by the genome". At present, proteomics has become an important field in life science research. Two-dimensional electrophoresis (2-DE) is one of the core separation technologies of proteomics[4], and is widely used in aquatic species research. It has been used in the studies of proteins ofPseudosciaenacrocea[5],Ctenopharyngodonidellus[6],Boleophthalmuspectinirostris[7], and GIFTOreochromisniloticusI[8]. There are no reports on proteomics ofS.sihamaForsskål in China.
In this paper, the extraction and two-dimensional electrophoresis of protein from spleen ofS.sihamaForsskål were optimized, and a two-dimensional electrophoresis system was established for spleen protein ofS.sihamaForsskål so as to lay a foundation for the next step of research onS.sihamaForsskål proteomics and provide a reference for spleen proteomics research in other fish.
2 Experimental materials
2.1MaininstrumentsandequipmentEttan IP Gphor III horizontal electrophoresis system, Image Scanner III scanner, Manifold strip holder, hydration tray, single standard holder, Immobiline Drystrip strip holder (7 cm, pH 4.0-7.0), GE Healthcare.
2.2MainreagentsIPG dry strip (pH 4.0-7.0), IPG Buffer (pH 4.0-7.0), protease inhibitor, ribozyme inhibitor, Coomassie brilliant blue R-250, urea, thiourea, iodoacetamide, silver staining kit, 2-D Clean-up Kit, 2-D Quant Kit (GE Healthcare); CHAPS (MD); acrylamide, methylene diacrylamide, TEMED, dithiothreitol (DTT) (AMRESCO); protein pre-stained marker (MBI); SDS (MBCHM).
3 Experimental methods
3.1PreparationofproteinsamplesSpleen tissue ofS.sihamaForsskål was collected, washed rapidly with 1 mL of wash buffer (10 mmol/L Tris Cl, pH 8.0, 5 mmol/L magnesium acetate) three times on rice, added with 500 μL of pre-cooled lysate (7 mol/L urea, 2 mol/L thiourea, 1% IPG Buffer pH 4.0-7.0, 4% CHAPS, 1% DTT, 1% protease inhibitor, 1% ribozyme inhibitor), ground, lysed with 500 μL of lysate on ice for 2 h, and centrifuged at 13 000×g for 1 h at 10 ℃ in success. The supernatant was transferred to a new centrifuge tube and stored at -80℃.
3.2PurificationandquantificationofproteinThe concentration of the protein samples was determined with 2-D Clean-up Kit and 2-D Quant Kit according to corresponding instructions.
3.3OrdinarySDS-PAGE
3.3.1Dissociating non-continuous buffer system vertical electrophoresis. A certain amount (10 μg) of the purified spleen protein ofS.sihamaForsskål was added to each of the holes. In 5% spacer gel, electrophoresis was performed at 80 V. After entering the separation gel, electrophoresis was performed at 180 V. Electrophoresis was ended till the protein was approximately far from the bottom of the gel.
3.3.2Coomassie brilliant blue G-250 staining. The gel that was peeled off from the glass plate was removed from fixing solution (10% glacial acetic acid, 40% methanol), placed in staining solution (10% glacial acetic acid, 45% methanol, 0.1% Coomassie brilliant blue R-250) for 4 h or overnight, and decolorized in decolorizing solution (5% glacial acetic acid, 20% methanol) for 3 h successively.
3.4Two-dimensionalelectrophoresisAccording to GE Healthcare’s two-dimensional electrophoresis operation manual, two-dimensional electrophoresis was performed.
3.4.1Hydration. For 7 cm pH 4.0-7.0 strips, 120 ug of protein was loaded to each strip. Hydration was performed in two ways, namely hydration focusing integrated method (using single standard strip holders) and hydration focusing two-step method (using a hydration tray). Each strip was hydrated at 20℃ for 12 h.
3.4.2One-dimensional isoelectric focusing (IEF). (i) 100 v 1 h, 200 v 1 h, 300 v 30 min, 1 000 v 1h, 5 000 v 2 h, 5 000 v 2 h to isoelectric focusing. (ii) After the isoelectric focusing was over, the IPG strips were quickly removed and equilibrated in equilibration solution A (6 mmol/L urea, 2% SDS, 50 mmol/L Tris-HCl pH 8.8, 30% glycerol, 0.2% DTT and bromophenol blue) and equilibration solution B (6 mmol/L urea, 2% SDS, 50 mmol/L Tris-HCl pH 8.8, 30% glycerol, 3% iodoacetamide and bromophenol blue) for 15 min separately.
3.4.3Two-dimensional SDS-PAGE. The equilibrated 7 cm strips were transferred into a small electrophoresis tank for second-direction electrophoresis. The concentration of the polyacrylamide gel was 12%. Spacer electrophoresis and separation electrophoresis were performed at 80 V (30 min) and 180 V, respectively. The test was repeated three times.
3.4.4Silver staining. After the electrophoresis was finished, the gel was taken out and stained with a silver staining kit. The procedure was as follows: fixative for 30 min, sensitizing solution for 30 min; washing with ultrapure water 3 times with 5 min per time; silvering solution for 20 min; washing with ultrapure water 2 times with 1 min per time; developing solution for about 2 h; stop solution for 5 min; washing with ultrapure water 3 times with 5 min per time; preservation solution for 30 min. The whole process was carried out on a horizontal shaker. The speed of the shaker was not too fast to avoid gel damage.
3.5ElectrophoregramscanningThe protein gel was stained and image-scanned using an Image Scanner III transmission scanner to obtain an electrophoregram.
4 Results
4.1OrdinarySDS-PAGEelectrophoregramofspleenproteomeofS.sihamaForsskålThe results of SDS-PAGE electrophoresis of the spleen protein ofS.sihamaForsskål are shown in Fig.1.
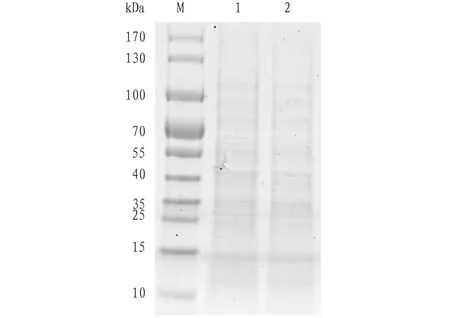
Note: M. marker; 1-2. Spleen protein ofS.sihamaForsskål.
Fig.1SDS-PAGEelectrophoregramofspleenproteinofSillagosihamaForsskål
4.2Conditionalexplorationoftwo-dimensionalelectrophoresisforspleenproteomeofS.sihamaForsskålUsing spleen protein ofS.sihamaForsskål as a sample, the hydration focusing integrated method and the hydrating focusing two-step method were used to explore the 2-DE conditions. The results showed that the 2-DE electrophoregram of the integrated method showed more horizontal stripes (Fig.2a), while the 2-DE electrophoregram of the hydration focusing two-step method showed clearer protein spots and less horizontal strips (Fig.2b). It shows that the hydration focusing two-step method is better.

Note: a. hydration focusing integrated method; b. hydration focusing two-step method.
Fig.2Conditionalexplorationoftwo-dimensionalelectrophoresisforspleenproteinofSillagosihamaForsskål
5 Discussions
In this study, SDS-PAGE electrophoresis was used to analyze the spleen protein ofS.sihamaForsskål, with 12% separation gel. It has an effective linear separation range of only 12-60 kD. The results showed that SDS-PAGE electrophoresis cannot resolve proteins with similar molecular weights and different isoelectric points.
Two-dimensional electrophoresis combines the isoelectric point and molecular weight of a protein. It can separate mixed proteins, and is one of the key technologies for proteomic analysis. It has the advantages of high resolution and good stability. At present, there are rare reports on the two-dimensional electrophoresis of spleen tissue at home and abroad. A set of methods for spleen proteomics research has not yet been systematically summarized. In this study, the two-dimensional electrophoresis technique was used to explore the conditions for separation of protein from spleen tissue ofS.sihamaForsskål. It was found that there are many factors affecting the 2-DE electrophoregram, and the conditions need to be optimized. First of all, sample preparation is the primary factor affecting the high resolution of 2-DE. The extraction of protein from the spleen tissue ofS.sihamaForsskål was completed at low temperature. Protease inhibitor and RNase inhibitor were added to effectively avoid protein degradation and try to get intact protein. DTT reducing agent was added for cleavage of disulfide bonds to make the spleen protein ofS.sihamaForsskål reduced, thereby increasing the solubility of the protein. The bands of the protein extracted from spleen tissue ofS.sihamaForsskål were clear and have no tailing (Fig.1). The results showed that the extracted spleen protein was of superior quality. Secondly, isoelectric focusing is an important factor influencing the high resolution of 2-DE. Fig.2a shows the electrophoregram of hydration focusing integrated method combining standard strip holder and isoelectric focusing electrophoresis. In the isoelectric focusing two-step method, the protein samples were hydrated in a hydration tray (about 20℃) for 16 h. Then, the gel strips were transferred to the Manifold strip holder, which was installed to the Ettan IP Gphor III electrophoresis instrument for isoelectric focusing. The focusing program was the same as the hydration focusing integrated method. Fig.2a shows more horizontal stripes, which might be due to incomplete focusing. The protein spots on the electrophoregram shown in Fig.2b had higher resolution, and the isoelectric focusing was relatively ideal, suggesting that the hydration focusing two-step method is better than the hydration focusing integrated method.
杂志排行
Asian Agricultural Research的其它文章
- Study of the Discount on Private Placements and Risk of Stock Market Crash in Listed Companies
- Breeding of a New Tussah Variety "Gaoyou 1"
- Impact of Equity Pledge Behavior on Cash Holdings
- Investigation and Analysis on Diversity of Lucanidae spp. in Fanjing Mountain National Nature Reserve
- Spatio-temporal Variability of Disastrous Convective Weather in China from 1961 to 2016
- Impact of Sino-US Trade Friction on Import and Export Trade Pattern of Soybean in Heilongjiang
