Circulating factors present in the sera of naturally skinny people may influence cell commitment and adipocyte differentiation of mesenchymal stromal cells
2019-04-24NicolaAlessioTizianaSquillaroVincenzoMondaGianfrancoPelusoMarcellinoMondaMariarosaABMeloneUmbertoGalderisiGiovanniDiBernardo
Nicola Alessio,Tiziana Squillaro,Vincenzo Monda,Gianfranco Peluso,Marcellino Monda,Mariarosa AB Melone,Umberto Galderisi,Giovanni Di Bernardo
Abstract
Key words: Mesenchymal stromal cells;Senescence;Cytokines;Adipogenesis;Brown fat
INTRODUCTION
Epidemiologic studies recorded an increase of overweight and obesity prevalence worldwide[1].Body mass index (BMI) is a parameter used to define obesity,even though it provides incomplete information about fat distribution and does not distinguish between fat mass and lean mass.BMI is the numeric value obtained by dividing the weight (expressed in kg) by the height square (m2).Physicians use BMI to classify overweight,obese and normal weight in adults.The World Health Organization defines “overweight” as a physiological condition with a BMI ranging from 24.99 to 29.99,while “obesity” is characterized by a BMI greater than or equal to 30.Normal weight exhibits a BMI ranging from 18.5 to 24.9[2].
Essential obesity is characterized by an increase in the volume of fat cells due to an imbalance between real energy requirement and daily caloric intake[3].In 2015,obesity contributed to 4.0 million deaths and to 120 million disability-adjusted life years[4].Cardiovascular disease was the leading cause of these deaths and these disabilityadjusted life years,followed by diabetes,chronic kidney disease,and,finally,cancers[5,6].
Multiple causes determine obesity,including biological,environmental,and behavioral factors.Genetic predisposition to the development of excess weight plays a very important role.At the same time,there are genetic/epigenetic factors that restrict the gaining of body weight,in spite of high-calorie intake,which by far exceeds the body requirements.People with these traits are commonly called skinny people (SP).These individuals have BMIs ranging from 18.5 to 24.9,typical of all normal weight people[7,8].Biological,genetic,and physiological studies on SP are in their infancy.Some authors claim that a correlation exists between body weight and a gene calledAMY1,which is responsible for the production of an enzyme present in human saliva called salivary amylase[9].The copy number ofAMY1gene is not the same in all people.Consequently,the more copies present,the better the digestion of carbohydrates,which appears to be typical of skinny individuals.Other findings on rats suggest that the muscles of SP may just work differently.Some authors have proposed that the neural pathway involved in skeletal muscle thermogenesis,which includes ventromedial hypothalamus,and the central melanocortin system,play an important role in determining fuel partitioning and usage in peripheral tissues[10].
Overall,these studies did not explain why an excess of caloric intake does not induce weight gain in SP.Further examination is necessary.These studies can pave the way to improve the cure of obesity.We aimed to address these issues by analyzing the effect of SP serum components on thein vitrobehavior of adipocyte precursors.
In mammalian evolution,three types of adipose tissues developed:white,brown,and beige[11].White adipose tissue (WAT) represents almost all reserve fat.WAT is specialized in storing and releasing lipids in response to a variety of signals controlling energy balance[12].WAT is involved in the production and secretion of inflammatory cytokines.
Brown adipose tissue (BAT) is involved in thermogenesis,a process mediated by catecholamine,adrenaline,and noradrenaline[13].Recently,a new type of brown-like adipocyte,called beige cells,can be found inside white adipocyte storage such as subcutaneous WAT and perirenal WAT in rodents.The function of beige cells is very similar to brown adipocytes and they are inclined to form small clusters in WAT storage after protracted cold stimulation,a process recognized as browning of WAT[14,15].
Adipocytes originate from mesenchymal stromal cells (MSCs),which can be isolated from human and animal sources.Human MSCs are a heterogeneous population containing multipotent stem cells with the capacity to differentiate into mesodermal derivatives,such as osteocytes,chondrocytes,and adipocytes.For the first time,scientists identified MSCs in bone marrow and then MSCs have been isolated from other tissues containing a stromal component,such as adipose tissue,endometrium,dental tissues,umbilical cord,and Wharton’s jelly[16].
Both white and brown adipocytes originate from mesodermal derivatives,although they derive from different progenitor cells.In particular,Parket al[17]describe two different ways to obtain white and brown cells starting from MSCs.During the final phase of differentiation (from preadipocytes into mature adipocytes),MSCs can be committed to either an adipogenic lineage (known as Myf5-negative cells) or a myogenic lineage (Myf5-positive cells).Myf5 is a protein with a key role in regulating muscle differentiation or myogenesis[18].White adipocytes are derived from the adipogenic lineage,whereas brown adipocytes are derived from the myogenic lineage.
In this research,we studied how circulating factors in the sera of SP may affect the biology of MSCs that we obtained from bone marrow of healthy donors with both normal BMI and caloric consumption.We focused our attention on bone marrow MSCs since they are precursor of fat cells present in this semi-solid tissue.Bone marrow adipocytes regulates functions of resident stem cells and may also play a role in energy storage and thermogenesis[19].Changes in circulating factors in obese,overweight,and SP may play role in the phenomena we investigated.Indeed,in a previous study,we provided evidence that serum factors in overweight people strongly affect thein vitroadipogenesis and osteogenesis of normal MSCs[19].
MATERIALS AND METHODS
Ethical approval
The experimental procedures followed the rules approved by the Ethics Committee of the University of Campania “Luigi Vanvitelli.” Specifically,patients were informed of the research and gave permission for the use of serum samples and bone marrow harvests.
Serum samples
Serum samples were collected from six adult male individuals (NP,normal people –BMI < 25;age range:20-30 years old) and six adult male skinny individuals (SP – BMI< 25;age range:20-30 years old),after informed consent.Whole blood samples (10 ml)were collected from patients in Vacutainer test tubes (BD Bioscience,Italy).After collection,the blood was left undisturbed at room temperature to allow clotting.The clots were removed by centrifuging at 4000 rpm for 10 min in a centrifuge.The resultant supernatants were designated sera and were collected with a Pasteur pipette.We pooled sera from the NP and SP to create two different experimental groups:“NP” and “SP”.
Isolation of human MSCs
Bone marrow samples were obtained from healthy donors after written informed consent.Cells were separated on Ficoll density gradient (GE Healthcare,Italy),and the mononuclear cell fraction was collected and washed in PBS.We seeded 1 to 2.5 ×105cells/cm2in alpha-minimum essential medium (alpha-MEM) (EuroClone,Italy)containing 10% fetal bovine serum (FBS) (EuroClone,Italy),100U/mL penicillin and 100 mg/mL streptomycin (EuroClone,Italy),1× L-Glutamine (EuroClone,Italy),and 3 ng/mL basic fibroblast growth factor (bFGF) (Preprotech,United Kingdom).After 72 h,non-adherent cells were discarded and adherent cells were further cultivated to confluency and amplified at P1.Then,the medium was changed every 3 d and cells harvested when they reached 70%–80% confluence using Trypsin-EDTA (EuroClone,Italy) and routinely sub-cultured at 1:3 dilution.
We evaluated on MSCs the expression of markers recognized as one of the criteria to identify MSCs[20].We verified by immunocytochemistry that MSCs expressed the surface antigens CD73,CD90 and CD105.
MSCs treatment with sera from NP and SP (serum priming)
For evaluation of the patients’sera effects onin vitroMSCs functions,cells were incubated for seven days in alpha-MEM containing 10% patients’sera and 3 ng/mL β-FGF.At the end of that time,cultures were used for further analyses.This procedure was named serum priming.All the experiments were carried out following this priming procedure.
Proliferation assay
Proliferation cells were detected with Cell Counting Kit-8 (CCK-8) colorimetric assay(Dojindo,Germany).This kit contains a highly water-soluble tetrazolium salt,WST-8,which is reduced by cell dehydrogenase activities producing an orange-color formazan dye.The amount of the formazan dye,generated by the activities of dehydrogenases in cells,is directly proportional to the number of living cells.Specifically,1 × 103cells after the treatment with sera were seeded in 96-wells and WST-8 was added.Twenty-four hours,48 h,and 72 h after the incubation,cell proliferation was evaluated by a microplate reader at 450 nm.
In situ senescence-associated b-galactosidase assay
Cells were fixed using a solution of 2% formaldehyde and 0.2% glutaraldehyde.After that,cells were washed with PBS and then incubated at 37 °C for at least 2 h with a staining solution [citric acid/phosphate buffer (pH 6),K4Fe(CN)6,K3Fe(CN)6,NaCl,MgCl2,X-Gal].The percentage of senescent cells was calculated by the number of blue,b-galactosidase-positive cells out of at least 500 cells in different microscope fields,as already reported[21].
Cell cycle analysis
For each assay,cells were collected and fixed in 70% ethanol,followed by PBS washes,and finally were dissolved in a hypotonic buffer containing propidium iodide.Samples were acquired on a Guava EasyCyte flow cytometer (Merck Millipore,Italy)and analyzed with a standard procedure using EasyCyte software.
Nexin-V assay
Apoptotic cells were detected using a fluorescein-conjugated with Nexin V kit on a Guava EasyCyte (Merck Millipore,Italy) flow cytometer,following the manufacturer’s instructions.The kit utilizes 2 separate dyes (Annexin V and 7AAD) to identify a broad spectrum of apoptotic and non-apoptotic cells.Annexin V (red) binds to phosphatidylserine on the external membrane of apoptotic cells,while 7AAD (blue)permeates and stains DNA in late-stage apoptotic and dead cells.Staining allows the identification of 3 cell populations:non-apoptotic cells (Annexin V- and 7AAD-);early apoptotic cells (annexin V+ and 7AAD-);and late-apoptotic or dead cells (Annexin V+and 7AAD+).In our experimental conditions,early and late apoptotic cells were grouped together.
Colony forming units assay
One thousand cells from MSC culture were plated in a 10 cm culture dish.Cells were maintained for two weeks in a growth medium.At the end of this period,the medium was eliminated and colonies were fixed with methanol (100%) for ten minutes at -20°C,then were stained with crystal violet (0.01% w/v) for 30–60 min.The number of colonies in culture dishes was counted under a light microscope.
Clonal differentiation
MSC cultures were trypsinized,then one cell/well was seeded in 96MW and incubated for 14 d in a growth medium.Subsequently,75 clones from NP and 75 clones from SP were collected and the cells were expanded individually to 70%–80%confluency before being to split in four wells of 24MW.Each well was induced to differentiate or in Adipo or in Osteo or in Chondrocyte or not differentiate[22].
In vitro adipocyte differentiation and Oil Red Oil staining
We plated MSCs (5 × 103cell/cm2) in six-well plates and grown them in DMEM medium.At 70%–80% cell confluence,we eliminated the growth medium and added the adipogenic induction medium (high-glucose DMEM,10% FBS,1 mmol/L dexamethasone,10 µg/mL insulin,200 µmol/L indomethacin,0.5 mmol/L 3-isobutyl-1-methylxanthine).MSC cultures were then incubated for 21 d.Oil Red O stain,which is an indicator of intracellular lipid accumulation,was used to confirm adipogenic differentiation.In brief,differentiated cultures were washed with PBS and then fixed with 4% formaldehyde for ten min at RT (room temperature).Cells were subsequently washed with isopropanol (3%),and treated with Oil Red O staining solution.Stained cultures were analyzed under the light microscope[21,23].All reagents were from Sigma-Aldrich (Saint Louis,United States).
In vitro osteogenic differentiation and Alizarin Red S staining
Osteogenic differentiation was performed by culturing 5 × 103cell/cm2in six-well with DMEM medium (EuroClone,Italy) supplemented with 10% FBS (EuroClone,Italy),0.05 mmol/L ascorbic acid (Sigma-Aldrich,Saint Louis,United States),10 mmmol/L β-glycerophosphate (Sigma-Aldrich,United States),and 100 nmmol/L dexamethasone (Sigma-Aldrich,United States) for 21 d,with changes of medium every 3 d.To visualize calcium sediments,cultures were stained with Alizarin Red S(Sigma-Aldrich,United States) and the microscope images were acquired[21].
In vitro chondrogenic differentiation and Alcian Blue staining
We plated MSCs (5 × 103cell/cm2) in chondrogenic medium (DMEM,1% FBS,0.1 mM dexamethasone,50 nmol/L ascorbate-2-phosphate,10 ng/mL human TGF-β1).We changed medium every 3 d.Alcian Blue staining was used to identify glycosaminoglycan formation on the cell surfaces,as already described[21].Briefly,we fixed cells in acetone:methanol solution (at 4 °C) for 3 min and then we incubated cells at room temperature in Alcian Blue solution (1%) for 30 min.After we rinsed cells thrice in acetic acid (3%) and then in water for 2 min.Stained cultures were analyzed under the light microscope.All reagents were from Sigma-Aldrich (Saint Louis,United States).
RNA extraction and RT-qPCR
We used TRIREAGENT (Molecular Research Center Inc.,United States) to extract RNA from cell cultures.We measured the mRNA levels by RealTime-PCR amplification,as already reported[21].From nucleotide data bank (National Center for Biotechnology Information),we retrieved mRNA sequences to design primer pairs for RT-PCR reactions (Primer Express,Applied Biosystems,United States).As controls,appropriate regions of GAPDH mRNA were used.Real-time PCR assays were carried out on an Opticon 4 machine (MJ Research,United States).Reactions were performed according to the manufacturer’s instructions.We used SYBR green PCR master mix and the 2-ΔΔCTmethod was employed as a relative quantification method.
ATP assay
ATP Fluorometric Assay Kit (BioVision,United States),was used to measure intracellular ATP levels.The procedure is based on glycerol phosphorylation to obtain a product that is quantified by fluorometric methods (Ex/Em = 535/587 nm).A microplate reader and 96-well black bottom plates were used to perform the measurements according the manufacturer's instructions.
MitoTracker and Bodipy staining
To label mitochondria,cells were incubated with 100 nmol/L of MitoTracker®(Invitrogen,Italy) probes,which passively diffuses across the plasma membrane and accumulates in active mitochondria.Cell samples were incubated for 30 min in DMEM free serum at 37 °C in the culture incubator.Then cells were acquired on a Guava EasyCyte flow cytometer (Merck Millipore,Italy) and analyzed with a standard procedure using EasyCyte software.
Cytokine array
The relative levels of 62 cytokines were analyzed in NP and SP groups by the Human Obesity Antibody Array C1 (RayBiotech,United States).A total of 2 mL of the serum samples for each of the two experimental groups was used for the hybridization with the nitrocellulose membranes provided by the manufacturer.Each membrane contains 62 antibodies spotted in duplicate on the surface.The reactions of hybridization and the signal evaluation were performed according the manufacturer’s instructions.We acquired array signals with the Chemidoc system (Bio-Rad Company,United States) and the related software QuantityOne.The images coming from hybridized arrays were used for signal quantification (pixel density).All the hybridized membranes were analyzed simultaneously.
Statistical analysis
Statistical significance was evaluated usingANOVAanalysis followed by Student’stand Bonferroni’s tests.We used mixed-model variance analysis for data with continuous outcomes.All data were analyzed with a GraphPad Prism version 5.01 statistical software package (GraphPad,United States).
RESULTS
SP sera did not affect the proliferation,cell cycle,and senescence rate of MSC cultures
Serum samples were collected from 12 adult men with normal BMIs.We selected only male since estrogen fluctuation may introduce further complexity to data analysis.Indeed,estrogen may influence MSC osteo-adipo commitment[24,25].Six had caloric intakes corresponding to their needs (NP) and six remained skinny while consuming high-calorie diets,which exceeded by at most 30%–40% of the daily caloric intake(SP).
Significant intra- or inter-group variations were not detected in the levels of the principal blood serum biochemical parameters (Table 1).For this reason,to compensate for the limited sample numbers and to reduce biological variation[26],we pooled sera from the normal weight and SP samples,creating two different experimental groups:NP and SP sera,respectively.Figure 1 describes the research strategy.Specifically,cells were primed for 7 d with SP or NP sera and then cultivated in canonical media for biological assays or differentiation analyses.
We evaluated whether somein vitrobiological properties of MSCs were differently affected by incubation with SP sera compared with NP sera.Proliferation rates and cell cycle profiles of MSCs incubated with SP did not differ significantly from those treated with NP (Figure 2A and D).Likewise,the senescence process was also unaffected by SP treatment,as detected by the acid beta-galactosidase assay as compared to the control (Figure 2B).Contrarily,the Annexin assay showed a significant reduction in the percentage of apoptotic cells in cultures treated with SP as compared to the controls (Figure 2C).
SP sera affect MSC cell commitment and adipogenesis
Colony forming units (CFU) assay evaluates cell clonogenicity,which is the capacity of a cell culture to expand at a single-cell level.We performed a CFU assay to ascertain whether MSC cultures can be perturbed by sera treatments.Sera samples from SP,compared with the control,can contain molecules that may influence MSC clonogenicity,i.e.,their stem-cell properties (self-renewal,multi-potentiality).CFU analyses showed no significant difference in colony numbers between MSCs treated with SP or NP sera (Figure 3,upper picture).
Cell commitment and differentiation of MSCs could be affected by serum.Individual MSC clones,obtained by limiting dilutions,were used to determine the multipotentiality.We took 150 clones from MSCs treated with SP or NP sera.MSC cultures are heterogeneous in their content of the progenitor cells.Indeed,MSC cultures present tri-,bi-,and uni-potent progenitors[27].The great majority of analyzed clones (85%) showed differentiation capacity either in samples treated with SP or NP.Specifically,in SP treated samples,we found an increase in tripotent clones (AOC)along with a reduction in adipo-osteo (AO) clones and a slight increase in adipochondro (AC) clones.These data showed that SP treatment may affect the adipogenesis process (Figure 3,lower picture and table).To gain further insight,we evaluated the ability of MSCs to spontaneously differentiate in adipocytes.Cells were incubated for 21 d with typical MSC growing medium;this step was preceded by 7 d priming with either NP or SP sera samples.In these conditions,we observed a reduced proneness at adipogenesis of SP primed MSCs cultures compared with control as evidenced by qRT-PCR analysis of differentiation markers (Figure 4).
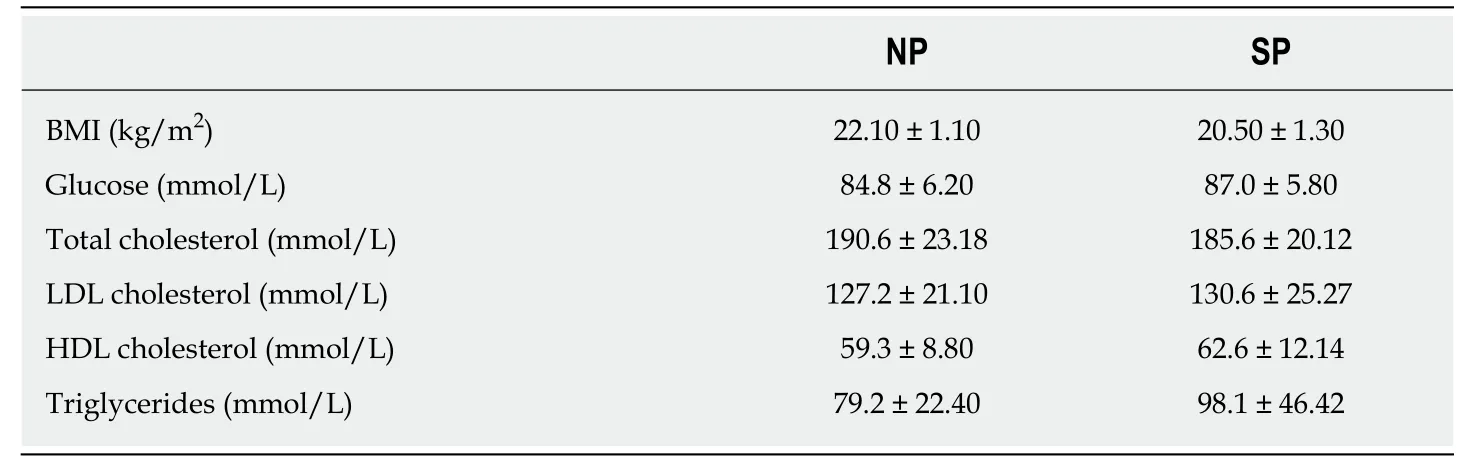
Table1 Normal and skinny patient parameters
SP sera affect exogenous-induced adipocyte differentiation
We further evaluated whether SP or NP sera treatments can perturb adipogenesis.MSC cultures,after an initial priming of 7 d with NP or SP sera,were stimulated(induced differentiation) for 21 d in mesenchymal stem cell adipogenic differentiation medium.SP and NP sera treatment induced the same percentage of differentiated adipocytes (40 ± 6%) and (45 ± 4%),respectively (Figure 5A).Nevertheless,Oil Red O staining method showed that in SP samples the size of lipid droplets is by far smaller than in NP (Figure 5B).We then performed gene expression analysis of early (C/EBPß and C/EBPδ) and late [PPARγ,C/EBPα,LPL,and adipose triglyceride lipase(ATGL)] adipocyte differentiation markers.
There were no significant differences between the two experimental conditions (NPvsSP).Strikingly,we detected significant differences concerning the ATGL mRNA level,which was higher in SP-treated cells compared to controls (Figure 5C).The enzyme ATGL is the primary triglyceride lipase that,within lipid droplets,breaks down triglycerides,releasing fatty acids and glycerol[28].These data agree with the presence of smaller lipid droplets in SP-treated samples,due to high ATGL expression than can degrade triacylglycerols.High ATGL activity in SP-treated samples should release a high content of fatty acids that cells could utilize for energy production[29].To corroborate this hypothesis,we evaluated ATP production under starvation conditions in SP and NP samples.ATP production in cells incubated in minimal medium without energetic supplements were significantly higher in SP pretreated cultures compared to control (Figure 5E).Also,data regarding number of mitochondria per cell are in agreement with our hypothesis.Indeed,SP pretreated cells showed a significant increment of mitochondria compared with control (Figure 5D).
The bio-physiological features of adipocytes we obtained by pretreating MSC cultures with SP sera suggest that the differentiation process produced brown adipocytes rather than white ones.We evaluated UCP1 expression in our samples to further validate our hypothesis.UCP1 is a mitochondrial protein responsible for thermogenic respiration,a specialized capacity of BAT.UCP1 is used as brown adipocyte differentiation markers,because this protein is not expressed in white adipocytes.As shown in Figure 5F,we observed a strong expression of UCP1 mRNA levels only in MSCs treated with SP serum.
Cytokine profiles of SP sera compared with NP ones
We performed a preliminary analysis on cytokine composition of SP sera compared with NP samples,in order to determine the factors present in the sera of SP that may influence MSC biological activities.Initially,we analyzed circulating molecules that can modulate adipose tissue functions[30].Adipose tissue responds both to afferent signals from hormones and the central nervous system but also produces factors with essential endocrine activities.Adipokines (including adiponectin,visfatin,leptin and resistin) and cytokines/chemokines (MCP-1,IL-6 and TNF-α) are among the key factors released by adipose tissue.These proteins play a role in maintaining health.We used the Human Obesity Antibody Array C1 to accurately profile the expression of 62 cytokines involved in obesity.The expression levels of several investigated cytokines did not differ significantly between the SP and NP samples.We identified 17 cytokines whose expression differed between SP and NP samples (ACE-2,Adiponectin (ACRP30),Adipsin (Complement Factor D),Angiopoietin-1,Angiopoietin-2,Fas (TNFRSF6/Apo-1),HCC-4 (CCL16),IGFBP-1,IL-1 alpha (IL-1 F1),LIF,MSP alpha/beta,PAI-1,RANTES (CCL5),Resistin,SAA (Serum Amyloid A),TNF RII (TNFRSF1B),TIMP-1,TIMP-2).We found statistically significant differences only for some of them.In particular,we observed an increased level of ACRP30,ANGPT1,FAS,HCC-4,IGFBP1,RANTES,RESISTIN,and SAA (Figure 6).Cytokine expression trends are consistent with physiological changes expected,as argued in detail in the discussion section.

Figure1 Experimental plan.
DISCUSSION
Adipose tissue,like almost every tissue,contains its own stem cells that represent the reservoir for tissue homeostasis and repair.Within adipose tissue,MSCs are present,which are a heterogeneous population containing multipotent stem cells that can differentiate into adipocytes,osteocytes,and chondrocytes.Stem cell behavior is affected by chemical and physical signals coming from the surrounding environment(niche) but also from distant districts through blood circulation or nervous system signaling.In patients suffering from metabolic syndrome disease and/or obesity,the MSCs,resident in adipose tissue,may show a function impairment.Previously,we showed that sera from overweight people might alter the differentiation potential of MSCs with a bias toward adipogenesis.Our data further confirmed that excessive caloric intake leading to overweight status does not imply a mere aesthetic issue but may have consequences for patient health.
At the same time,it is of great interest to understand why some people who consume high-calorie diets remain thin or skinny (SP),while other individuals become obese under the same conditions.Investigating SP’s status could contribute to the cure of obesity.We then decided to evaluate whether circulating factors present in the sera of SP affect the biology of MSCs.If this hypothesis is proven,we will add new evidence in support of a key role for humoral factors in regulation of adipose stem cell compartment.

Figure2 Mesenchymal stromal cell proliferation,cell cycle analysis,apoptosis,and senescence,following treatment with skinny people and normal people sera.
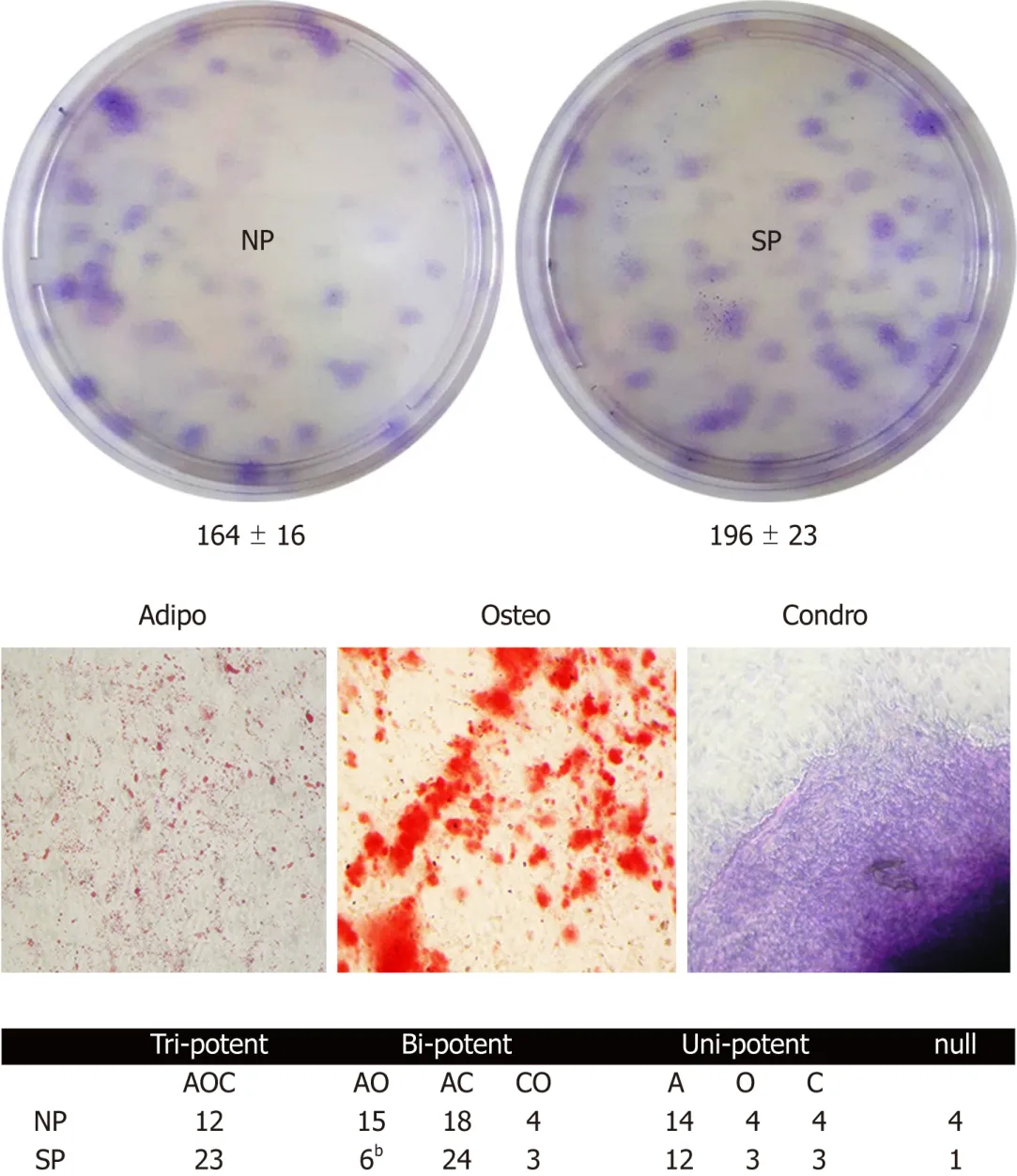
Figure3 Colony Forming Units assay to evaluate the effect of sera on mesenchymal stromal cell stemness.
Our data showed that priming of MSCs with SP can influence cell commitment and differentiation process of MSCs.Specifically,SP sera probably contain molecules that affected adipocyte cell commitment and reduced spontaneous adipogenesis.Besides,apparently,the exogenously induced adipogenesis appeared to occur at the same level in both SP- and NP-primed samples (an equivalent number of differentiated adipocytes,no discrepancies in the expression of differentiation markers).An indepth analysis of differentiation showed,indeed,striking differences between adipocytes differentiation in SP-primed samples compared with NP ones.In adipocytes from SP cultures we observed a reduced size of lipid droplets and an increased expression of ATGL,along with high mitochondria content and ability to produce ATP in starvation conditions.These data,together with expression of UCP1 protein,indicated that SP pretreatment produced a bias toward brown adipocyte differentiation.
The fact that sera from SP may promote brown adipogenesis rather that white adipocyte differentiation could explain why these people present normal body compositions and BMIs,in spite of an excess of caloric intake.We hypothesize that some circulating components present in the blood of these individuals may favor brown adipogenesis at the expense of white adipocyte production.This hypothesis agrees with studies showing that BAT has a key role in blood triglyceride clearance and glucose disposal.Decreasing plasma lipids and lowering plasma glucose may fight obesity and avoid weight gain[31].Future studies should further analyze the body fat composition of SP and the contribution of their BAT to the release of circulating factors,which could contribute to fighting obesity by controlling MSC cell commitment and adipocyte differentiation.
In the above described scenario,we performed a preliminary analysis of SP cytokine content to identify possible molecules that may play a role in the physiopathology of adipogenesis and related phenomena.In sera of skinny individuals,we identified an upregulation of several factors governing phenomena associated with obesity of metabolic syndrome disease.We focused on four factors (ACRP30,ANGPT1,IGFBP1,and RANTES) that could be related to the physiological status of SP:Eating an excess of calories without weight gain.

Figure4 qRT-PCR analysis of adipocyte differentiation markers in mesenchymal stromal cell cultures treated with either normal people or skinny people.
Specifically,in SP samples we found an increased level of Adiponectin/ACRP30(Adipocyte complement-related protein of 30),which is a protein expressed only in adipocytes.Indeed,the serum level and its expression is reduced in obesity status and in patients with insulin resistance.In the last years,some studies have demonstrated the role of adiponectin in the lipid homeostasis and regulation of glucose[32,33].In particular,Fruebis and co-workers demonstrated that a chronic injection of ACRP30 in mice induced a reduction of body weight,independently of food intake.This study supports the assumption that adiponectin contributes to regulating energy homeostasis[32].Other authors have revealed that adiponectin levels increase with weight loss[34].Our results are perfectly in agreement with these observations,because in SP serum we note high levels of ACRP30.
In our analysis,SP exhibited slightly higher levels of ANGPT1 compared with the control.This protein is a vascular maturation regulator expressed in adipocytes.ANGPT1 mRNA levels inversely correlated with the rates of change in body weight.Injecting a plasmid to overexpress ANGPT1 in obese mice induced a reduction of the body weight and fat pads;therefore,it is possible to state the role of angiopoietin-1 in the regulation of adipose tissue growth,suggesting that the vascular remodeling could modify tissue plasticity.
Insulin-like growth-factor-binding protein-1 (IGFBP-1) is involved in the regulation of glucose metabolism,which is associated with insulin resistance and glucose intolerance.High levels of insulin are associated with low IGFBP-1 levels.Low levels of IGFBP-1 are also related with aspects of the metabolic syndrome,obesity,and the progression of cardiovascular disease[35].In our condition,we observed the presence of IGFBP1 only in SP sera.
RANTES,also known as CCL5,has been investigated for its chemokine proinflammatory role;indeed,it is described as a T cell-specific protein.Some years ago,a study[36]highlighted an alternative function for RANTES.This protein modulates the function of Langerhans’islets through its interaction with GPR75 receptor.The interaction between RANTES ligand and GPR75 receptor increases intracellular calcium in beta cells via Gq protein and stimulates insulin secretion useful to reduce hyperglycemia by helping glucose uptake and storage.In our samples,we observed an increase of RANTES levels in SP compared with NP.
Overall,our preliminary findings showed that SP present humoral factors that affect the adipogenesis process by acting on stem-cell commitment and adipocyte differentiation.This process,in turn,may play a major role in controlling body weight and health.Further studies are needed to identify serum key factors that play a role in this process.This could shed light on studies aiming at fighting obesity and related pathologies.
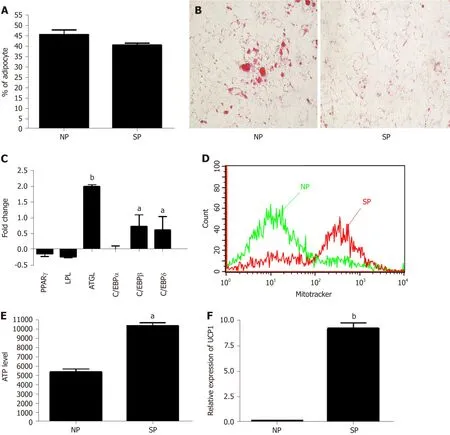
Figure5 Analysis of mesenchymal stromal cell induced adipocyte differentiation after initial priming of 7 d with skinny people sera and subsequently treated with adipogenic differentation medium.
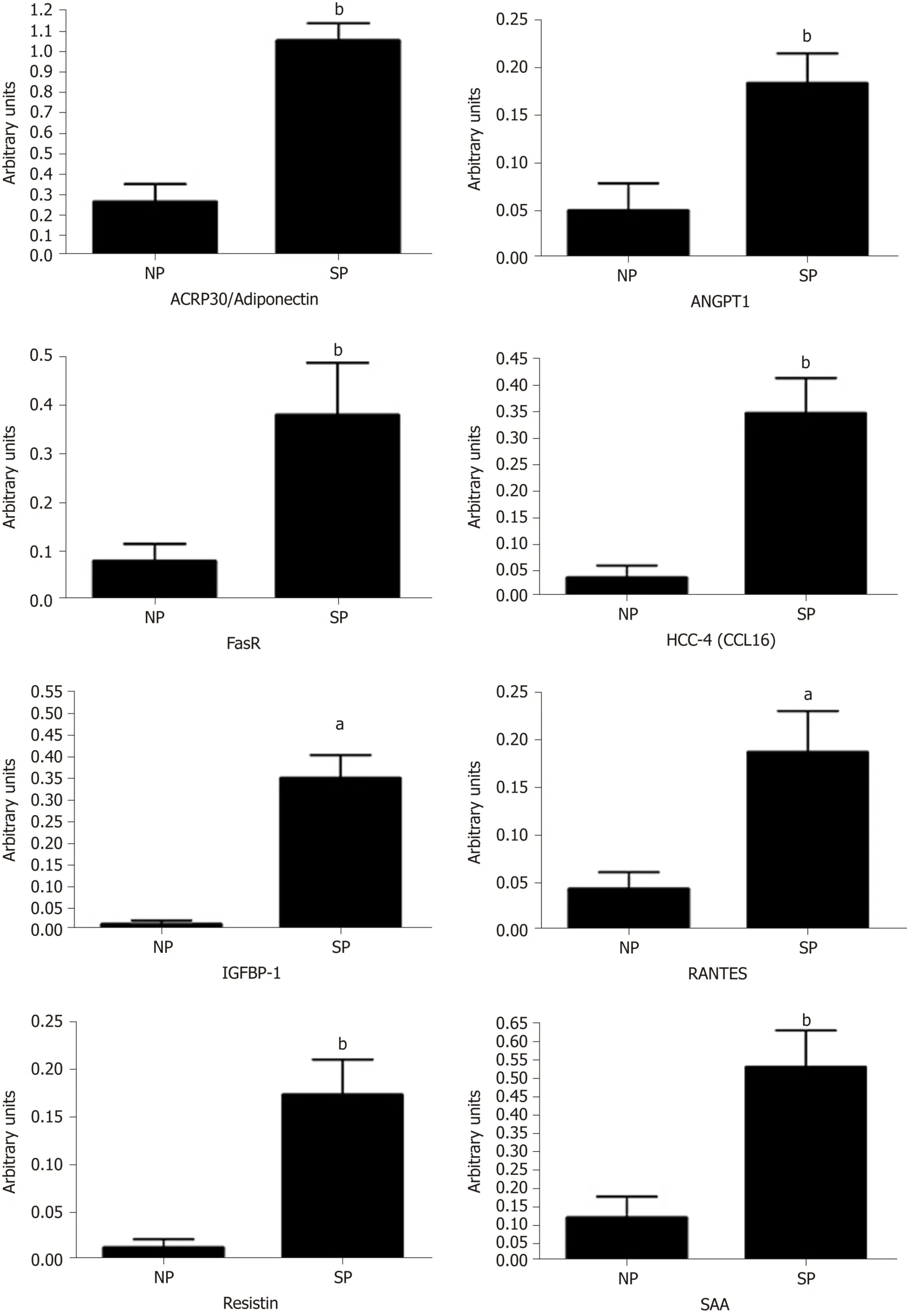
Figure6 Cytokines detection in sera.
ARTICLE HIGHLIGHTS
Research background
Research on physiopathology of obesity may receive new hints from studies on skinny people(SP).These are individuals who show a poor or null gaining of body weight,in spite of highcalorie intake,by far exceeding the body requirements.
Research motivation
It is of great interest to understand why some people who consume high-calorie diets remain thin or skinny (SP),while other individuals become obese under the same conditions.Investigating SP’s status could contribute to the cure of obesity.
Research objectives
We evaluated how circulating factors present in the SP sera may affect adipogenesis of mesenchymal stromal cells (MSCs).
Research methods
We isolated MSCs from bone marrow of healthy donors with both normal body mass index and caloric consumption.MSC cultures were primed with sera collected from SP or normal people(NP).Then biomolecular assays were performed to evaluate effect on proliferation,apoptosis,senescence,cell commitment,and differentiation.
Research results
SP priming affected adipocyte cell commitment and reduced spontaneous adipogenesis.Moreover,an in-depth analysis of exogenous-induced adipocyte differentiation showed striking differences between differentiation in SP-primed samples compared with NP ones.
These data and the expression of UCP1 protein,indicated that SP pretreatment produced a bias toward brown adipocyte differentiation.
Research conclusions
Our data suggest that sera from SP may promote brown adipogenesis rather that white adipocyte differentiation.This finding could explain why SP present normal body composition in spite of an excess of caloric intake.
Research perspectives
Our study could contribute at fighting obesity and related pathologies.
ACKNOWLEDGEMENTS
We thank Dr.Vincenzo Condè (Servizio di Oncologia Pediatrica,AOU,Università della Campania Luigi Vanvitelli) for bone marrow harvests and technical assistance.
