Long non-coding RNA: The functional regulator of mesenchymal stem cells
2019-04-24ZhongYuXiePengWangYanFengWuHuiYongShen
Zhong-Yu Xie,Peng Wang,Yan-Feng Wu,Hui-Yong Shen
Abstract
Key words: Mesenchymal stem cells;Long non-coding RNA;Regulator;Multipotent stroma cells
MESENCHYMAL STEM CELLS
First identified by Friedensteinet al[1]in 1974,mesenchymal stem cells (MSCs) were found to support the hematopoietic stem cell niche in the bone marrow microenvironment.With the advancement of research through these years,it has been established that MSCs are multipotent progenitor cells located in various tissuesin vivo.Moreover,MSCs possess two critical capacities,immunoregulation and multilineage differentiation[2].On the one hand,MSCs modulate the functions of different kinds of immunocytes,such as T cells,B cells and dendritic cells,which greatly contribute to the homeostasis of the immune system[3,4].On the other hand,under different circumstances,MSCs could differentiate into osteoblasts,chondroblasts or adipoblasts,which play an important role in body development and tissue repair[5,6].Benefiting from self-renewal and low immunogenicity characteristics,MSCs with these powerful capacities have been widely used in clinical practice[7-9].
MSCs exhibit diverse capacities under various circumstances (i.e.,inflammation,stress or other factors)[10-12].These external stimuli induce changes in transcriptional regulation in the cells,which modulate the activation of signaling pathways and finally determine the immunoregulatory ability and differentiation direction of MSCs.On the other hand,abnormal transcriptional regulation causes MSC dysfunction,which is also involved in disease progression.Therefore,illuminating the mechanism of the transcriptional regulation of MSCs could not only elucidate the pathogenesis of diseases but also improve their curative effects.Recently,many studies on the mechanism of transcriptional regulation in MSCs have been conducted,and the importance of long noncoding RNA (LncRNA) is coming into focus.
LncRNA
General cell function is under the precise control of various epigenetic mechanisms,including DNA methylation,histone methylation and noncoding RNA[13,14].Noncoding RNA,although spanning more than 98% of DNA transcripts,is not translated into proteins.Rather,these molecules spatiotemporally regulate proteincoding gene expression.Noncoding RNA is defined into two classes,those less than 200 nt,including microRNA,small nuclear RNA and small interfering RNA,and those larger than 200 nt,called lncRNA[14].
LncRNA used to be considered as dark matter.Recently,more evidence has indicated a role for LncRNA as a new player in the functional regulation of various eukaryotes[15].According to its different characteristics,LncRNA is divided into several categories,including sense,antisense,intronic,intergenic and bidirectional LncRNA.These LncRNAs are mainly located in the cell nucleus or cytoplasm,affecting the status and fate of cells through different transcriptional and posttranscriptional mechanisms[16].Nuclear LncRNA guides chromatin modifiers,such as DNA methyltransferase,histone methyltransferase and heteronuclear ribosome protein,to a specific genetic locus and induces chromatin structure remodeling,which in turn regulates gene expression either positively or negatively[17].In addition,some nuclear LncRNAs bind to specific genetic loci by complementary sequences and affect their expression.Moreover,the location and transcriptional status of other nuclear LncRNAs will directly affect their nearby or partial overlapping gene expression mainly through bringing transcriptional enhancers closer to the promoters of a specific gene.Based on the difference in action range,these nuclear LncRNAs are distinguished intocis-acting LncRNAs,which affect the gene expression in the vicinity of the same chromosome,andtrans-acting LncRNAs,which function over great distances as “intercontinental missiles”[17].For cytoplasmic LncRNA,the regulation mechanisms are fundamentally different[18].The most common and widely studied mechanism of cytoplasmic LncRNA is to act as a competitive endogenous RNA (ceRNA).MicroRNA,another type of noncoding RNA,negatively controls the translation of mRNA into protein.LncRNA can act as a microRNA sponge through binding to specific complementary sequences and competitively protect the target mRNA being repressed by microRNA[19].In addition,cytoplasmic LncRNA can act as a decoy for cytoplasmic mRNA by base pairing and then degrading or conversely stabilizing these specific mRNAs.This mode of action may also function at the mRNA translation level when cytoplasmic LncRNA binds to translationally controlled proteins and indirectly modulates target mRNA translation[18].Recently,a novel mechanism of cytoplasmic LncRNA was identified.These cytoplasmic LncRNAs can either block the functional site or alter the structure and modification of specific proteins,thereby regulating the function and stabilization of these proteins.This process leads to the up- or downregulation of signaling pathways and ultimately alters the fate and function of cells[20].These protean and powerful functions allow the wide involvement of LncRNA in all aspects of cell processes.
LNCRNA REGULATION OF MSCS FUNCTION
A number of studies have indicated that LncRNA modulates various functions of multiple cells.In stem cells,LncRNA contributes not only to stemness maintenance but also to multilineage differentiation and other aspects[21-24].In recent years,the role of LncRNA in MSCs has been widely studied,and several critical LncRNAs have been identified with regard to differentiation,immunoregulation,proliferation and apoptosis.In this review,we provide a systematic overview of these studies to provide new insights on the functional role of LncRNA in MSC regulation.
Osteogenic differentiation
MSCs are the major origins of osteoblastsin vivo[25].Under specific conditions or in specific microenvironments,MSCs perform osteogenic differentiation,and this process is under the precise control of a series of transcriptional and posttranscriptional mechanisms.To date,investigation into the expression and regulatory function of LncRNA during MSC osteogenic differentiation has provided the most insight into the role of LncRNA in MSCs.Songet al[26]was the first to detect the LncRNA expression profile of immortalized MSCs on day 28 of osteogenic differentiation with RNA sequencing.Hundreds of differentially expressed mRNAs and LncRNAs and several signaling pathways have been identified.However,the genome of immortalized MSCs has been artificially reconstructed by gene editing technology to infinite multiplication.Therefore,compared to normal MSCs,immortalized MSCs may have partially different transcriptomes.Several studies on bone marrow MSCs undergoing osteogenic differentiation were then conducted to study the LncRNA expression profile using a microarray[27,28].The results using MSCs directly isolated from human bone marrow may reflect the real LncRNA expression profile during osteogenic differentiation.However,studies with microarrays mainly detect identified LncRNAs,while RNA sequencing assays could detect larger changes to identify unknown LncRNAs.Considering that a large number of LncRNAs have not yet been identified,RNA sequencing technology has a better prospect of application in further studies of LncRNA.
Osteogenesis is a dynamic process that results in inconsistent changes in LncRNA expression profiles at any time point.Therefore,the continuous monitoring of LncRNA profiles during the osteogenic differentiation of MSCs is commanded.Recently,Qiuet al[29]reported the global LncRNA expression on days 7,14 and 21 of osteogenic differentiation and identified 665 LncRNAs that were continuously differentially expressed.This result provides a more comprehensive view of LncRNA expression patterns and is helpful in identifying critical LncRNAs.Notably,either the number or the category of the differentially expressed LncRNAs in these described studies is different.This discrepancy could result from the different experimental conditions in these studies.It is necessary to determine the mutual differentially expressed genes in these results,which could represent critical LncRNAs in the osteogenesis of MSCs.
Although the expression profile provides a comprehensive view,identifying the functional LncRNAs that function during the osteogenesis of MSCs is of great importance.H19,a paternally imprinted noncoding DNA,positively regulates the osteogenic differentiation of MSCs via microRNA-657.This complex inhibits TGF-β1 and the phosphorylation of Smad3,therefore promoting MSC osteogenesis[30].This mechanism as a ceRNA to microRNA is common in MSCs during osteogenic differentiation.Jianget al[31]demonstrated that LncRNA-HULC,acting as a ceRNA to microRNA-195,promoted the osteogenic differentiation of MSCs.Moreover,another well-known LncRNA,MALAT1,also up-regulates the critical osteogenic transcription factor Osterix by targeting microRNA-143,which eventually promotes MSC osteogenesis[32].Other regulatory mechanisms also function during the osteogenic differentiation of MSCs,such as DANCR and BDNF-AS[33,34].However,several questions still exist.First,most studies focus on the regulatory mechanisms and downstream targets of these LncRNAs.The upstream regulator that induces expression changes in these LncRNAs during the osteogenic differentiation of MSCs is still unknown.Moreover,a myriad of LncRNAs are related to the osteogenesis of MSCs to a certain degree;nevertheless,vital LncRNAs need to be identified.Last,whether and how these LncRNAs function in bone metabolismin vivoare also unclear.Recently,we determined that LncRNA-OG interacted with hnRNPK and accelerated MSC osteogenesisin vitroandvivoby modulating the BMP signaling pathway.Surprisingly,hnRNPK promoted the transcription of LncRNA-OG by affecting its H3K27 acetylation,therefore forming a positive feedback loop[35].This result indicates that specific proteins,such as hnRNPK,could be both upstream regulators and downstream targets of LncRNA,suggesting a novel mechanism of LncRNA.
The osteogenic differentiation of MSCs is affected by many factors[36].It is very likely that these factors influence MSCs by regulating their LncRNA expression.Osteogenic growth peptide,a short and linear tetradecapeptide in serum,enhances the osteogenic differentiation of MSCs through lncRNA-AK141205[37].In addition,MSCs undergoing osteogenic differentiation in an inflammatory microenvironment caused by Staphylococcal protein A have the specific expression profile of LncRNAs,among which LncRNA-NONHSAT009968 may play a leading role[38].In addition,high glucose conditions inhibit LncRNA-AK028326 expression and therefore prevent MSC osteogenic differentiation[39].Therefore,the conclusion could be made that not only the nutrient content but also the osteo-inductive and inflammatory factor in the culture microenvironment could affect LncRNA expression,leading to a subsequent change in MSC osteogenesis.However,even when different stimulations result in the same tendency of differentiation,these factors still exhibit functions through various LncRNAs and different mechanisms.
Apart from the culture medium,culture materials influence the osteogenic differentiation of MSCs.A large number of biomaterials,including nanomaterials,piezoelectric materials and hydrogels,could enhance the osteogenic differentiation ability of MSCs,some of which have been widely used in the clinic[40,41].Recently,Lvet al[42]found that TiO2nanotube materials could alter the epigenetic profile,including the LncRNA expression of MSCs during osteogenic differentiation.Moreover,nanofibers modulated the LncRNA network to accelerate MSCs osteogenesis[43].These studies confirm that the differentially expressed LncRNA in the culture materials mediate the osteogenic differentiation of MSCs.Clarifying the concrete mechanisms of these LncRNAs can allow us to precisely control the osteogenic ability of MSCs and help to improve their curative effect in bone reconstruction.
Adipogenic differentiation
Adipose tissue comprises a substantial amount of biologically active tissues,and nonobese men and women have approximately 12 kg and 14 kg of adipose tissue,respectively[44].Previously,some studies have demonstrated that LncRNA contributes to the process of adipogenesis[45,46].The objects of these studies were adipocytes,indicating that these LncRNAs may function in the later phase of adipogenic differentiation.Adipocytes are the basic components of adipose tissue,and MSCs are a main source of adipocytes in many tissues.It could be more important to focus on the LncRNAs that initiate the adipogenic differentiation process.Recently,we found that lncRNA-GAS5 expression decreased gradually during the adipogenesis of MSCs.GAS5 negatively regulated adipogenic differentiation through the microRNA-18a-CTGF axis as a ceRNA,forming a negative feedback loop to prevent excessive adipogenesis[47].In addition,Kalwaet al[48]reported that LncRNA-HOTAIR formed a triple helix structure to impact the adipogenic differentiation of MSCs through DNA methylation.These studies revealed the precise mechanism of adipogenesis,even though their rolesin vivoneed to be further discussed.
In regard to research on MSC differentiation,an unavoidable question is the balance between osteogenesis and adipogenesis.Although MSCs can undergo both osteogenic and adipogenic differentiation,one differentiation direction inhibits the other[49,50].Similarly,osteo-inductive factors always accelerate MSC osteogenic differentiation but inhibit adipogenic differentiation and vice versa as lipogenic factors[51].Determining the balance point and regulatory mechanisms of this process is the focus academic research in recent years.Recent studies suggest that LncRNAs can control this process.To promote osteogenesis,LncRNA-H19 was proven to inhibit adipocyte differentiation through a histone deacetylase mechanism in MSCs[30,52].These results suggested that H19 could be one of the critical points in this differentiation balance.In addition to LncRNA-H19,a newly identified LncRNA named TCONS_00041960 could also enhance osteogenic differentiation but inhibit adipogenic differentiation.This LncRNA interacted with two competitive microRNAs,thereby modulating the balance between osteogenesis and adipogenesis of MSCs isolated from rats[53].It could be assumed that the regulatory mechanism mediated by LncRNA is common and relatively conserved in many different species.Nevertheless,a challenge to these results is that all these studies are only performedin vitro.Recently,Liet al[54]identified LncRNA-BMNCR in MSCs and then constructed overexpressed and depleted transgenic mice.The results showed that the BMNCRdepleted mice had decreased bone mass but increased bone marrow adiposity,and overexpressed transgenic mice had completely opposite features[54].To our knowledge,this study is the first concerning lncRNA in controlling the osteogenic and adipogenic differentiation directionin vivo.Nevertheless,it is worth noting that the complete knock-in or out of a gene in mice could possibly affect the functions of other cells,except the target cells,so conclusions regarding this phenotypic feature of MSCs alone should not be made at this time.Conditional knock-in/out transgenic mice targeting MSCs may help to illustrate the function of lncRNA in MSCsin vivoin the future.
As MSCs can be isolated from many different tissuesin vivo,such as bone marrow,adipose tissue and Wharton’s jelly,the differentiation capacities of MSCs from various tissues are explicitly different[55].Briefly,bone marrow-derived MSCs have stronger potential in osteogenic differentiation,but adipose-derived MSCs tend to undergo adipogenic differentiation.The reason and mechanism of this tissue specificity remain unknown.Interestingly,a recent study showed that bone marrowderived MSCs preferred osteogenic differentiation,and LncRNA-MEG3 inhibited their osteogenic differentiation[56].However,another study showed that LncRNAMEG3 inhibited adipogenesis but promoted the osteogenesis of adipose-derived MSCs[57].In addition,the expression profile of LncRNA-MEG3 in these two kinds of MSCs was distinctly different during differentiation.Therefore,we suggest that on the one hand,the difference in the differentiation potential of MSCs from various sources may result from the expression and function of LncRNA,and on the other hand,the same LncRNA could exhibit different functions in MSCs from various origins.
Chondrogenic differentiation
Apart from osteogenic and adipogenic differentiation,studies on LncRNA functions in the chondrogenic differentiation of MSCs have been widely carried out.The process of MSCs differentiating into chondrocytes contains two steps,chondrogenic and hypertrophic phases[58].On day 14 of induction,as the chondrogenic phase,Wanget al[59]detected the expression profiles of LncRNA in MSCs and identified a total of 3638 differentially expressed LncRNA.Furthermore,Caoet al[60]determined the LncRNA expression profiles at the hypertrophic phase and found three critical LncRNAs that exerted functions from the chondrogenic phase to the hypertrophic phase.SOX9 is one of the most important transcription factors in chondrogenesis.Focused on SOX9 expression,Barteret al[61]identified that LncRNA-LOC102723505,termed ROCR,participated in the differentiation of MSCs into chondrocytes.However,the underlying mechanism of ROCR in modulating SOX9 expression is still unclear.
MSCs from joint tissues,including synovium and synovial fluid,are considered to have a stronger capacity for chondrogenic differentiation.This kind of MSC in the joint can directly take part in cartilage repair regeneration.A recent study reported that divergent LncRNA-ZBED3-AS1 promoted the chondrogenic differentiation of synovial fluid-derived MSCs by activating the Wnt/β-catenin signaling pathway[62].Another LncRNA-DANCR,which is also the positive regulator of MSC osteogenesis,accelerated the chondrogenesis of MSCs isolated from synovium through a microRNA-1305/Smad4 axis as a ceRNA[34,63].Notably,these LncRNAs and their mechanism can help to improve the therapeutic effect of MSCs in osteoarthritis.
Neurogenesis,myogenesis and endothelial differentiation
As the major origins of mesodermal lineage,MSCs possess multipotent differentiation ability.Other than their well-known osteogenic,adipogenic and chondrogenic differentiation,MSCs could also differentiate into endotheliocytes and myocytes in the mesoderm under conditional induction[2].Compared to the research performed in the field of osteogenesis,adipogenesis and chondrogenesis described above,studies on the concrete roles of LncRNAs in the endothelial and myogenic differentiation of MSCs are relatively rare.
Endothelial cells,forming a monolayer endothelium,are an important part of the vesselsin vivothat separates the circulating blood from the tissues[64].The regular quantity and function of endothelial cells guarantee angiogenesis;otherwise,their dysfunction results in the development of many pathological disorders.Therefore,it is of great importance to investigate,in depth,the mechanism of MSC differentiation into endotheliocytes.LncRNA-MIAT,which was considered to contribute greatly to development and disease,facilitated the endothelial differentiation of MSCs by targeting microRNA200a and vascular endothelial growth factor (VEGF)[65,66].Moreover,MEG3,a well-studied LncRNA in MSCs as described above,was also found to participate in the endothelial differentiation of MSCs.This LncRNA facilitated the ubiquitination and subsequent degradation of FOXM1,which therefore reduced angiogenic VEGF expression and finally promoted the endothelial differentiation of bone marrow-derived MSCs[67].This result suggests that in the same kind of cells,an LncRNA,such as MEG3,could possess various regulatory functions.Under specific stimulation,this LncRNA exhibits different functions through different mechanisms,which in turn regulate the different capacities of MSCs to meet various needsin vivo.This precise regulation is mediated by LncRNA,making MSCs universally functional from development to disease.
Myocytes contain skeletal muscle cells,myocardial cells and smooth muscle cells,which perform different functionsin vivo.Although previous studies have claimed that MSCs could differentiate into all kinds of myocytes,only studies concerning LncRNA in the smooth muscle differentiation of MSCs have been reported thus far[68-70].Liet al[71]reported that LncRNA-HULC is a promoter of the smooth muscle differentiation of adipose-derived MSCs through BMP9 expression and the activation balance between the Wnt/β-catenin/Notch signaling pathways.Several other LncRNAs,including H19,MEG3 and MALAT1,were also considered to be related to the myogenic differentiation of MSCs,although some of these studies were performed using C2C12 cells,an immortalized mouse myoblast cell line[72].Since there is a vast difference between MSCs and C2C12 cells,the role of these LncRNAs in the myogenic differentiation of MSCs needs to be further confirmed.
Neural cells,derived from an ectoderm lineage,are the foundation of the nervous system but have relatively weaker regeneration capacityin vivo.Therefore,treatment for neurological disorders and nerve injury remains a worldwide problem.In recent years,studies have suggested that MSCs could be used as a novel and effective therapy for nervous system disease[73,74].This therapeutic effect,on the one hand,is due to the immunoregulatory ability of MSCs and neuroprotective factor secretion from these cells,while on the other hand,this effect may result from MSCs differentiating into neural cells.Although whether MSCs can transdifferentiate into neural cells of other lineagesin vivois still controversial,many studies have demonstrated that MSCs could gradually differentiate into neural-like cellsin vitrounder specific induction conditions[75,76].To study the in-depth mechanism,Wuet al[77]detected the LncRNA expression pattern of MSCs during their neurogenesis differentiation.Moreover,Farzi-Molan further reported that the neural-like differentiation of MSCs was under the regulation of LncRNA-H19,which functioned through the miRNA-675/IGFR axis[78].In vivoexperiments should be performed to confirm MSC neurogenesis and these mechanisms,which could help to improve their therapeutic effect on nervous system disease.
Other aspects of MSCs functions
Similar to other cells,MSCs have other capacities,such as proliferation and antiapoptosis.As we suggest that LncRNA could affect various aspects of cells,Liet al[79]reported that LncRNA-HULC promoted MSC proliferation and inhibited apoptosis.In addition,this LncRNA also accelerated the migration of MSCs.Other LncRNAs,including H19 and MALAT1,were determined to regulate proliferation and apoptosis simultaneously.Moreover,these two LncRNAs affect the angiogenesis of MSCs[80,81].However,detailed regulatory mechanisms were not clarified in these studies.The Wnt/β-catenin signaling pathway contributes to cell proliferation and differentiation.LncRNA-LET was proven to negatively modulate the proliferation of MSCs by inhibiting TGF-β expression and subsequent Wnt/β-catenin signal pathway activation[82].In addition,LncRNA-p21 devitalized the Wnt/β-catenin signaling pathway and therefore participated in MSC senescence[83].In another study,LncRNAp21 was involved in the apoptosis of MSCs through the Wnt/β-catenin signal pathway[84].
A lingering issue in our mind is the question of why fewer studies about LncRNA in the immunoregulatory abilities of MSCs were performed.Except for a study of LncRNA-MALAT1-overexpressing MSCs that promote M2 macrophage polarization,no other studies have been reported to date[80].Considering that the immunoregulatory ability of a series of immune cells is one of the most critical functions of MSCs,it is of great importance to investigate the role of LncRNA in this function,which could improve the clinical use of MSCs in the treatment of inflammatory disease.
LNCRNA OF MSCS IN DISEASES DIAGNOSIS AND TREATMENT
As MSCs greatly contribute to every physiological process through their powerful capacities,the regular functions of MSCs are critical to maintaining homeostasisin vivo.Therefore,it is not surprising that the abnormal function of MSCs has a great effect on our body or even leads to diseases.To date,MSC dysfunction has been demonstrated to be the critical pathogenesis in several diseases.For example,disorders in the immunoregulatory capacity of MSCs are related to autoimmune diseases,such as systemic lupus erythematosus (SLE) and rheumatoid arthritis(RA)[85,86].Additionally,the dysfunction in MSC differentiation contributes to diseases with structural abnormalities,including osteoporosis and ectopic ossification[87].Ankylosing spondylitis (AS) is a kind of autoimmune disease characterized by chronic inflammation and pathological osteogenesis[88].Through our studies,we demonstrate for the first time that MSCs from AS patients have reduced immunoregulation ability,which leads to disproportionality between Th17 cells and Treg cells.This dysfunction of MSCs contributes to the pathogenesis of chronic inflammation in AS[89].Furthermore,we further determined that enhanced osteogenic differentiation ability was observed in the MSCs from AS patients,which is a critical reason for pathological osteogenesis in AS[90](Figure 1).As LncRNA is a major regulator of MSC functions,it could be speculated that LncRNA may also participate in these processes.
Many studies have proven that LncRNA contributes to various diseases[14].To confirm this hypothesis specifically in the MSC setting,we first detected and compared the LncRNA expression profiles between AS patient MSCs and normal MSCs during osteogenic differentiation and found that 520 LncRNAs were abnormally expressed during the osteogenesis of MSCs from AS patients.Among these 520 LncRNAs,Lnc-ZNF354A,Lnc-LIN54,Lnc-FRG2C,and Lnc-USP50 had tight relationships with the imbalance between BMP2 and Noggin,which leads to enhanced osteogenic differentiation ability and the subsequent pathological new bone formation[91](Figure 2).In contrast,several other studies showed that abnormal LncRNA expression resulted in reduced osteogenic differentiation and related diseases.Zhuanget al[92]determined that LncRNA-MEG3 expression,an LncRNA that modulates MSC osteogenesis,as reported in a previous study,was downregulated in MSCs from patients with multiple myeloma.This downregulation of MEG3 inactivated the transcriptional activity of BMP4 and then led to the reduced osteogenic differentiation of MSCs from multiple myeloma patients.MSC dysfunction was a critical mechanism of osteoporosis,one of the pathological features in multiple myeloma[92].Consistent with this study,a recent study demonstrated that LncRNAMEG3 participated in the pathogenesis of osteoporosis through the same mechanism[93].In addition,Wanget al[94]identified that the impaired osteogenic differentiation of MSCs from periodontitis patients resulted from the lower expression of LncRNA-POIR through a ceRNA mechanism.These results indicated that the abnormal expression of LncRNA resulted in disorder in MSC osteogenic differentiation,therefore contributing to abnormal bone metabolism in diseases.As other MSC dysfunctions have been tightly related to the pathological features of several diseases,especially the abnormal immunoregulatory ability of MSCs in inflammatory diseases,further research should be performed to identify critical LncRNAs and illustrate their mechanisms.These studies could help us to gain a deeper understanding of the occurrence and development of diseases.
A recent study showed that the exosomes from lung cancer altered the LncRNA expression profile of MSCs[95].As MSCs interact with tumor cells and affect tumor progression,LncRNA is very likely to participate in this process[96].In addition,this study emphasized that under the specific microenvironment of diseases,MSCs could be educated and their LncRNA expression profiles could be unique to specific conditions.Circulating MSCs have been found in peripheral blood and could be used as an auxiliary diagnosis method[97].Therefore,we suggest that these educated MSCs with special LncRNA in blood circulation could be treated as a natural diagnostic kit for diseases.Detecting the specific LncRNA or distinct LncRNA groups in educated circulating MSCs in peripheral blood will be a novel and accurate method to diagnose these diseases.

Figure1 Long non-coding RNA in the tri-lineage differentiation of mesenchymal stem cells.
To date,clinical studies of MSCs have been carried out extensively,and these results demonstrated that MSCs could be an effective treatment for some autoimmune diseases,including SLE and graft-versus-host disease[98,99].Moreover,MSCs with differentiation potential could be used as seed cells in tissue and regeneration engineering such as fracture repair and osteoarthritis[100,101].In 2011,we performed clinical research on AS and demonstrated that the intravenous infusion of allogenic MSCs was an effective and safe treatment for active AS patients who failed nonsteroidal anti-inflammatory drug treatment[102].However,the clinical efficacy of MSC treatment could be further improved by enhancing the capacities of MSCs.Recently,a study determined that LncRNA was involved in MSC treatment for airway allergic inflammation in mice[103].In addition,lncRNA MEG3 and MIAT could affect MSC treatment for erectile dysfunction[66,67].As LncRNA is a critical functional regulator of MSCs,regulating specific LncRNA expression through gene editing technology may be a novel way to improve the clinical efficacy of MSCs.In autoimmune diseases,upregulating or downregulating LncRNA could induce the secretion of specific anti-inflammatory cytokines that target key pathogenic factors and then promote the immunoregulatory capacity of MSCs.On the other hand,modulating specific LncRNA could guide the directional differentiation of MSCs into specific cells,which may be committed to special use in tissue and regeneration engineering.
CONCLUSION
In this review,we highlight the critical role of LncRNA in the function of MSCs.Based on the discoveries described above,we conclude that LncRNA is an important functional regulator of MSCs,including their immunoregulation and differentiation capacities,which can greatly improve the clinical use of MSCs in the diagnosis and treatment of diseases.However,limitations still exist in these current studies.First,the detailed mechanism of LncRNA in MSCs warrants further study.What does control the LncRNA expression profile when MSCs exhibit functions? Several studies have reported that RNA modifications may be critical to this question,but the detailed mechanisms remain unknown.In addition,how does an LncRNA affect the ability of MSCs? Or does the LncRNA have tissue specificity in MSCs from various sources? Although many LncRNAs have been identified,their spatiotemporal specificities and functional mechanisms need to be clarified in the future.Second,in vivoexperiments should be performed to ensure the actual function of these LncRNAs.Benefiting from the development of CRISPR/Cas9 technology,it is now possible to construct conditional knock-out (CKO) mice,which precisely inhibits the target LncRNA in MSCs.These CKO mice will help to illustrate the role of LncRNAin vivo.Finally,clinical research should be conducted to confirm the clinical application value of LncRNA.Clinical-based studies can help us to not only understand the actual pathogenesis of diseases but also improve the clinical application of MSCs in disease diagnosis and treatment.
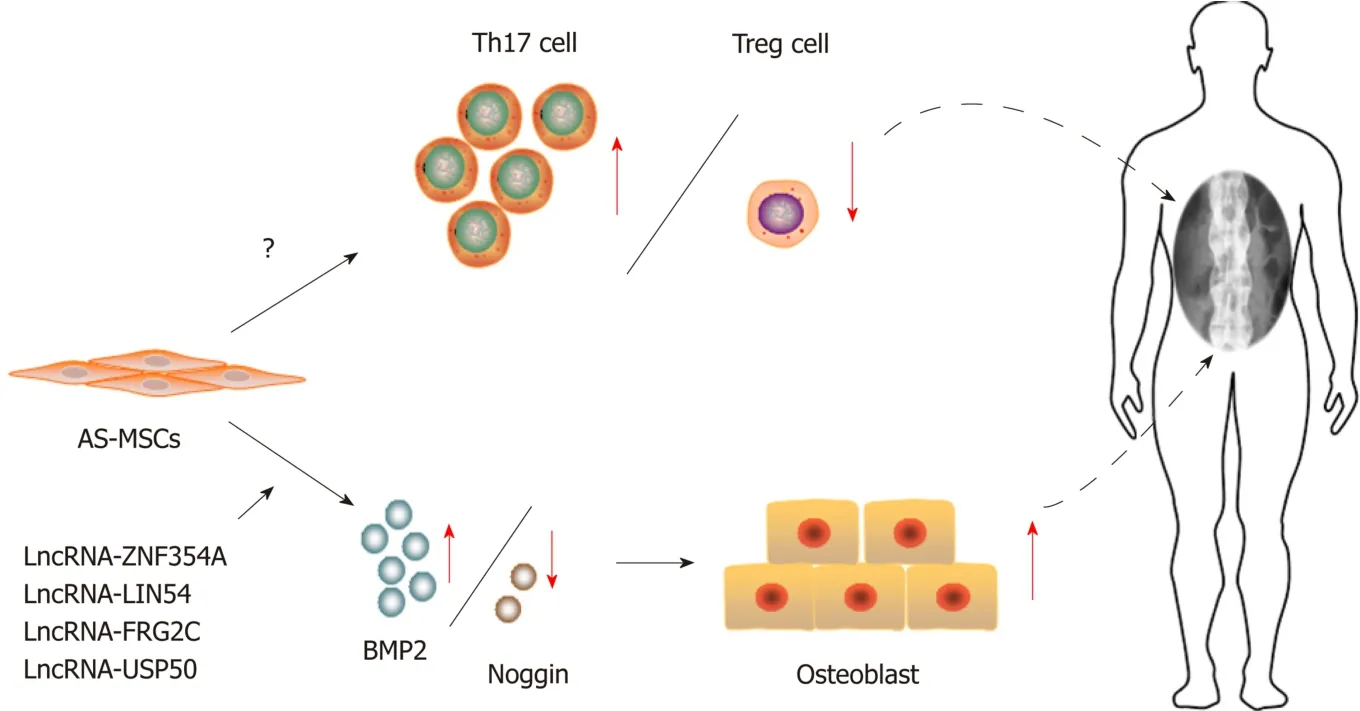
Figure2 Mesenchymal stem cells in ankylosing spondylitis.
杂志排行
World Journal of Stem Cells的其它文章
- Adipose-derived stromal/stem cells from different adipose depots in obesity development
- Circulating factors present in the sera of naturally skinny people may influence cell commitment and adipocyte differentiation of mesenchymal stromal cells
- Stromal cell-derived factor-1α promotes recruitment and differentiation of nucleus pulposus-derived stem cells
