Adipose-derived stromal/stem cells from different adipose depots in obesity development
2019-04-24KarinaRibeiroSilvaLeandraSantosBaptista
Karina Ribeiro Silva,Leandra Santos Baptista
Abstract
Key words: White adipose tissue;Metabolic diseases;Obesity;Adipose-derived stromal/stem cells;Adipose depot;Inflammation
INTRODUCTION
The World Health Organization defines obesity as abnormal or excessive fat accumulation that represents a risk to health.Obesity can develop due to an imbalance between energy intake and expenditure by the organism,and it is strongly related to environmental factors,such as high caloric food consumption and sedentary lifestyle.In addition,the intestinal microbiota,stress levels,endocrine and genetic profiles can also contribute to increase an individual’s susceptibility to obesity[1,2].The increasing prevalence of obesity is alarming because it is a risk factor for several diseases,including hypertension,ischemic cardiovascular disease,dyslipidemia,insulin-resistance,diabetes,metabolic syndrome[3-7]and also cancer[8-11].
The primary site for energy storage in humans is white adipose tissue (WAT)[12].The discovery that metabolic diseases such as obesity and type-2 diabetes arise from WAT dysfunctions has revealed immune and endocrine non-classical functions of WAT,which strongly impact on metabolism,insulin sensitivity and promote local and systemic inflammation[13-15].Mammalian WAT depots found in distinct anatomical locations are highly heterogeneous in their morpho-phenotypic profiles[16,17].The differential accumulation of fat in specific anatomical depots (rather than the total body fat mass) is the crucial factor that determines the clinical outcomes of obesity and other metabolic diseases.Depot-specific adipose-derived stem/stromal cells(ASC) could be pivotal to determine the different pathophysiological roles of each depot,by modulating the depot’s gene expression profile and its adipogenic and immunomodulatory potentials.Therefore,a deep understanding of the contribution of depot-specific ASC to the differential properties and pathogenicity of WAT depots can be crucial for developing new therapeutic approaches against metabolic disorders.
In this review,we discuss the current knowledge on depot-dependent ASC properties and how they can contribute to the pathophysiology of obesity and metabolic disorders.The discussion here aims to provide guidance for researchers and clinicians in the future use ASC in therapeutic strategies against obesity and related pathologies.
WHITE ADIPOSE TISSUE DEPOTS: A MATTER OF ANATOMICAL LOCATION OR INHERENT PROPERTIES?
In most mammals species,fat storage occurs mainly in WAT,inside specialized cells called adipocytes[18],which accumulate triglyceride molecules (consisting of glycerol and fatty acid chains).Adipocytes can dramatically alter their size according to changes in metabolic demand.After a meal,insulin stimulates WAT to store energy in the form of neutral lipids,mainly triacylglycerol,in a process known as lipogenesis.Conversely,adipocytes provide free fat acids to be metabolized by the organism through lipolysis,in periods of fasting[19].
In humans,WAT is distributed in two main depots – the subcutaneous and the visceral WAT - with distinct structure,cell content,gene expression and secretion profiles,as well as responsiveness to neuro-endocrine stimuli.The subcutaneous WAT is distributed along the body surface,forming the hypodermis,with distinct depots in the abdominal,femoral,gluteal,facial and cranial regions.On the other hand,visceral WAT surrounds the organs of the abdominal cavity,and is also found in smaller amounts around the heart (epicardial visceral WAT),stomach (epigastric visceral WAT) and blood vessels (perivascular visceral WAT)[16,17,20].
Evidence links obesity and metabolic dysfunction to the total body fat mass,particularly in the abdominal region[5].In the abdominal WAT,subcutaneous WAT is subdivided by the Scarpa’s fascia into superficial and deep depots[21,22],while visceral WAT is subdivided into omental (surrounding the surface of the intestines),mesenteric (deeply within the intestines) and retroperitoneal (near the kidneys,at the back) fat depots[16,23].In the 1950s,Vague[24]showed that the anatomical fat distribution could have important metabolic implications,with certain distributions favoring diabetes and atherosclerosis.Krotkiewsket al[25]showed that subjects with a higher waist-to-hip ratio had increased blood pressure,low carbohydrate tolerance and high insulin plasma levels.By connecting clinical,epidemiological and physiological evidence with WAT measurements,different research groups concluded that visceral fat accumulation (central obesity) is more strongly associated with higher metabolic and cardiovascular risk,while subcutaneous fat accumulation in the thighs and hips(peripheral obesity) is associated with a lower risk of these diseases[26-30].
However,it remained unclear whether the differential impact on systemic metabolism was due to the anatomical location of the WAT depot,to intrinsic properties of the cells in each depot,or both.WAT depot transplantation in mice shed light on the influence of depot anatomical location on systemic metabolism.Both lean and obese mice had increased glucose tolerance,insulin sensitivity and reduced body weight after receiving a transplant of subcutaneous WAT from lean mice into the visceral cavity[31-34].The metabolic improvement exerted by subcutaneous WAT transplanted into a different anatomical location suggested that subcutaneous and visceral WAT depots are intrinsically different.
The studies mentioned above triggered the search for intrinsic biological differences between depots that could explain the link between depot heterogeneity and metabolic complications,both in lean and obese rodents and humans.Indeed,gene expression analysis revealed significant differences in hundreds of genes between distinct adipose tissue depots[35-37].Moreover,visceral WAT has a higher triglyceride turnover compared to subcutaneous WAT,probably due to a higher sensitivity to the lipolytic function of catecholamines and a lower sensitivity to the antilipolytic effects of insulin[38-40].
Thus far,the vast majority of studies on the heterogeneity of abdominal WAT depots focused on the comparison between subcutaneous and visceral WAT.However,abdominal WAT comprises not only these two types of depots but also the preperitoneal (also known as endoabdominal or extraperitoneal) WAT,located between the transverse fascia and the parietal peritoneum[41].Interestingly,the preperitoneal WAT has the highest size variation during weight loss by dieting,compared with subcutaneous and visceral WAT[42].Like subcutaneous and visceral WAT,preperitoneal WAT can also be identified in non-obese and obese subjects by computer-tomography and ultrasonography[43-46].
Suzukiet al[43]suggested that the abdominal wall fat index (AFI) determined by ultrasonography could be a novel indicator of visceral fat deposition.This study showed that the AFI - which represents the ratio between the preperitoneal WAT maximum thickness (Pmax) and the subcutaneous WAT minimum thickness (Smin) -positively correlates with the visceral to subcutaneous WAT ratio (V/S).These data indicate that the thickness of the preperitoneal WAT depot is positively associated with the visceral depot mass.Moreover,the AFI correlated positively with the plasma levels of triglycerides and with the basal insulin levels in obese individuals,but was inversely correlated with high density lipoprotein levels[43].Whether preperitoneal and visceral WAT depots have similar properties or even similar impact on metabolic dysfunctions remains controversial.While some studies showed that the preperitoneal WAT maximum thickness or the AFI are associated with cardiovascular risk factors[47,48],others indicated that visceral WAT thickness showed a better association with cardiovascular risk factors compared with subcutaneous and preperitoneal WAT thickness[49,50].Similarly to visceral WAT,the preperitoneal WAT is covered by the peritoneum;however,visceral (but not preperitoneal) WAT contains portal vein circulation[51,52].A functional comparison of the preperitoneal WAT depot with subcutaneous and visceral WAT,both in lean and in metabolically disrupted patients,is necessary to clarify the impact of each WAT depot on metabolic and cardiovascular disease risks.
Therefore,the relationship between different WAT depots and systemic homeostasis and the development of metabolic diseases is mainly dependent on the intrinsic properties rather than the anatomical location of each depot.The metabolic and genetic differences observed between abdominal whole WAT depots could be related to the behavior of the cells that dwell in each depot.
STROMAL-VASCULAR FRACTION AND THE INHERENT PROPERTIES OF WAT DEPOTS
WAT is composed of two main cell fractions:mature unilocular adipocytes and stromal-vascular cells,known as the stromal-vascular fraction (SVF).After enzymatic digestion of the adipose tissue and centrifugation,the adipocytes float to the surface,while SVF cells sediment to the pellet[53].
Adipocytes have the fundamental role of accumulating triacylglycerols during periods of caloric excess,and then breaking this reservoir into free fatty acids when energy consumption is required.Mature adipocytes are equipped with enzymes and regulatory proteins to perform lipolysis and lipogenesis,which are orchestrated by hormones,cytokines and other factors involved in energy metabolism[16].
The adipose SVF is highly heterogeneous,and can be sub-divided into hematopoietic and stromal compartments[53].The hematopoietic compartment comprises cells that express CD45,including lymphocytes (Natural Killer,helper and regulatory T cells,and B cells)[54],eosinophils[55],neutrophils[56],hematopoietic progenitors[57],mast cells[58]and macrophages.Notably,the presence of macrophages has been repeatedly reported in human and murine adipose tissue[59-61].The percentage of macrophages varies according to the presence of pathophysiological conditions,such as obesity,which is characterized by monocytic/macrophagic infiltration into adipose tissue[62,63].
The stromal compartment of the adipose SVF is composed of mesenchymal and endothelial cells associated with blood vessels.Zimmerlinet al[64]distinguished the following four cell subpopulations in the stromal SVF compartment,using a combination ofin situimmunolabeling and cell sorting:(1) Pericytes/mesenchymal stem cells (MSC;CD146+/CD34-/CD31-);(2) Adipocyte progenitors/Pre-adipocytes(CD146-/CD34+/CD31-);(3) Endothelial progenitor cells (CD31+/CD34+);and (4)Mature endothelial cells (CD31+/CD34-).All cells in the stromal compartment are negative for the pan-hematopoietic marker CD45.In the adipose tissue,MSC give rise to endothelial progenitors and pre-adipocytes,which differentiate into endothelial cells and adipocytes,respectively.Therefore,adipose MSC can maintain or increase adipocyte numbers,thereby modulating the adipose tissue lipid store capacity,as well as its ability for homeostasis or regeneration through adipogenesis[65].
SVF culture generates a population of adherent cells characterized by the expression of mesenchymal markers including CD44,CD73,CD90 and CD105,but negative for CD45 and CD31[66,67].These cells can differentiatein vitrointo mature cells of mesodermal lineages,such as adipocytes,osteoblasts and chondrocytes[66-69].These combined phenotypic features and differentiation properties are diagnostic of ASC[70].These cells can also lead to angiogenesis,by differentiating directly into endothelial cells[71],by interacting with endothelial cells to induce vascular formation[72],or by secreting angiogenic factors such as VEGF,HGF,FGF and PDGF[73-75].The angiogenic potential of ASC has important therapeutic implications.ASC secrete different types of chemical mediators,including cytokines and growth factors,which have paracrine activities that stimulate local cell survival and proliferation,angiogenesis,differentiation of local stem cells,and reduce apoptosis[75-77].Moreover,ASC can suppress mixed lymphocyte reaction[78]and their low immunogenicity could enable their safe use in allogeneic transplants,as part of cell-based regenerative therapies[79].Therefore,the differentiation capacity of ASC and their trophic effects directly contribute to adipose tissue homeostasis,cell renewal,tissue repair and tissue immunogenic balance[80].
Lafontanet al[81]postulated that metabolic and genetic differences observed between abdominal whole WAT depots could be related to the unique properties of the cells that dwell in each of these depots.Besides,these unique cell properties could also account for the different responses of each depot to metabolic challenges[81,82].Proteomic analysis of adipocytes and SVF cells isolated from subcutaneous and visceral WAT from lean subjects showed that the SVF could have a higher contribution to the functional differences observed between these depots[83].
Thein vivocounterparts of the cultured ASC still remain to be defined and studies sometimes refer to these cells as mesenchymal stem/stromal cells.Throughout this review the term “ASC” only will be used for the adherent cells derived from the SVF with the diagnostic features mentioned previously,which we use as criteria for ASC identification[70].In contrast,when describing resident adipose cells with progenitor potentialin vivo,the term “adipose progenitors” will be used instead.Given the ability of ASC/adipose progenitors to govern adipose tissue development and homeostasis,some studies have suggested that depot-specific ASC with unique cellautonomous properties could be responsible for the morpho-functional heterogeneity of WAT depots[84-86].
RELATIONSHIP BETWEEN OBESITY-INDUCED INFLAMMATION AND ADIPOGENESIS
WAT inflammation in obesity
The ability of adipocytes to increase in size (adipocyte hypertrophy) during lipogenesis was believed to be the only mechanism by which adult WAT expands upon insulin stimulation.However,it is now widely accepted that an increase in adipocytes number - or adipose tissue hyperplasia - also contributes to WAT mass gain through the recruitment and differentiation of adipose progenitors,in a process known as adipogenesis[2].Therefore,the ability of WAT to expand during life in response to metabolic needs depends not only on adipocytes,but also on the adipogenic potential of adipose progenitors.Other factors such as vasculature and extracellular matrix remodeling also contribute to the plasticity of adipose tissue and influence adipocyte hypertrophy and adipogenesis from stem cells[87].
During the development of obesity,WAT expands to an extent that leads to chronic tissue inflammation[62],which is associated with an increased risk of type-2 diabetes and cardiovascular disease[88].The first functional connection between obesity and inflammation was the observation that obese WAT secretes large amounts of the proinflammatory cytokine tumor necrosis factor (TNF)-α,and that this cytokine had a direct role in obesity-induced insulin resistance[89,90].As well as increased levels of proinflammatory cytokines,obese WAT also exhibits low level of anti-inflammatory mediators[89,91].The discovery that obesity is characterized by macrophage accumulation in adipose tissue added a new dimension to our understanding of how obesity propagates inflammation,as macrophage recruitment is an important factor in promoting insulin resistance[62,63].A clue to the origin of these recruited macrophages came from the observation that,in CD45.2 mice transplanted with bone marrow cells from CD45.1 mice,85% of the adipose tissue macrophage (F4/80+) cell population had the CD45.1 marker.Therefore,during obesity development,the expanding WAT secretes chemoattractants (such as the mouse chemoattractant protein-1,MCP-1,and the macrophage inflammatory protein-1α,MIP-1α) that recruit monocytes from the bone marrow to adipose tissue[62,63].
In obesity,the infiltrating macrophages adopt a proinflammatory (“M1”)phenotype,becoming a source of proinflammatory cytokines such as IL-1β and TNFα[63],which trigger local and systemic insulin resistance[62].These infiltrating macrophages differ from adipose tissue resident (“M2”) macrophages,which exhibit anti-inflammatory characteristics[92,93].In mice,high-fat diets turn the secretion pattern of M2 macrophages into M1,by the reduction of IL-10 and arginase levels,and the increase in TNF-α and iNOS levels[94].Diet-induced obesity increases the expression of the M1 marker CD11c in WAT,while decreasing CD206 expression,which is typical of M2 macrophages[95].
The poorly-defined mechanisms that initiate inflammation and connect the inflammatory scenario of obese WAT to other diseases are the subject of intense investigation,in a research area known as “metabolic inflammation”[96].Metabolically altered adipose tissue cells may interact with immune cells to initiate the inflammatory process.Interactions between immune and metabolic cells occurs in other metabolic tissues and organs (liver,muscle and pancreas) in obese individuals,suggesting that metabolic inflammation could be a systemic feature of obesity[97].
Immune-metabolic interactions occur in obesity between adipocytes or SVF cells and macrophages.Indeed,adipocyte hypertrophy is a potential trigger for macrophage accumulation in WAT[98].In association with the large increase in protein synthesis,hypertrophied adipocytes display mitochondrial and endoplasmic reticulum stress,which could lead to the activation of inflammatory signaling pathways[99-101].In line with this hypothesis,hypertrophied adipocytes in obese individuals change their intrinsic secretion profile towards a proinflammatory phenotype (characterized by high TNF-α and low adiponectin levels)[19,102,103].TNF-α could stimulate pre-adipocytes and endothelial cells to secrete MCP-1,attracting monocytes from the bone marrow[62,63].In addition,pro-inflammatory cytokines and fatty acids secreted by hypertrophic adipocytes can lead recruited macrophages towards an M1 proinflammatory phenotype[104].Moreover,groups of hypoxic and hypertrophic adipocytes undergo necrosis,and are cleared by macrophage phagocytosis.Indeed,macrophages form crown-like structures around necrotic adipocytes in obese WAT,in a typical chronic inflammatory response[95,105].
While the M1 profile is pro-inflammatory,the potentiation of M2 pathways in macrophages appears to reduce metabolic inflammation (or “metainflammation”),improving insulin sensitivity[103].The M2 phenotype of resident adipose-tissue macrophages is maintained by the paracrine action of lymphocytes and eosinophils;however,in obesity,the recruitment of these cells to WAT is suppressed[106,107].Tolerogenic CD4+T-regulatory cells (Tregs) are also downregulated in WAT during obesity,which could lead to metainflammation[108,109].Aside from Tregs,other leukocytes,including NK,NKT and mast cells,have a yet poorly-defined role in metainflammation[110-112].Further studies on the temporal and spatial immunemetabolic interactions between leukocytes and WAT cells should shed light on the mechanisms underlying inflammation in obesity,to identify potential targets for clinical intervention.
Complex molecular signaling pathways may link metabolic challenges (e.g.,excessive fat storage) with inflammation in obesity[113],including pathways involving the NLRP3 inflammasome,a cytoplasmic protein complex that promotes the conversion of pro-cytokines into active cytokines,which are then secreted[114].NLRP3 inflammasome activity can be modulated by several metabolites,including fatty acids,and the activation of this complex can interfere with insulin signaling[115,116].Inflammasome activity can be triggered by endogenous or exogenous stress signals(e.g.,cytokines,free fatty acids,glucose,reactive oxygen species,ATP),which function as “pathogen-associated molecular patterns” that interact with pattern recognition receptors,especially toll-like receptors (TLRs),in WAT cells.The interaction of stress signals with TLR4,for example,activates the nuclear factor-κB pathway,which increases NLRP3 expression[116-118].
Adipose progenitors could be key regulators of macrophage recruitment and activation in WAT[84].Indeed,human ASC express active TLRs,including TLR4,whose activation results in the secretion of the pro-inflammatory cytokines IL-6 and IL-8[119].Moreover,adipose progenitors express molecules that favor immune differentiation,such as osteopontin,which was identified as one of the factors involved in macrophage accumulation during diet-induced obesity[120].In line with this notion,we showed that human ASC secrete MCP-1in vitro[121],and that mouse ASC populations enriched in pre-adipocytes (CD34+ASC) could be responsible for most of the MCP-1 secretion in mice[122].In addition,we observed that ASC can supportin vitrohematopoiesis,with a tendency to generate macrophages from hematopoietic progenitors[67].Moreover,while adipocytes are the main source of hormones that regulate energy metabolism (such as adiponectin and leptin),inflammatory cytokines are mostly secreted by cells from the SVF[123].Therefore,adipose progenitors can be key players in the regulation of the metabolic inflammation established during obesity,acting as a key source of secreted immunemediators in adipose tissue,both in normal and in pathological conditions[124].
Although macrophage infiltration in obese adipose tissue potentiates inflammation and favors the development of comorbidities,the pro-inflammatory cytokines secreted by infiltrating macrophages with an M1-phenotype could also decrease WAT mass by stimulating adipocyte lipolysis and inhibiting adipogenesis[98].In fact,classically activated M1 macrophages impair insulin signaling and adipogenesis in adipocytes,by both direct and paracrine signals[94].The immune and metabolic interactions that occur within WAT may have evolved as a mechanism to regain homeostasis,in order to prevent the obesity-associated mobility impairment that makes animals more vulnerable to predators[125].
The mechanisms regulating adipogenesis and inflammatory responses from stromal cells have been the subject of several studies,using variousin vivoandin vitromodel systems[126-129].These studies have shown that the TNF-receptor superfamily molecule CD40 is expressed during adipogenic differentiation and interacts with surrounding immune cells,modulating adipocyte inflammatory responses and insulin resistance[127,128].Additionally,a study by Touset al[129]identified sphingosine kinase-1 as a potential therapeutic target to attenuate chronic inflammation in obesity and related metabolic diseases,as this molecule regulates the pro-inflammatory response in adipose progenitors.
Impact of inflammation induction on ASC functionality
ASC functionality is directly affected by obesity-induced inflammation[121,130].Some studies have reported an inverse correlation between the body-mass index (BMI,a commonly used obesity indicator) and ASC differentiation capacity[130-132].In agreement with these data,our studies and those of others demonstrated that ASC from obese subjects have decreased ability to differentiate into adipocytesin vitro,when compared with those from lean subjects,as assessed by intracellular lipid accumulation and/or the expression of adipogenic genes[121,130-133].Isaksonet al[134]suggested that the inflammatory state in adipose tissue may be responsible for the impaired adipocyte differentiation observed in obesity.Indeed,inflammatory cytokines are anti-adipogenic[135],and it is possible that ASC from obese patients carry a “memory” of differentiation inhibition from the inflammatory environmentin vivo,and which manifests itself as impaired adipogenesisin vitro.Pro-inflammatory macrophages secrete factors that impair human adipogenesis from ASCin vitro[136,137],and there is a negative correlation between the adipogenic capacity of obese ASC and the up-regulation of inflammatory genes[130,138].In contrast,some studies reported that ASC from obese donors showed higher expression of adipogenic genes,suggesting that obese ASC are more potent in adipogenesis[138,139].A recent study showed that ASC from obese pigs (given a high-fat diet) exhibited increased adipogenic potential relative to those from lean pigs,at the onset of obesity[140].The discrepancies between studies on the impact of inflammation on the adipogenic potential of ASC could be due to differences in the methods used to evaluate adipogenesis,or to the use of donors with different adiposity grades,or at different stages of obesity development.
The pro-angiogenic potential of ASC is also altered in obesity.ASC from morbidly obese individuals have higher mRNA and protein expression of the anti-angiogenic factor TSP-1 than ASC from lean individuals[130].In addition,“lean” ASC (i.e.,those differentiated from adipose tissue of lean individuals) had increased capacity to form tube-like networks while “obese” ASC (derived from obese individuals) were not responsive to angiogenic stimuli[141],showing a reduced capacity to form capillary-like structures[142].Moreover,extracellular vesicles from obese ASC exhibited lower levels of angiogenic-related factors and,consequently,reduced angiogenic potential compared with those derived from lean ASC[143].
The ASC differentiation capacity is also disrupted in patients with type-2 diabetes mellitus.Global gene expression profiling revealed that ASC from type-2 diabetes donors have low levels of adipogenic genes compared with those from non-diabetic donors[144],indicating a decreased potential for adipogenic differentiation in diabetes.Additionally,ASC from diabetic rats were less effective at forming microvesselsin vivothan those from non-diabetic animals[145].
Obesity also alters the immunomodulatory properties of ASC,and their ability to secrete chemical mediators.ASC isolated from patients with different adiposity grades exhibit different secretion patterns[146,147].In particular,we demonstrated that ASC from morbidly obese patients secrete more proinflammatory cytokines,such as IL-6 and IL-8[121],which is in agreement with data from other groups showing that obese ASC display up-regulation of inflammatory genes (including IL-6,IL-8,IL-10 and MCP-1) compared with lean ASC[138,148].In addition to the increased expression of inflammatory markers,obese ASC had increased migration and phagocytosis capacity compared with lean ASC.Besides,ASC from obese individuals show reduced capacity to activate the M2 macrophage phenotype and to suppress lymphocyte proliferation[149].Therefore,the immunomodulatory properties of ASC are altered in obesity,which may be related to the role of adipose progenitors as key regulators of the immune response during obesity development.As well as in obesity,alterations in immunomodulatory properties are observed in patients with type-2 diabetes mellitus[149],and global gene expression profiling revealed that genes involved in inflammation are upregulated in ASC from type-2 diabetes patients[144].Recently,Liu and colleagues[150]showed that ASC derived from mice with type-2 diabetes are less effective at restricting CD4+T lymphocyte proliferation and pro-inflammatory“polarization” (during pro-inflammatory immune phenotype acquisition) than ASC from lean mice.
Collectively,these data show that obesity and other immune metabolic pathologies disrupt ASC/adipose progenitor functionality,favoring a pro-inflammatory response.This response,in turn,impairs ASC adipogenic capacity,which may reduce the ability of adipose progenitors to generate new adipocytes in WAT depots,ultimately leading to ectopic fat storage.Overall,evidence from a large number of studies indicate that ASC/adipose progenitors are key regulators of the immune response in obesity and other metabolic disorders,highlighting the potential of ASC use in cellbased regenerative therapies.
REGIONAL DIFFERENCES IN ASC FUNCTIONALITY IN OBESITY AND THEIR EFFECT ON FAT EXPANSION AND DISTRIBUTION PATTERNS
ASC behavior in WAT depots in obesity
Numerous studies evaluated the behavior of ASC derived from different WAT depots(in both rodents and humans;Table 1),to test the hypothesis that the properties of depot-specific ASC could account for some of the differences in morphology,function and response to metabolic challenges observed between WAT depots.
Baglioniet al[85]reported that,for both lean and overweight subjects,ASC derived from subcutaneous WAT depots have higher growth rate and adipogenic potential than those derived from visceral WAT depots.In addition,adipocytes derived from subcutaneous ASC have greater capacity to secrete adiponectin and are less susceptible to lipolysis than adipocytes derived from visceral ASC.Therefore,functional differences between subcutaneous and visceral WAT depots could originate from differences in depot-specific stem cells.Moreover,microarray analysis revealed that the genes differentially expressed between subcutaneous and visceral ASC are implicated in energy and lipid metabolism;importantly,genes involved in cholesterol biosynthesis and triacylglycerol metabolism were upregulated in visceral ASC[151].Genome-wide expression profiles of ASC derived from subcutaneous and visceral depots are highly distinct,in particular for the expression of genes responsible for early development,which gave rise to the idea that adipose depots exist as individual mini-organs[152,153].
Numerous studies have compared depot-specific ASC from lean and obese subjects,to investigate if the differences between depot-specific ASC may account for the differential responses of adipose depots during the development of metabolic dysfunctions.We have recently demonstrated that ASC from the visceral depot secreted the highest levels of IL-6 and IL-8 compared with ASC derived from subcutaneous and preperitoneal WAT depots[86].Other studies also reported increased secretion of pro-inflammatory,pro-angiogenic and pro-migratory molecules (IL-6[154,155],IL-8[154],CCL-5[154],MCP-1[86,154,155],G-CSF[86],GM-CSF[155],eotaxin[155],IL-1ra[155]and VEGF[155]) by ASC from the visceral depot,when compared with those derived from the subcutaneous depot.Therefore,visceral ASC appear to secrete more proinflammatory cytokines than subcutaneous ASC,both in obese and in non-obese states,which is in line with the stronger pro-inflammatory pattern adopted by visceral WAT in response to metabolic challenges.
Fernándezet al[156]were the first to report the isolation of ASC from the preperitoneal WAT depot.These authors observed that preperitoneal ASC have a higher adipogenic potential than those derived from the subcutaneous WAT depot.Comparing ASC from abdominal subcutaneous,preperitoneal and visceral WAT depots of morbidly obese women,we demonstrated that preperitoneal ASC have the highest ability to differentiate to the adipogenic lineagein vitro.In addition,we observed that ASC derived from the visceral depot had the lowest adipogenic potential[86],which could be explained by the strongly pro-inflammatory milieu established in this depot during obesity.For example,IL-6 production in visceral WAT is 3 fold higher than in the subcutaneous depot[146,157].Moreover,the macrophage accumulation observed in WAT depots during obesity development[62,63]is particularly high in the visceral compared with the subcutaneous WAT depot[158].We have recently demonstrated that,compared with subcutaneous SVF cells,visceral SVF populations have higher numbers of CD14+CD206-cells,a phenotype associated with M1 macrophages[86].
Although some studies showed that visceral ASC have higher adipogenic potential than those from subcutaneous depots,others studies reported the opposite,both in humans[85,86,151,153,159-166]and in mice[152,167-169],with no differences reported in two studies in humans[131,170].Thus,there is currently no clear consensus regarding the differences in adipogenic potential between depot-specific ASC populations.Differences in donor adiposity grades and sex,in ASC isolation and adipogenic induction protocols,as well as in methods of adipogenic evaluation could account for this discrepancy,and highlight the importance of technical standardization in this area[173].Nevertheless,as most studies suggest that there are differences in the adipogenic capacity of ASC derived from distinct WAT depots,together with differences regarding their proinflammatory potential (Figure 1),it is likely that ASC/adipogenic precursors contribute to establish distinct fat distribution and expansion patterns between depots,and the balance between hypertrophy and hyperplasia during obesity development.
Fat distribution and expansion capacity of different WAT depots
As mentioned earlier,WAT can expand through increases in the size of adipocytes(hypertrophy),as well as by increases in the number of adipocytes (hyperplasia,through adipogenesis).Obese individuals where the visceral WAT is expanded preferentially have a greater risk of developing other metabolic and cardiovascular diseases than those who have more subcutaneous WAT expansion[174-176],which has aprotective role against the metabolic complications of obesity induced by high-fat diets[177,178].
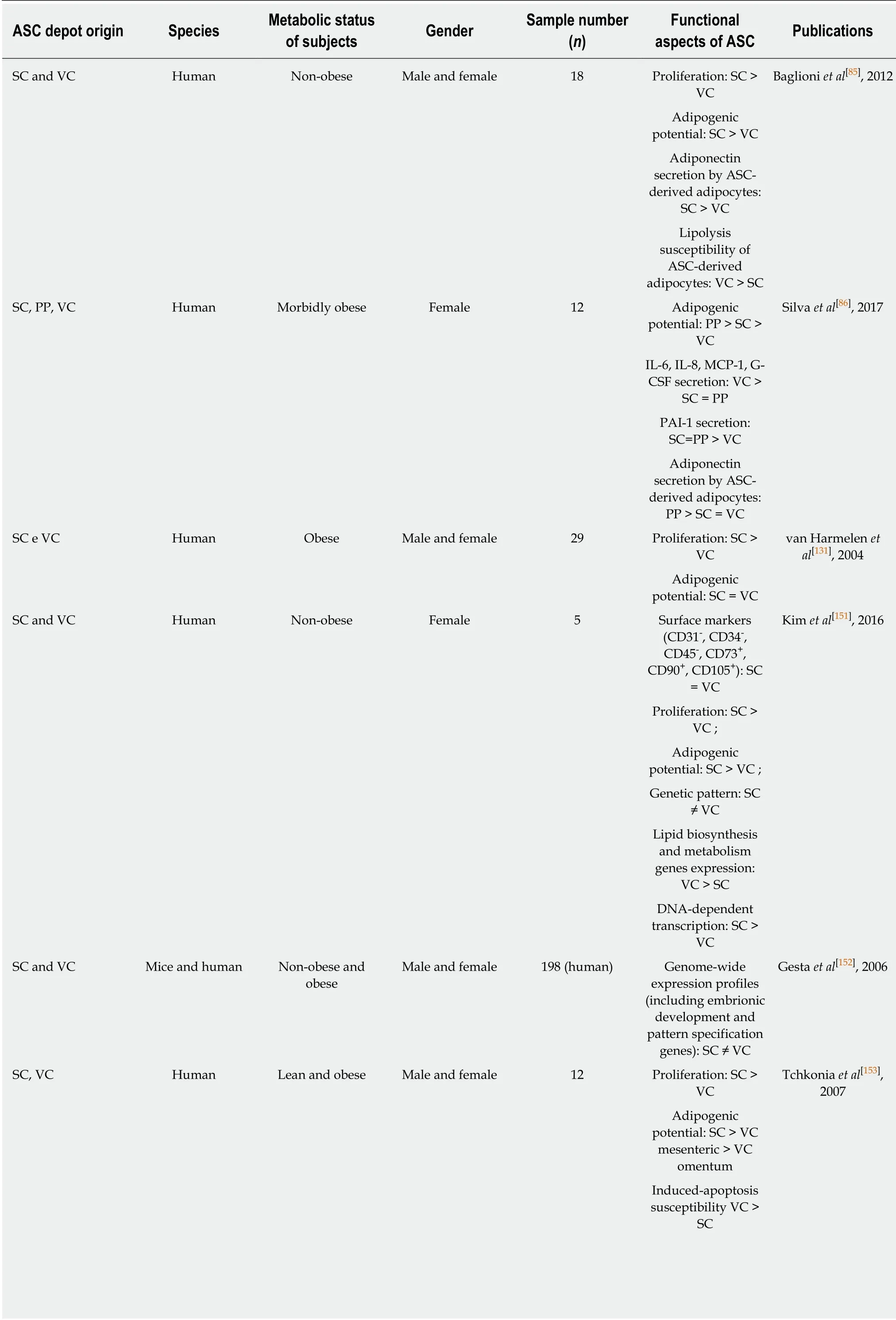
Table1 Functional aspects of human adipose-derived stem/stromal cells derived from different adipose depots

Genome-wide expression profiles(including early development genes):SC ≠ VC SC and VC Human Obese Female 8 MCP-1,IL-6,IL-8,CCL-5 secretion:VC>SC Zhu et al[154],2015 SC and VC Human Non-obese Male and female 15 MCP-1,eotaxin,IL-1ra,IL-6,GM-CSF,VEGF secretion:VC>SC Perrini et al[155],2013 SC and PP Human Non-obese and obese Male 8 Proliferation:SC >PP Fernández et al[156],2010 Adipogenic potential:PP >SC SC and VC Human Lean and obese Female 14 Adipogenic potential:SC >VC Hauner et al[159],1988 SC and VC Human Not stated Not stated Not stated Adipogenic potential:SC >VC Adams et al[160],1997 SC and VC Human Non-obese and obese Male and female 12 Adipogenic potential:SC >VC Niesler et al[161],1998 Susceptibility to induced apoptosis:VC >SC SC and VC Human Non-obese and obese Male and female Not stated Adipogenic potential:SC >VC Digby et al[162],2000 SC,VC Human Obese Male and female 16 Adipogenic potential:SC >VC mesenteric >VC omentum Tchkonia et al[163],2002 SC,VC Human Obese Male and female 18 Adipogenic potential:SC >VC omentum Tchkonia et al[164],2005 Resistance to induced apoptosis:SC >VC omentum Proliferation:SC =VC mesenteric >VC omentum SC,VC Human Overweight and obese Male and female 31 Adipogenic potential:SC >VC Tchkonia et al[165],2006 Resistance to induced apoptosis:SC >VC mesenteric>VC omentum SC and VC Human Not stated Not stated 21 Proliferation:SC =VC Toyoda et al[166]2009 Adipogenic and osteogenic potential:SC >VC SC and VC Mice Non-obese and obese Not stated Not stated Proliferation:SC >VC Macotela et al[167],2012 Adipogenic potential:SC >VC SC and VC Mice Not stated Male Not stated Adipogenic potential:SC >VC Meissburger et al[168],2016 SV and VC Mice High-fat diet Male and female Not stated Proliferation in response to high-fat diet:SC >VC Joe et al[169],2009 Adipogenic potential:SC >VC SC and VC Human Non-obese and obese Male and female 18 Adipogenic potential:SC = VC Shahparaki et al[170],2002 SC and VC Human Non-obese Male and female 13 ASC-derived adipocytes C/EBP,AP-2 and adiponection expression:SC >VC Perrini et al[171],2008

ASC:Adipose-derived stem/stromal cells;G-CSF:Granulocyte colony-stimulating factor;GM-CSF:Granulocyte-Macrophage colony-stimulating factor;IL:Interleukine;MCP-1:Monocyte chemoattractant protein-1;MMP:Matrix metaloproteinase;PP:Preperitoneal;SC:Subcutaneous;VEGF:Vascular endothelial growth factor;VC:Visceral.
Hypertrophic adipocytes are associated with adipose tissue dysfunction and inflammation[179-181],while adipocyte hyperplasia is associated with improved insulin sensitivity and other metabolic parameters[182],indicating that the balance between hypertrophy and hyperplasia during WAT expansion can determine the effect of adipose tissue expansion on metabolic disease development.A comparison of WAT depots suggested that hyperplasia contributes to subcutaneous WAT expansion more than to the expansion of visceral WAT,after a high-fat diet[169].Given the association of hypertrophic adipocytes with adipose tissue dysfunction,the preferential expansion of visceral WAT by hypertrophy rather than hyperplasia could represent the mechanism underlying the link between visceral WAT expansion and obesity.On the other hand,the preferential expansion of subcutaneous WAT in humans by hyperplasia may explain why subcutaneous WAT expansion is considered comparatively “healthier” than visceral WAT expansion.
However,lineage-tracing experiments in transgenic male mice have challenged this view,by detecting an increase in the formation of new adipocytes in the epididymal visceral WAT,with no measurable adipocyte formation in the subcutaneous WAT,in mice given a high-fat diet[183,184].Later studies demonstrated thatin vivohyperplasia in WAT varies according to the specific depot and the sex,being influenced by sex hormones[185].While males have higher potential for expansion by hyperplasia in the visceral WAT only,females exhibit WAT hyperplasia in both visceral and subcutaneous depots after a high-fat diet[185].This may also occur in humans,since obesity development in men is associated predominantly with visceral WAT expansion,while obesity development in women involves subcutaneous WAT expansion[22,186].Adding further complexity to this issue,Tchoukalovaet al[187]reported that overfeeding in humans induces different mechanisms of WAT expansion in upper- and lower-body subcutaneous WAT depots:while upper-body abdominal subcutaneous WAT predominantly expands by adipocyte hypertrophy,lower-body subcutaneous WAT preferentially expands by adipocyte hyperplasia.Moreover,differences in preadipocyte replication or apoptosis could explain the differential patterns of expansion between upper- and lower-body subcutaneous WAT depots.
The numerousin vitroandin vivostudies described in this review suggests that the different physiopathological properties of distinct WAT depots could be attributed to the intrinsic properties - including gene expression,adipogenic and angiogenic potentials,and inflammatory behavior - of adipose progenitors cells within each adipose compartment.However,Jefferyet al[185]recently challenged this hypothesis by demonstrating,in a series of elegant transplantation experiments in transgenic mice,that donor adipose progenitor cells behave as resident progenitors after transplantation.As previously described,levels of hyperplasia were only detected in visceral WAT of male mice fed with a high-fat diet,but not in subcutaneous WAT,indicating that subcutaneous adipose progenitors did not enter adipogenesis[184].Importantly,when subcutaneous adipose progenitors were injected into the visceral WAT depot,they proliferated in response to the high-fat diet,but neither subcutaneous nor visceral adipose progenitors proliferated when transplanted into the subcutaneous WAT depot[185].These exciting data may suggest that adipose progenitors from distinct WAT depots,despite having different developmental origins[188,189],are functionally plastic and capable of responding to high-fat diets according to cell-extrinsic factors of the depot microenvironment.Therefore,these new data suggest that,irrespective of their origin,adipose progenitors behave according to the WAT depot in which they dwell.Although it is clear that ASC from distinct fat depots contribute differently to obesity,further studies are now necessary to clarify the contribution of cell-extrinsic and/or intrinsic factors in obesity development.

Figure1 Adipogenic and pro-inflammatory potentials of adipose-derived stem/stromal cells derived from different abdominal adipose tissue depots.
CONCLUSION
Adipose progenitors play an important role in obesogenic WAT growth and the regulation of adipogenesis by these cells may be used in novel therapeutic strategies against obesity and related diseases.There is no doubt that ASC from different WAT depots have distinct properties,which are not totally autonomous,as the distinct microenvironments of each WAT depot influence the function of adipose progenitor in WAT expansion.Moreover,distinctin vivoniches of adipose progenitors may account for the differential susceptibilities of adipose depot to the development of metabolic dysfunction.Future studies on adipose progenitor niches,considering the depot-specific microenvironment and the influence of sex influence on adipose progenitor activation,should elucidate the regulatory signals that govern adipose progenitor function.Ultimately,these studies may allow adipose progenitors to be targeted in therapeutic approaches to prevent obesity development or to allow obese individuals to reach a healthier metabolic status.
ACKNOWLEDGEMENTS
The authors thank the National Council for Scientific and Technological Development(CNPq),the Carlos Chagas Filho Foundation for Research Support of the State of Rio de Janeiro (FAPERJ) and the Coordination of High Education Personnel Improvement(CAPES) for financial support.
杂志排行
World Journal of Stem Cells的其它文章
- Long non-coding RNA: The functional regulator of mesenchymal stem cells
- Circulating factors present in the sera of naturally skinny people may influence cell commitment and adipocyte differentiation of mesenchymal stromal cells
- Stromal cell-derived factor-1α promotes recruitment and differentiation of nucleus pulposus-derived stem cells
