Management of pancreatic head adenocarcinoma:From where to where?
2019-04-24KemalDolayFatmaUmitMalyaSamiAkbulut
Kemal Dolay,Fatma Umit Malya,Sami Akbulut
Abstract
Key words: Pancreatic head cancer;Standard pancreatectomy;Extended pancreatectomy;Regional lymphadenectomy;Extended lymphadenectomy
INT RODUCTION
The challenges associated with pancreatic head adenocarcinoma (PHAC) include higher frequencies of regional or distant lymph node (LN) metastases and positive resection margins in the pancreatic and retroperitoneal tissues.Despite advances in imaging,staging,adjuvant therapy,aggressive surgery,and down staging with neoadjuvant therapy,overall survival (OS) has not improved.Surgical resection followed by adjuvant therapy is associated with disease relapse rates of > 70%[1].Only 30%-40% of pancreatectomies achieve R0 resections,even in experienced hands,because the tumors spread early into and along neural sheaths[2].
Given mortality rates of 0.7-3% and morbidity rates of 36%-41%,standardizing surgical procedures and centralizing pancreatic surgery within high-volume institutions are essential[3].The median OS for patients with primary resectable tumors is 20-24 mo,while that for patients with locally advanced,nonmetastatic PHAC is 9-13 mo.However,a 5-year OS rate of 25% is possible for patients who are suitable for resection;hence,surgery offers the only chance of a cure and long-term survival[4].The extent of regional LN invasion by PHAC is a powerful prognostic factor after resection that is independent of the histology[5];therefore,lymphadenectomy is a crucial step during pancreaticoduodenectomy (PD).
Halsted reported the first successful periampullary cancer resection in 1899.Subsequently,the procedure was described by Codivilla in 1898,Kaush in 1909,and Whipple in 1935[6].In the 1960s and 1970s,publications describing large patient series without operative deaths became available.As the short-term results became more predictable,attention turned to modifying the operation to improve the long-term oncologic outcomes.In 1973,Fortner[7]defined regional pancreatectomy as a new technique that increased tumor resectability and improved patient outcomes.The procedure comprised a total PD with a subtotal gastrectomy and mesenteric-portal confluence resection or mesenteric venous axis and superior mesenteric artery (SMA)resections with reconstruction.This technique is extremely complex,does not improve life expectancy significantly,and has not been adopted in western countries.However,Japanese surgeons inspired by this technique have described extended LN dissections.Furthermore,multiorgan,peripancreatic nerve plexus,portal venous,and arterial resections comprise radical surgery for pancreatic cancer.In this review,we aimed to evaluate the basic concepts and the role of radical surgery in PHAC.
LYMPHADENECTOMY:ANATOMY,DEFINITIONS,AND GENERAL CONSIDERATIONS
Several routes drain lymph from the pancreatic head,and the three main lymphatic drainage pathways are a superior pathway that drains lymph into the celiac axis LNs,and two inferior pathways that drain lymph into the LNs around the SMA.Other minor lymphatic channels drain lymph into the thoracic duct directly or through the paraaortic LNs.Many studies' findings and consensus meetings have standardized LN nomenclature and definitions according to the Japan Pancreas Society definitions[3].Accordingly,LN metastases are divided into three groups (Figure1).Group 1 (N1) comprises the LNs at stations 13a,13b,17a,and 17b located at the posterosuperior,anteroinferior,posteroinferior,and anterosuperior portions of the pancreatic head,respectively.Group 2 (N2) comprises the LNs at stations 6,8a,8p,12a,12p,12b,14p,and 14d,located at the infrapyloric,anteriosuperior aspect of the common hepatic artery (CHA),posterior aspect of the CHA,hepatic artery,portal vein (PV),bile duct,proximal SMA,and distal SMA nodes,respectively.Group 3 (N3)comprises the LNs at stations 1,2,3,5,7,10,11p,11d,15,16a2,16b1,and 18 located at the right cardiac node,left cardiac node,lesser curvature of the stomach,suprapyloric node,left gastric artery,splenic hilum,proximal splenic artery,distal splenic artery,middle colic artery,abdominal aorta from the superior margin of the celiac artery(CA) to the inferior margin of the left renal vein,abdominal aorta from the inferior margin of the left renal vein to the superior margin of the inferior mesenteric artery,and inferior margin of the pancreas,respectively.
Lymphadenectomy is the most important and fundamental step in PD.Indeed,70.5%-77% and 18%-26% of resected pancreatic specimens have LN metastases and paraaortic LN metastases,respectively[8-10].Nagakawaet al[9]showed that metastases were most prevalent in station 13,followed by stations 17 and 14,and 12 and 16.On average,3.2% of LNs examined had metastases in station 16,but Kayaharaet al[8]determined that 13% had metastases in this station.While the extent of LN metastases tends to increase with tumor size,the relationship between tumor size and the risk of metastasis to the paraaortic region is weak.Retroperitoneal tumor invasion predicts LN metastasis.Hence,the paraaortic metastasis pathway may take a retroperitoneal lymphatic route from stations 13 to 14 before it reaches station 16;these findings led authors to consider extensive LN dissections for curative resections that included stations 14 and 16.
Standard PD successfully removes 80% of the most commonly involved LN sites[11];however,Ishikawaet al[12]suggested that if perineural invasion is a pathway for the lymphatic spread of cancer cells,perineural and lymphatic invasion may signify lymphatic metastases before the cancer cells enter LNs,and that microinvasions might occur in the N2 region,even when nodal involvement was limited to the N1 region.Consequently,they suggested that a simple lymphadenectomy without resection of the surrounding connective tissue might be inadequate for lymphatic clearance.
The findings from a multicenter,randomized controlled trial (RCT) that compared standard and extended lymphadenectomies during PD[13],showed that while there was no difference between standard and extended lymphadenectomy regarding survival,there was a possible trend towards longer survival for node-positive patients who underwent extended lymphadenectomies;these findings concurred with those from several retrospective studies.These investigators also found that extended lymphadenectomy did not increase the morbidity and mortality rates significantly,and they noted that disabling watery diarrhea was not a problem[13],despite Ishikawaet al[12]reporting results to the contrary.
The definitions of standard and radical or extended surgery and the usage of terminology differ among studies;these include the extended radical Whipple resection,regional pancreatectomy,extended PD,extended lymphadenectomy,anden-blocresection.A consensus statement from a meeting held in 1998 to unify the surgical terminology explained that a standard PD includes regional lymphadenectomy around the duodenum and pancreas,a radical PD includes regional lymphadenectomy plus skeletonization of the hepatic arteries,the SMA between the aorta and inferior pancreaticoduodenal artery and the CA,and the dissection of the anterolateral aspect of the aorta and vena cava,including Gerota's fascia,and an extended radical PD includes radical PD and clearance of the anterior aorta between the diaphragmatic hiatus around the CA and the origin of the common iliac arteries[14].
Yeoet al[15]compared radical and standard resections,and showed no significant differences regarding the 1-,3-,and 5-year survival rates or the median survival times(30 movs28 mo).While the perioperative mortality rates were similar for the radical(2%) and standard (4%) procedures,the morbidity rates were higher following radical resection,because of the higher delayed gastric empting (16%vs6%) and pancreatic fistula (13%vs6%) rates.Farnellet al[16]compared extended and standard lymphadenectomies,and found that while the morbidity and mortality rates were comparable,the mean number of LNs resected was greater in the extended lymphadenectomy group (36vs15),and that there were no significant differences in the 1-,3,and 5-year survival rates.However,the long-term follow-up assessment showed that the patients who had undergone extended lymphadenectomies had significantly more diarrhea,and worse bowel control and body appearance.
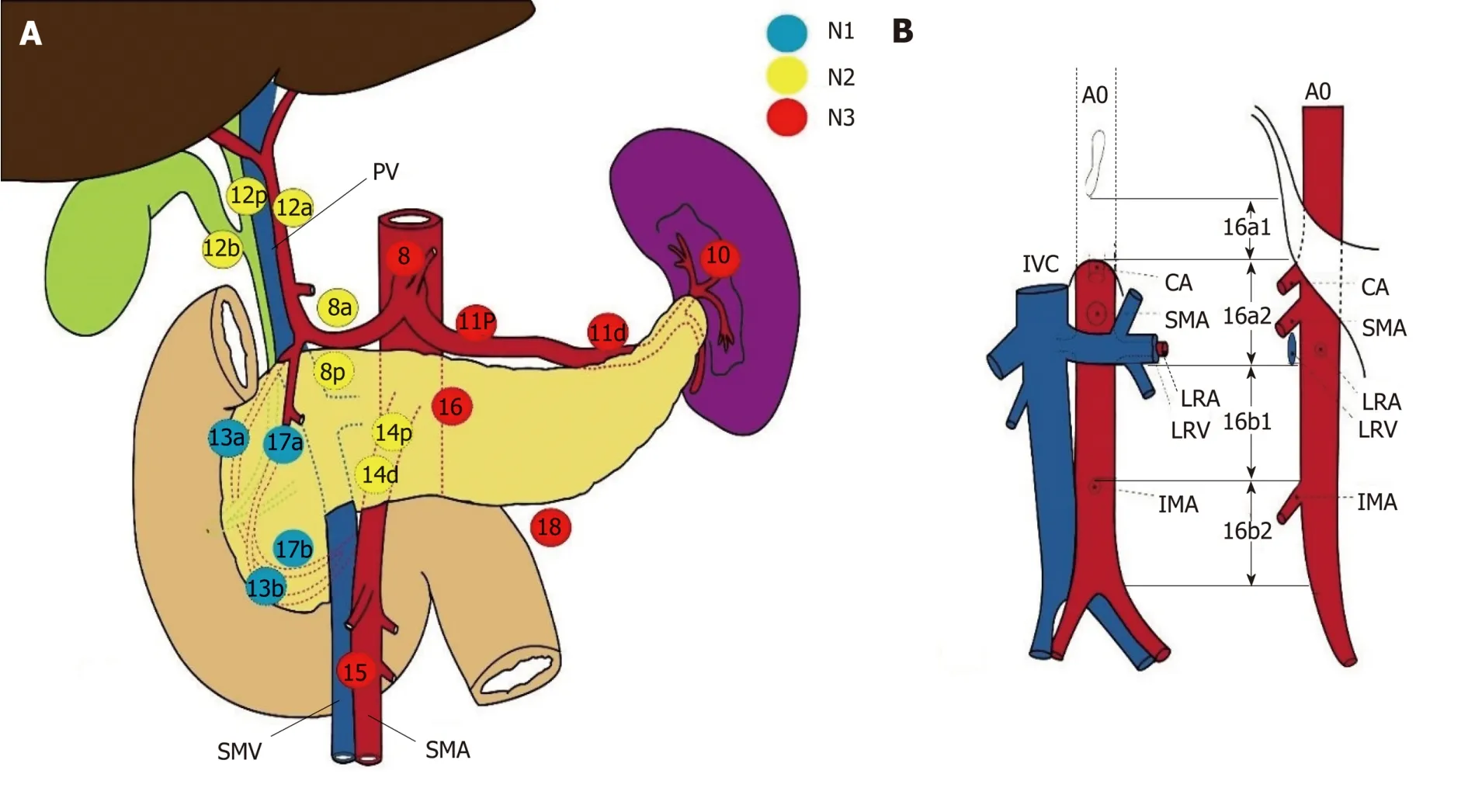
Figure1 Japan Pancreas Society classification of the regional lymph nodes stations of the pancreas.
Nimuraet al[1]designed a multicenter RCT of 101 patients with PHAC who underwent PD and were grouped into regional or extended lymphadenectomy groups.Significant differences were found between the regional and extended lymphadenectomy groups regarding the numbers of LNs harvested (13.3vs40,P<0.001),but not regarding median survival (19.9 movs13.8 mo) or the 1- (78%vs54%),3- (28%vs18%),and 5- (16%vs6%) year OS rates.Since these RCTs generated equivocal results,Pawliket al[17]and Farnellet al[18]evaluated the value and principles of RCTs,and they developed a biostatistical model that accurately calculates the population size needed for powerful and meaningful statistical analyses.Their data showed that only three in 1000 patients might show a survival benefit from extended lymphadenectomy,which was based on the fact that only patients with secondechelon LN involvement could benefit from extended lymphadenectomy.Hence,>200000 patients would be required to adequately power a trial that would detect any OS benefit.
Five metaanalyses evaluated RCTs and cohort studies that investigated the effects of extended pancreatectomy on OS,disease-free survival,and intraoperative and postoperative complications,and their findings showed no benefits associated with extended lymphadenectomy[19-23].These conclusions concur with the findings from an RCT from Japan that showed no long-term survival benefit after extended lymphadenectomy in patients with resectable PHAC[1].
The International Study Group on Pancreatic Surgery (ISGPS) evaluated the extent of a standard lymphadenectomy for pancreatic cancer[24],and the members proposed using the Japanese Pancreas Society's nomenclature that classifies the nodal stations[25].Further,the ISGPS proposed a meaningful definition of a standard lymphadenectomy for PHAC that included LN stations 5,6,8a,12b1,12b2,12c,13a,13b,14a right lateral side,14b right lateral side,17a,and 17b,and it concluded that no strong recommendation could be made regarding the routine resection of stations 8p and 16b1.
The numbers of LNs harvested and the LN ratios of metastatic/resected LNs in gastrointestinal cancer have important roles[26,27].Likewise,the clinical importance of the LN count and LN ratio for survival following surgery for pancreatic cancer has been described[28,29].Contreraset al[30]suggested that examining higher LN numbers is a multivariable predictor of a negative microscopic margin,and it can thus be accepted as a quality measure.Therefore,data exist that describe the importance of the number of LNs analyzed in relation to survival following surgery for pancreatic cancer.Schwarzet al[31]postulated that the LN ratio and the number of LNs examined are important prognostic factors,and that to optimize operative benefits,≥ 15 LNs in total or approximately 10 negative LNs should be examined for curative-intent pancreatectomy.The LN ratio was introduced to characterize the lymphatic tumor load and create a prognostic parameter independent of rough estimations based on N0vsN1 or the overall number of positive LNs.In general,an LN ratio of 0.2 is the accepted cutoff value that indicates poor survival.An LN ratio > 0.2,vascular or perineural invasion,and a positive resection margin are independent prognostic factors that determine long-term survival in patients undergoing surgery for PHAC[32,33].However,other studies' findings suggest an LN ratio of 0.4 as the cutoff value[34].Hence,further studies involving larger numbers of patients are necessary to determine the prognostic LN ratio cutoff value for PHAC.
An R0 resection is the only chance of long-term survival for patients with PHAC.The International Union Against Cancer,American Joint Committee on Cancer criteria,and College of American Pathologists reporting guidelines define an R1 resection as microscopic tumor tissue at the surgical resection margin.In Europe,the Royal College of Pathologists' guidelines define an involved margin as tumor cells within 1 mm of the resection margin.Recent analyses of resected pancreatic specimens using standardized pathologic reporting protocols showed higher R1 rates[35-44].Consequently,other issues aside from extended lymphadenectomy have been discussed regarding the achievement of R0 margins[45-50].
NERVE PLEXUS AND RETROPERITONEAL TISSUE RESECTION
Pancreatic tumors tend to spread along nerves,but the underlying mechanism is not completely understood.Growth factors secreted by tumors may increase perineural connective tissue degradation,resulting in early nerve invasion[51-53].Several trophic factors,including glial-derived neural factors,artemin,and tyrosine kinase receptor-1,have been described[54-56].Extrapancreatic neural involvement may be related to early recurrence and poor outcomes[57-59].En-blocremoval of the peripancreatic neural tissue with the specimen may reduce local recurrence rates;however,it is difficult to isolate the neural tissue surrounding the SMA from the lymphatic tissue[60].The right half of the SMA plexuses,in particular,may show malignant cell invasions in patients with pancreatic cancer.Removing the right half of this plexus during standard lymphadenectomy without interfering with intestinal function may be possible and worthwhile[61].The authors of studies from westernized countries have commented on the significance and prognostic implications of these neural plexuses[62,63].Hence,a standard lymphatic dissection aims to resect all the lymphatic and neural tissue on the right-hand side of the SMA and all the retropancreatic tissue anterior to the aorta and vena cava,which may reduce the positive retropancreatic resection margin risk,and increase the chance of a cure.
MESOPANCREAS RESECTION
Given the tendency of cancer cells to infiltrate the retropancreatic tissue,the term“mesopancreas” has been coined to describe the perineural lymphatic layer located dorsally to the pancreas.Complete mesopancreatic resection may minimize locoregional recurrences and improve outcomes[64].Moreover,the epithelialmesenchymal transition-related phenomenon underlying cancer progression has been depicted[65]that involves tumor budding,which describes the presence of dedifferentiated,isolated single cells or small cell clusters scattered within the stroma at the invasive tumor front,and the formation of tumor deposits (TDs),which are macroscopic or microscopic nests or nodules found in the lymph drainage areas of a primary carcinoma without evidence of residual LNs in the nodules;these TDs may cause local recurrences.
Consequently,several mesopancreatic resection techniques have been described.Adhamet al[66]defined a “mesopancreas triangle” as an inverted triangle with its base on the posterior surfaces of the superior mesenteric vein (SMV) and PV,apex on the anterior surface of the aorta between the CA and the origin of the SMA,and its lateral boundaries limited by the right semicircumferences of the CA and SMA plexuses,and the mesopancreatic boundaries on the sagittal plane extend to the paraaortic area[66].Kawabataet al[67]expanded this definition to include the entire paraaortic and perisuperior mesenteric arterial area.While many new “artery first” or “posterior first”en-bloctechniques have been described that extend the resection of the retropancreatic lymphatics,negative mesopancreatic resection margins and lower locoregional recurrence rates have not improved survival[68],and the findings from a cadaveric anatomic-pathologic study[69]demonstrated that the mesopancreas concept is anatomically unsubstantiated and thaten-blocmesopancreas removal is not possible,because no fibrous sheath or fascia exists around the retropancreatic loose areolar and adipose tissue.Subsequently,this areolar tissue has been called the“pancreatic head plexus II”,which is the most accurate definition[70].A greater understanding of this area's boundaries and the importance of TDs would improve surgery.
Although appropriate lymphadenectomy is important to control tumors effectively,it may not be sufficient,and it should be combined with adjuvant or neoadjuvant chemotherapy with or without radiotherapy;several studies are investigating the role of neoadjuvant therapy[71],which seems feasible for borderline and locally advanced disease.Importantly,the likelihood of delivering full-dose chemotherapy is higher before surgery and it may be more effective than postoperative therapy,because resected tumor beds are associated with poor drug delivery and low sensitivity to radiation as a consequence of reduced oxygenation.
A promising development was extended life expectancies after chemotherapy,especially in groups that had undergone surgery.The advantages of neoadjuvant chemotherapy for borderline resectable tumors seem to be important,but its efficacy is not as high as that for tumors with good responses,and further studies are necessary.
TOTAL PANCREATECTOMY
Total pancreatectomy (TP) was introduced by Ross and Porter in the 1950s to avoid pancreatic anastomosis-related complications.TP emerged as a consequence of high recurrence rates following the Whipple procedure and a belief that pancreatic cancer might be a multicentric disease.It was subsequently considered radical surgery,because it was based on the idea of removing all residual potential tumor tissue[35].Furthermore,it was thought that extended radical pancreatectomies could include appropriate lymphatic dissections and R0 surgical margins.The main problems associated with TP were insulin-dependent diabetes mellitus with difficult-to-control blood glucose levels and malabsorption that caused severe mortality and morbidity and significant quality of life reductions.Several centers reported perioperative mortality and morbidity rates equal to those associated with the Whipple procedure,and no improvements in long-term survival.Hence,TP was largely abandoned as part of radical resections[37].Sometimes,PD is required,for example,for diffuse intraductal papillary mucinous neoplasms,hereditary pancreatitis,recurrent pancreatic cancer,or pancreatic cancer,or as a rescue treatment for severe pancreaticoduodenal anastomotic leakage.
VASCULAR RESECTION
Given that an R0 resection is the cornerstone of a potential cure,determining the relationship between the primary tumor and the mesenteric vasculature,and detecting metastatic disease in the liver or peritoneum using abdominal multidetector row computed tomography or magnetic resonance imaging are crucial for planning appropriate treatment for PHAC.
PORTAL VEIN-SUPERIOR MESENTERIC VEIN RESECTION
Locally advanced disease in PHAC caused by vascular invasion occurs in up to 35%of patients,and PV resection is an important curative option that improves resectability and the R0 rate in selected patients that may be accompanied by survival benefits.Child,who first highlighted the importance of PV resection in PHAC,described a two-step PV resection during PD in 1950 that involved PV ligation,followed by PD with PV resection[41].This procedure did not become popular,because of its high complication and mortality rates.Several techniques were described subsequently.In addition to autologous or homologous vessel grafts for PV reconstruction,the use of artificial grafts has been reported,and end-to-end PV anastomosis following resection is the safest procedure.
PV invasion is most frequently diagnosed using computed tomographic portography,but intraportal endovascular ultrasonography requires further research and greater operator proficiency[38].Classifying PV invasion radiologically informs surgeons about the appropriate resection technique and resectability,and PV invasion is classified as follows:type A:absent (normal);type B:unilateral narrowing;type C:bilateral narrowing;and type D:stenosis or obstruction with collateral circulation[39].Pathologic findings are also classified to demonstrate correlations between invasion and OS,and the radiologic findings correlate with the pathologic findings.The pathologic findings associated with PV invasion are classified as:grade 0:no invasion;grade 1:tunica adventitia invasion;grade 2:tunica media invasion;and grade 3:tunica intima invasion[40].
PV or SMV resections are usually required in patients with borderline-resectable pancreatic cancer (BR-PDAC).BR-PDAC was initially defined as marginal resectability[41].Borderline resectability was defined by the National Comprehensive Cancer Network in 2006,and was noted as a high risk for a margin-positive status in these patients.Subsequently,several groups have proposed different definitions of BR-PDAC,and a symposium held during the International Association of Pancreatology meeting in 2016 defined BR-PDAC as tumor abutment or invasion of the SMV/PV with bilateral narrowing or occlusion not exceeding the inferior border of the duodenum,tumor contact with the SMA and/or CA of < 180° without stenosis or deformity,or tumor abutment of the CHA without tumor contact with the HA and/or CA proper[42].All of the meeting attendees accepted that PV resection is indicated only if cancer-free surgical margins were considered achievable.The findings from a study of survival after pancreatectomy with PV resection for pancreatic cancer showed that the 1-,3-,and 5-year survival rates were 50%,16%,and 7%,respectively,in 40 studies involving 1351 patients[43].
Nakaoet al[40]found that patients with Type A PV invasion have a significantly higher OS rate than patients with Types B,C,D,or unresectable disease.Authors note that patients with grade 0 PV invasion had a significantly higher OS rate than patients with grades 1,2,3,or unresectable PV invasion.The authors also reported that patients who underwent PV resection had significantly worse prognoses than those who did not undergo PV resection,because of the advanced stage of the disease,and they showed that a cancer-free surgical margin was obtained from 80.2%,73.2%,56.7%,and 39.7% of patients with Types A,B,C,and D PV invasion,respectively.These authors suggested that invasions of the pancreatic head nerve plexus or of the plexuses around the arteries are the main causes of cancer-positive surgical margins.Although OS is low among patients with Types C or D PV invasion,it is higher than that among patients with unresectable tumors;hence,patients with Types C or D PV invasion deserve surgery.
A recent meta-analysis that evaluated the role of PV/SMV resections on survival[44]showed that the R1 resection rates were higher in patients with type C or D PV invasion;consequently,these authors suggested that OS would not improve following resection.The authors also pointed out that while the evidence suggested that PV/SMV resections are feasible with acceptable morbidity and mortality rates,the margin status and long-term survival improvements remained unclear.However,the studies included in this meta-analysis were retrospective and nonrandomized,and subject to selection bias and confounding.In contrast,other studies' findings,particularly those from the ESPAC 3 trial,have shown that the resection margin is not an independent prognostic indicator of survival.Hence,periarterial nerve plexus and lymphovascular invasion indicate systemic disease,but tumors requiring PV/SMV resections are bigger and more advanced,which could explain the lower survival rates.The main reason for suggesting PV/SMV resections during PDs,especially for BR-PDACs,is that OS is better compared with that for patients who do not undergo resections;resection is recommended by the ISGPS.Importantly,patients who are expected to achieve R0 resections should be selected by undertaking multidisciplinary preoperative evaluations.
ARTERIAL RESECTION
According to the current guidelines,arterial involvement requiring SMA,CHA,or CA resections defines local irresectability[72].However,the few studies that have investigated the outcomes of patients who underwent arterial resections for pancreatic cancer have generated inconsistent results[72].In their systematic review,Mollberget al[73]evaluated 26 studies' results,and they noted significant increases in the risks of perioperative mortality and morbidity,and in the reoperation rates for patients who underwent pancreatectomies with arterial resections.Moreover,they showed that the OS of patients who underwent pancreatectomies and arterial resections was significantly worse than that of patients who underwent pancreatectomies without arterial resections,and that their long-term survival rate was lower than that of patients who underwent venous resections.While these results were considered to be related to the high R1 resection rates associated with advanced tumors,the R1 resection rates did not differ between the arterial resection and nonarterial resection groups.Although the life expectancy results were not satisfactory,the long-term survival rate was better in the arterial resection group than that in the patients with inoperable tumors.The authors concluded by suggesting that arterial resections should be performed on selected groups of patients at specialized centers,and that the results should be recorded.The general condition of these patients should be suitable for a major resection,and arterial resections should be performed if anastomoses are possible following resection.
Nakaoet al[74]performed curative resections in 289 (65.2%) of 443 patients with pancreatic cancer,and,of these,200 (69.2%) underwent curative resections with vascular resections.Furthermore,PV resections without arterial resections were performed on 186 patients,and combined PV and artery resections were performed on 14 patients.Operative deaths occurred in 11 patients who underwent curative resections (3.8%),one (1.1%) of 89 patients who did not undergo vascular resections,five (2.7%) of 186 patients who underwent PV resections without arterial resections,and five (35.7%) of 14 patients who underwent PV and arterial resections.The authors noted that the patients who underwent surgery for locally advanced pancreatic cancer with HA or SMA resections had high postoperative morbidity and mortality rates and worse prognoses.Furthermore,OS in the arterial and venous resection group was similar to that observed among the patients whose tumors were unresectable.
Katoet al[75]performed radical surgery on 12 patients,including HA,SMA,or CA resections,following neoadjuvant treatment,and noted significantly higher 5-year survival rates among those patients who achieved R0 resections.Stitzenberget al[76]studied 12 patients with pancreatic cancer who underwent pancreatectomies with HA or CA resections after neoadjuvant chemotherapy,and found that the 60-d mortality rate was high (17%),and that the median survival duration after diagnosis was only 20 mo;they suggested that HA or CA resection with reconstruction might prolong survival for selected patients after neoadjuvant therapy.Christianset al[77]evaluated 10 patients who underwent major arterial resections after neoadjuvant therapy,and they noted that planned arterial resection at the time of pancreatectomy was safe for patients who were stable or responding to chemotherapy.To summarize,arterial resection during pancreatectomy is not recommended,because it does not provide long-term survival advantages and it increases mortality and morbidity.However,if the preoperative arterial invasion signs are suspicious,the presence of arterial invasion should be confirmed,especially in patients receiving neoadjuvant therapy.
MULTIVISCERAL RESECTION
Approaches to neighboring organ involvement as part of radical surgery are discussed in relation to different visceral organ cancers,and multiorgan resections are effective for most.Regarding pancreatic cancer,and,especially,if the cancer is in the body or tail,the multiorgan involvement rate is as high as 35%[78].Several studies'findings have shown that the morbidity associated with extended resection increased and the survival benefit was limited following multiorgan resections.However,the findings from more recent studies have shown thaten-blocresections of contiguously involved organs can be performed safely in selected patients[79].Compared with standard resections,no differences were reported regarding perioperative morbidity(35%) and mortality (3%).Extended resections may include the mesocolon,colon,adrenal glands,liver,and stomach;the main goal of these procedures is to achieve R0 resections,because this is the most important predictor of long-term survival.Given the high morbidity rate,these radical procedures involving hepatobiliary and gastrointestinal surgery,must be undertaken in specialized centers.
LIVER METASTASES
Up to 70% of patients with PHAC present with liver metastases at the time of diagnosis or they develop liver metastases[80].The role of hepatectomy in patients with liver metastases from PHAC is controversial[81-84].Andreouet al[84]examined patients'postoperative outcomes and long-term survival following pancreatic resection for PHAC and concomitant liver resection for synchronous liver metastases,and found postoperative morbidity and mortality rates of 50% and 5%,respectively,1-,3-,and 5-year OS rates of 41%,13%,and 7%,respectively,and 1-,3-,and 5-year disease-free survival rates of 39%,9%,and 5%,respectively.
Tachezyet al[82]studied 69 patients with pancreatic cancer and synchronous liver metastases,and following simultaneous resections,the 5-year OS rate was 5.8% and the median survival duration was 14.5 mo,which was significantly higher than that in the nonresected control group (median survival duration:7.5 mo) (P< 0.001).Similarly,85 patients who underwent curative resections for pancreatic cancer and liver metastases,had a median survival duration of 12.3 mo and a 5-year OS rate of 8.1%[85].
The most important factors influencing OS following synchronous metastasis resection are an R1 margin status at liver resection,a T4 tumor,regional LN metastases,poorly differentiated cancer,and an absence of preoperative or postoperative chemotherapy.Therefore,perioperative adjuvant treatment modalities may be essential to improve survival.Finally,prolonged survival might be possible for patients with liver metastases associated with pancreatic cancer if a tumor's biology is favorable.However,these treatments must be customized,and should be offered by highly specialized centers only.
杂志排行
World Journal of Gastrointestinal Surgery的其它文章
- Liver preservation prior to transplantation:Past,present,and future
- Classification and guidelines of hemorrhoidal disease:Present and future
- Laparoscopic celiac plexus ganglioneuroma resection:A video case report
- Single incision laparoscopic fundoplication:A systematic review of the literature
- Learning curve of enhanced recovery after surgery program in open colorectal surgery
- Conduit necrosis following esophagectomy:An up-to-date literature review
