Oligometastases in prostate cancer: Ablative treatment
2019-04-11AmaliaPalaciosEitoAmeliajarLuqueMilagrosaRodrguezLiSoniaGarcCabezas
Amalia Palacios-Eito, Amelia Béjar-Luque, Milagrosa Rodríguez-Liñán, Sonia García-Cabezas
Abstract
Key words: Oligometastases; Metastasis-directed therapy; Stereotactic body radiation therapy; Stereotactic ablative radiotherapy; Prostate cancer
INTRODUCTION
The hypothesis that local therapies may cure metastatic disease arose from the description by Hellman and Weichselbaum[1]in 1995 of the so-called oligometastatic state. Based on their clinical experience, these authors described an intermediate state of distant spread, reflecting disease with a low, slow and late metastatic spreading capacity. There is progressively increasing confirmation of the correlation between the clinical behavior and molecular characteristics of oligometastatic patients[2]. The metastatic process is becoming increasingly well known. Based on the identification of cellular clones in metastatic tissue biopsies, it has been seen that dissemination to form new metastases is a frequent phenomenon, and that metastatic spread does not always originate from the primary tumor[3]. This has given rise to the idea that the early elimination of metastases can avoid subsequent disseminations[4,5]. Such knowledge of the metastatic cascade has contributed to the interest in performing ablative local therapies targeted to all the metastatic sites amenable to eradication.
Prostate cancer (PCa) metastasizes mainly to bone and lymph nodes. Visceral involvement is infrequent. Eradication treatment of visceral metastases is mainly surgical. While that of lymph node metastases is variable and conditioned to many factors, use is indistinctly made of surgery as well as stereotactic body radiation therapy (SBRT) and conventional external beam radiation therapy (cEBRT), mainly for the elective irradiation of certain lymph node chains. This need to associate several local treatment options has led to emergence of the concept of metastasis-directed therapy (MDT) in oligometastatic patients.
PCa is characterized by a long natural course, and in most cases is initially hormone-sensitive. Thus, in oligometastatic patients there are at least three scenarios involving different therapeutic approaches: oligometastases synchronous to the primary tumor; oligorecurrences; and oligoprogression situations, which conceptually constitute castration-resistant patients.
As a locally ablative tool, SBRT has been little studied in the first of the aforementioned scenario, though it plays a relevant role in oligorecurrences and oligoprogression.
The treatment of these patients remains a challenge. Multiple systemic treatment options are available, and the introduction of an ablative local treatment option increases the complexity and controversy of optimum treatment timing.
The present review aims to compile the main results published in the literature and examines aspects where gaps in knowledge persist in the use of SBRT,e.g., the optimum schemes, response assessment, and the identification and diagnosis of oligometastatic patients.
OLIGOMETASTATIC DISEASE
The definition of oligometastatic disease comprises at least three controversial points:the identification of oligometastatic behavior; which patients should be included under the concept of oligometastasis; and the optimum imaging techniques allowing its detection.
Identification of oligometastatic state and concept of oligometastasis
In the last consensus document on advanced PCa published in 2017, a total of 10% of those surveyed claimed not to believe in the existence of an oligometastatic state.
Identifying oligometastatic patients is crucial both in order to offer local treatment with curative intent and to optimize resource utilization and avoid needless iatrogenic problems[6]. On the other hand, patients with a tendency towards polymetastatic disease will not benefit from MDT, and the intensification of systemic therapy should be contemplated in such cases.
PCa is characterized by a broad spectrum of clinical aggressiveness. A number of biomarkers are under study, including plasma cell-free nucleic acids (e.g., cell-free DNA and circulating tumor cells), with a view to establishing their usefulness in treatment monitoring and for establishing a prognosis. However, none of them have been shown to be able to identify those patients that will exhibit oligometastatic behavior.
In bone metastases of castration-resistant PCa (mCRPC), aberrations of DNA repair genes, BRCA1, BRCA2 and ATM have been identified more frequently than in primary tumors[7]. Some studies suggest that oligometastatic progression may be regulated at least in part by epigenetic alterations and potentially by microRNA[2].MicroRNA is RNA composed of 19-22 nucleotides that regulates gene expression. A study of tumor samples from oligometastatic patients subjected to radiotherapy found that those individuals who did not develop polymetastases exhibited a different microRNA profile, including the microRNA-200 family.
A number of genic platforms have been marketed that are able to predict which PCa patients are likely to develop metastases after primary treatment. An oligometastatic molecular fingerprint therefore will probably soon become available.Until then, these platforms, together with clinical parameters such as advanced age,the Gleason score and a rapid prostate-specific antigen (PSA) doubling time are our only tools for predicting oligometastatic behavior[8]. Another point of controversy is the number of metastases to be included under the term “oligometastasis”. Based on the published series, there is a tendency to include up to 5 metastases in one or several organs under the term oligometastasis[9].
The hypothesis of a differential behavior of PCa between patients with few metastases and those with generalized lymph node metastases was proposed by Singhet al[10]in 2004. These authors found that patients with 5 or fewer metastatic lesions had longer overall survival (OS) than patients with more than 5 metastatic lesions (73%vs45% at 5 years and 36%vs18% at 10 years), as well as longer metastasis-free survival.
Another sometimes neglected term that needs to be used because it contextualizes the clinical situation is “oligorecurrence” as defined by Niibeet al[11]in 2010 to identify oligometastatic patients with a controlled primary tumor.
The number of metastases to be included under the term oligometastasis is even more crucial in the case of lymph node metastatic spread, where the quantification of either isolated nodes or lymph node areas increases screening variability. The solution to this dilemma is to define an oligometastatic patient as an individual in which all the tumor locations are amenable to MDT with radical intent.
Diagnosis of (oligo)metastatic disease
The number of metastases detected, and consequently classification as oligometastatic disease, depends on the method used for detection. The most recent European Association of Urology (EAU) guidelines recommend at least one cross-sectional abdominopelvic imaging study [computed tomography (CT) or magnetic resonance imaging (MRI)] in conjunction with a bone scan (BS) for the screening of metastases in intermediate- and high-risk primary PCa[12]. After biochemical relapse (BCR), and given the low detection rate, BS and abdominopelvic CT are only recommended in patients with serum PSA > 10 ng/mL or a PSA doubling time < 6 mo. In addition,multiparametric MRI may be useful in the event of BCR after prostate radiotherapy to assess local rescue possibilities[13]. However, these conventional imaging modalities have low sensitivity in detecting small-volume disease and may underestimate the disease burden.
Advances in molecular and biological imaging directly targeting tumor cells have resulted in greater efficacy in detecting PCa. In recurrent PCa, choline positron emission tomography-computed tomography (choline PET/CT) is the preferred restaging technique, with a pooled sensitivity and specificity of > 85%[14].Unfortunately, these figures probably decrease in the context of lymph node metastases[15], and sensitivity in application to micrometastatic disease is low. Choline PET/CT has demonstrated superiority in detecting local relapse and bone metastases versus whole-body MRI (including diffusion-weighted imaging), though with similar accuracy in detecting lymph node metastases[16]. The two techniques are presently regarded as complementary diagnostic options rather than alternatives[17].
A new radiotracer targeting prostate-specific membrane antigen (PSMA) has recently been developed and has demonstrated potentially higher detection rates than the conventional imaging modalities. PSMA is a protein expressed on dysplastic prostate cells, with expression levels 100-1000 times higher than in normal cells. These expression levels increase even further in higher disease stages and grades[18]. Recent meta-analyses show 68Ga-PSMA PET to offer excellent diagnostic performance in primary and secondary staging, due to its ability to detect lesions even in the presence of very low serum PSA levels[19]. As an example, in the meta-analysis published by von Eybenet al[19], the pooled detection rate was 50% even in a subgroup of studies evaluating patients who showed BCR with PSA levels of 0.2-0.49 ng/mL. The technique has been shown to modify the treatment proposal in approximately onehalf of the patients[20]. The most recent EAU guidelines therefore recommend PET/CT using PSMA together with choline in patients with BCR and low serum PSA levels (<1 ng/mL). The use of androgen deprivation therapy (ADT) may affect interpretation of the PSMA-PET explorations, since the expression of PSMA on the part of prostate cells increases with androgen receptor inhibition and may result in increased sensitivity of PSMA-PET after the administration of ADT. The new imaging techniques, and in particular PSMA-PET scans, may play an important role in the diagnosis of limited metastatic disease. Such techniques should be used, when available, in patients considered candidates for SBRT, in order to better define the extent of the disease and screen patients suitable for MDT[21,22].
SCENARIOS IN OLIGOMETASTATIC PCa
Three scenarios can be found in oligometastatic PCa: (1)De novooligometastatic disease, corresponding to patients diagnosed with synchronous metastases; (2)Oligorecurrent disease, corresponding to the appearance of metachronous metastases after local control of the primary tumor (with either surgery or radiotherapy), in which the metastases are usually detected from images requested after the occurrence of BCR; and (3) Oligoprogressive disease, corresponding to metastatic patients with systemic treatment, who at some point show progression of a limited number of metastases. These patients are subjected to ADT, either alone or combined with other systemic drugs, and therefore may be classified as castration-resistant cases (Figure 1).
Synchronous - de novo metastases
Local ablative treatment of oligometastatic disease with curative intent does not make sense if radical treatment of the primary tumor is not applied at the same time.Radical treatment of this group of patients includes a combination of surgery, cEBRT,SBRT and systemic therapy.
Retrospective studies suggest a survival benefit from prostatectomy or radical cEBRT in patients with metastatic PCa. Clinical trials evaluating MDT, including SBRT, in the metastatic disease setting are ongoing.
Oligorecurrent or metachronic metastases
The current basis for the treatment of metastatic hormone-sensitive PCa is systemic therapy, starting with ADT[23]with or without docetaxel[24], and more recently abiraterone acetate[25]. There is some controversy as to whether these aggressive drug combinations should be used for oligorecurrences, given their greater side effects compared with ADT in monotherapy[13]. Both initial observation with delayed ADT and immediate ADT are even considered as standard of care (SOC).
ADT has important side effects, and it is being evaluated whether SBRT in this group of oligorecurrent patients may serve to delay the start of hormone therapy.Two prospective studies[20,26]have shown SBRT as MDT to be able to prolong ADTfree survival (ADT-FS). However, we do not know whether the prolongation of this period will have an impact on OS.
It is somewhat contradictory that currently the addition of SBRT in oligometastatic PCa allows a delay in ADT while on the other hand research is being made or advocated to intensify therapy with the addition of drugs such as docetaxel and abiraterone to ADT - with the resulting increase in side effects. We need to identify prognostic factors with a view to screening patients who will benefit from such aggressive therapy, and to identify those in whom MDT with SBRT can postpone the introduction of ADT until disease progression. Studies comparing these two regimens and their impact upon OS are needed, since the quality of life repercussions are obvious.
The detractors of delaying the introduction of ADT in oligorecurrent disease point to the persistent difficulty of detecting metastases, and underscore that untreated metastatic disease remains despite the introduction of MDT.
Oligoprogression (oligoprogressive disease)
Oligoprogressive disease includes patients with oligometastatic progression following systemic therapy. Although the latter constitutes the basis of treatment for metastatic cancers, the effect is usually temporary, with the subsequent development of resistant clones and disease progression.
In the event of mCRPC, multiple systemic therapies improve survival. These include the chemotherapeutic agents docetaxel and cabazitaxel, androgen-targeting agents such as abiraterone and enzalutamide, a vaccine (sipuleucelT), and a radiodrug (radium-223)[13]. However, mCRPC remains an incurable disease associated with a life expectancy of 2-3 years.

Figure 1 Scenarios in oligometastatic prostate cancer.
In the event of oligoprogression, SBRT associated to systemic treatment has been shown to delay the start of a second hormonal line. Since each of these agents affords a mean increase in OS of 3-5 mo, it has been postulated that combining SBRT in the event of oligoprogression may prolong patient survival.
MDT
According to the hypothesis of Hellman and Weichselbaum[1], the application of ablation treatments to all tumor sites in oligorecurrent patients could cure oligometastatic disease. Based on cohort studies, the resection or ablation of oligometastatic disease has become standard therapy for other tumors such as colorectal cancer and renal cell carcinoma[27].
Prostate cancer mainly spreads to bone and lymph nodes, with few visceral metastases. The ablative treatment options for these locations are fundamentally surgery, cEBRT and SBRT. Most studies involve a combination of these treatment modalities. As a result, and in order to establish the contribution of local treatment considered globally, these therapies have been grouped under an emergent concept called MDT.
SBRT as MDT
SBRT or stereotactic ablative radiotherapy (SABR) is an external radiotherapy technique that delivers ablative doses [biologically effective dose (BED) > 100 Gy] in a few fractions (1-8 fractions). A high dose is administered with each fraction, and there is a large dose gradient between the tumor and the healthy tissues. This is a high precision technique that requires guided imaging systems and strict immobilization in accordance with the treated site. The terms SBRT and SABR are firmly rooted in clinical nomenclature and are difficult to replace. They prove confusing, however,since they do not truly reflect the technique performed, which actually should be referred to as extreme imaging guided hypofractionation. The technique is performed on an outpatient basis and involves only a few sessions, with no acute toxicity. It is also convenient for the patient and has no impact upon quality of life.
Radiotherapy technique and SBRT schemes as MDT in oligometastatic PCa
There is no consensus on the definition of SBRT volumes in application to bone oligometastases. The largest series published to date[28]included 106 metastases in 81 patients, of which 32% had PCa. The gross tumor volume was defined as the lesion evidenced on CT and/or MRI images. They added a clinical target volume of 5 mm over the surrounding tissue and generated the planning target volume (PTV) with an expansion of 2-5 mm.
Table 1 shows the schemes used in the main series published to date. The schemes are heterogeneous, with a predominance of those using one or three fractions, with a dosing range of 20 Gy/1 fraction to 50 Gy/5 fractions. Ostet al[29]reported significantly higher local control rates with BED > 100 Gy. Muldermanset al[30], in their multivariate analysis, found only the SBRT dose to be significantly correlated to local control (LC). Lesions treated with 16 Gy had a LC rate of 58%, while those receiving ≥18 Gy had LC rate of 95% at two years (P< 0.001). No patient treated with ≥ 18 Gy in a single fraction or with any fractionated scheme experienced local failure.
Muacevicet al[31], in the same way as Sivaet al[26]in the POPSTART study,concluded that a single fraction of 20 Gy over the bone lesion or affected lymph node proves effective and safe.
Given the diversity of schemes, at the consensus meeting of the Spanish Society of Radiation Oncology (SEOR)[32]it was agreed to use either 6 fractions of 7.5 Gy or three fractions of 10 Gy for lymph node SBRT, according to medical criterion and depending on the tolerance of the surrounding structures.
In lymph node oligometastases, the irradiation technique, as well as the treatment volumes used, range from SBRT only at macroscopic disease relapse[20,33-36]to irradiation of the entire chain in which the affected lymph node is located, or even irradiation of all the pelvic chains with boost targeted to the affected lymph nodes[37].The optimum irradiation volume is not clear. Because of the high risk of subclinical disease in pelvic lymph nodes beyond what PET is able to detect[38]and the consequent risk of relapse in the adjacent lymph nodes[34], the recommendations of the Australian and New Zealand Radiation Oncology Genito-Urinary group of 2018 advocated irradiation of all the pelvic lymph nodes at risk[21].
It should be remembered that surgical resection is the SOC for lymph node relapse in patients with a controlled primary tumor, provided the disease is amenable to complete resection, with or without ADT[21].
Response assessment /definition of local control
Most metastases treated with SBRT in PCa are bone metastases. A limiting factor in all SBRT studies involving bone metastases is the lack of a standard and objective method for measuring treatment response or failure. Such evaluation remains a challenge and should be based on a combination of the changes in the images referred to the location of the PTV, the PSA kinetics, and the variations in associated symptoms. Various radiological changes in CT images have been described after SBRT, including remineralization of lytic bone metastases, demineralization of sclerotic bone metastases, progression and response in different lesions. Studies relating the radiological changes to clinical outcomes are not available. Except in situations with measurable tumor spread to soft tissues, the Response Evaluation Criteria In Solid Tumors (RECIST 1.1)[39]do not offer consistent response criteria and are therefore of little value. Functional imaging and the PERCIST (Positron Emission tomography Response Criteria In Solid Tumors) have also been used, defining LC as no increase in uptake (11 choline PET, PSMA) or the absence of lesion growth as determined by MRI. The response in the case of bone metastases is usually investigator-dependent, which makes it difficult to compare the different therapeutic schemes and thus the efficacy of treatment. In the case of vertebral SBRT, such uncertainty has led to the development of a consensus sponsored by the SPINO group[40], where among other conclusions MRI has been classified as the optimum imaging test for assessing response to SBRT in the spine, and response has been defined as the absence of progression.
Most of the published series included LC among their endpoints. The concept of LC comprised lesions classified as stable disease, and partial response or complete response,i.e., the absence of progression. Consistent response criteria need to be developed to compare the results and evaluate the efficacy of new treatment approaches such as SBRT, similar to those established by the Neuro-Oncology group(SPINO) for the evaluation of response in spinal metastases[40].
SBRT RESULTS
A summary of different published studies is presented in table format (Tables 1 and 2). There is a predominance of retrospective studies that analyze SBRT jointly in bone and lymph node metastases. Few series analyze the two types of metastases separately. The primary endpoints assessed are LC, toxicity, the imaging method used for diagnosis, ADT-FS, progression-free survival (PFS), and OS on a point basis.
Local control
Local control is commonly defined as the absence of progression in PTV based onserial images. Tables 1 and 2 show the reported LC rates to range from 82%-100% at two years. This is consistent with a systematic review of SBRT in the treatment of oligometastatic PCa, which reported LC rates of > 90% and isolated cases of severe toxicity[41]. These high LC rates have been described for both bone and lymph node metastases. A relevant proportion of patients (25%-38%)[26,34]progress and remain amenable to ablative SBRT. However, after lymph node SBRT, new lymph node relapses frequently occur outside the treated field, accounting for 67% of all relapse cases over a median follow-up of two years[34]. SBRT may be used in selected patients,though they should be informed of the high risk of recurrence, which may prove more difficult to treat through re-irradiation with curative intent.

Table 1 Summary of selected publications reporting stereotactic body radiation therapy for mixed and bones oligometastatic prostate cancer
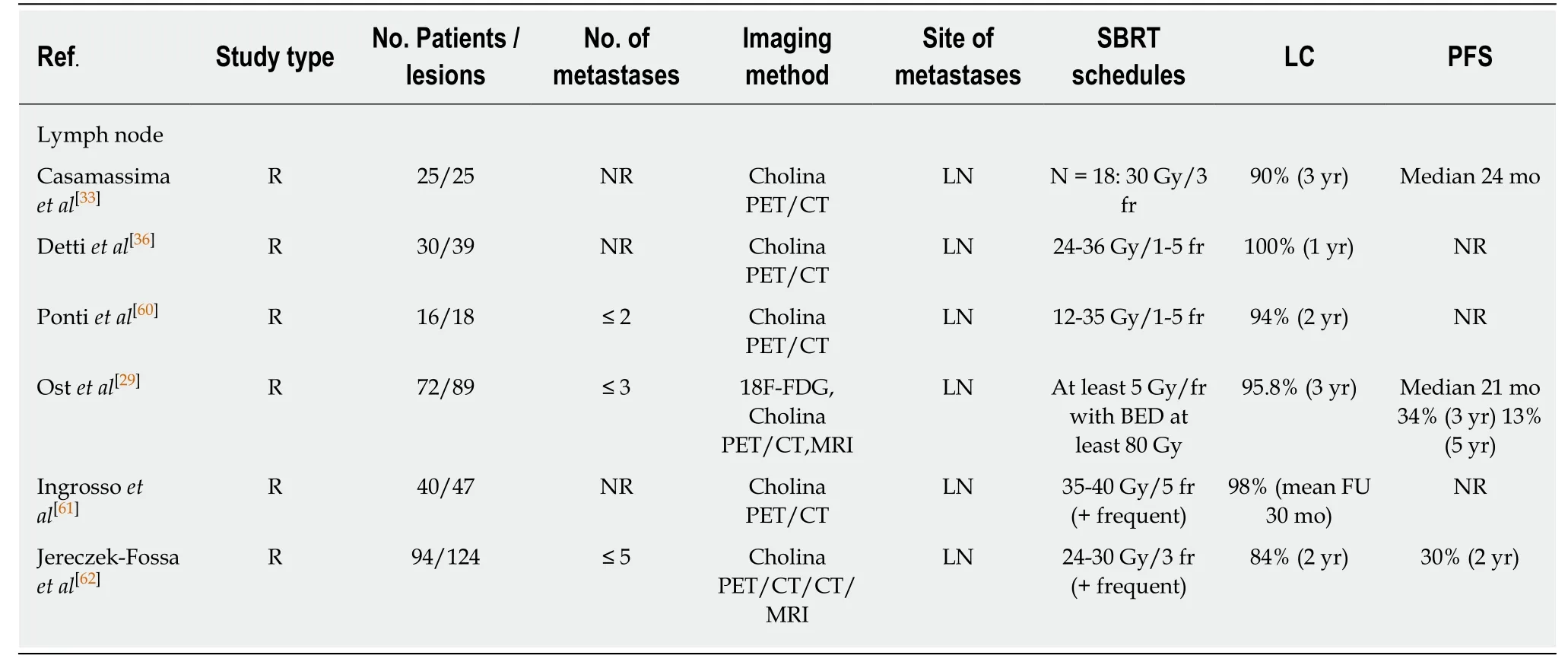
Table 2 Summary of selected publications reporting stereotactic body radiation therapy for lymph nodes oligometastatic prostate cancer
Toxicity
All the published series found toxicity to be low (Tables 1 and 2). The most relevant problem after SBRT of bone metastases is fracture - this being the cause underlying the only reported case of grade 3 toxicity[26]. Low-grade toxicity was generally limited to gastrointestinal effects such as nausea, and was consistently observed in < 20% of the treated patients. The largest published series on SBRT applied to non-spinal bone metastases documented a fracture rate of approximately 8.5%, and concluded that SBRT is safe, since the risk of pathological fracture after cEBRT was estimated to be approximately 4%-5%[42].
The Spinal Instability Neoplastic Score (SINS)[43]predicts fracture risk and should be assessed prior to vertebral SBRT in all cases. The criteria for assessing SINS include the level of the metastasis targeted for irradiation, the type of pain, spinal malalignment, the presence of baseline vertebral compression fractures, the type of lesion, and whether the tumor involves the posterior wall. The SINS classifies patients as stable (SINS 0-6), potentially unstable (SINS 7-12), or unstable (SINS 13-18). In the case of SINS ≥ 7, the risk of fracture is increased and vertebroplasty or surgical stabilization prior to SBRT is recommended[44].
ADT-FS
Five studies in oligorecurrent patients[34,45-48]analyzed ADT-FS as primary endpoint -this parameter being defined as the time interval between the first day of SBRT and the start of ADT. The reported range in median ADT-FS was 15.6-39.7 mo. The studies included second and subsequent cycles of SBRT, delaying palliative treatment with ADT and its side effects[34,45]. After a median follow-up of three years, the STOMP study[20], as the first prospective, randomized trial of MDT and delayed ADT in oligometastatic PCa versus observation, found the median ADT-FS to be 13 mo for the observation group and 21 mo for the MDT group. In the POPSTART study[26]the ADT-FS rate at two years was 48%.
PFS
The PFS rates at 1-2 years are shown in Table 1, and range from 40%-72% at one year to 35%-45% at two years. The median PFS values range from 7.3-31.6 mo. The use of hormone treatment is also highly variable. In contrast to cEBRT, the contribution of ADT used in combination with SBRT is not known, though it may improve tumor control by exerting a synergistic effect.
The largest reported series[29], a multi-institutional study pool, used PFS (defined as the absence of new metastatic lesions) as the primary endpoint, with rates of 31% and 15% at 3 and 5 years, respectively, and with a median PFS of 21 mo.
Oligoprogressive patients:Special mention must be made of the assessment of SBRT in oligoprogressive patients. Five series[30,47,49,50]include oligorecurrent and oligoprogressive, castration-resistant patients. Stereotactic body radiation therapy appears to be useful also in this group of patients with a poorer prognosis. In the POPSTAR trial[26]the PFS rate at one and two years was 58% and 39%, respectively. In the prospective study by Ahmedet al[49], 6 of the 11 castration-resistant patients achieved undetectable or decreasing PSA levels with a median follow-up of 4.8 mo.
The third study is a multicenter trial[47]describing 41 oligoprogressive patients (70 lesions) - this being the largest number of patients reported to date. With a median follow-up of 23.4 mo, the PFS rate at two years was 22% in this subgroup versus 43%in oligorecurrent patients of the same series.
In the Spanish phase II of the GICOR group[50], all patients had at least two years of ADT prior to SBRT, and 12 cases of oligoprogression were included, of which 66%with a median follow-up of 9.8 mo remained progression-free without the need for a new line of systemic treatment (hormonal or chemotherapy).
ONGOING STUDIES
Different ongoing studies assess the benefit of treatment of the primary tumor in the setting of oligometastatic disease, associated to SBRT of all the metastatic sites.However, we here focus on ongoing trials in oligorecurrent (hormone-sensitive) and oligoprogressive patients (castration-resistant). A summary of these trials is provided in Table 3. All of them are phase II trials, including mostly 1-5 bone and/or lymph node lesions, and their primary endpoints are fundamentally time to disease progression.
Among these studies, mention should be made of the different randomized trials.The ORIOLE study (NCT02680587)[51]is the first randomized study to evaluate the efficacy of SBRT as measured by the quantification of circulating tumor cells in hormone-sensitive oligometastatic PCa. Its preliminary findings have been presented at the ESTRO 2018 Congress[52]. The ongoing CORE study compares the best available best SOC with or without SBRT (NCT02759783)[53], although it also includes patients with breast cancer and non-small cell lung cancer. The PEACE V study (STORM,NCT03569241)[54]randomizes patients to MDT (lymphadenectomy or SBRT) versus MDT plus pelvic radiotherapy (45 Gy in 25 fractions). It will attempt to establish the standard treatment in lymph node oligorecurrent PCa. Lastly, the PCS IX(NCT02685397)[55]will analyze the role of enzalutamide associated to SBRT.
CONCLUSION
SBRT is safe and effective. It has been able to offer excellent LC rates, with minimal toxicity. It has also been shown to slow disease progression and therefore to delay the introduction of ADT and its associated side effects. The impact of these results upon OS in oligometastatic patients is not known. It is obvious that we need phase III trials to answer these questions, though on the basis of the ongoing trials such answers are not to be expected for several years.
However, due to the LC and symptoms control achieved, the convenience of administration, the delaying of side effects of ADT or the delaying of second systemic therapy lines, SBRT has become increasingly widely used in radiation oncology units and should be offered to well-informed patients who request such treatment.Knowing this situation, the European Organisation for the Research and Treatment of Cancer and the European Society for Radiotherapy and Oncology have launched the OligoCare Project, a broad registry of standard practice that will provide information on the contribution of SBRT to oligorecurrence and oligoprogression in PCa, among other tumor sites. In the absence of strong evidence, treatment should be personalized, established by agreement with well-informed patients, and the patient circumstances and preferences should be taken into account.

Table 3 Summary of clinical trials investigating treatment with stereotactic body radiation therapy in oligometastatic prostate cancer
杂志排行
World Journal of Clinical Oncology的其它文章
- Rational-emotive behavioral intervention helped patients with cancer and their caregivers to manage psychological distress and anxiety symptoms
- Hong Kong female’s breast cancer awareness measure: Crosssectional survey
- Impact of conditioning regimen on peripheral blood hematopoietic cell transplant
- Retrospective evaluation of FOLFlRl3 alone or in combination with bevacizumab or aflibercept in metastatic colorectal cancer
- Pancreatic cancer screening in patients with presumed branch-duct intraductal papillary mucinous neoplasms
- Existing anti-angiogenic therapeutic strategies for patients with metastatic colorectal cancer progressing following first-line bevacizumab-based therapy
