Efficacy and safety of Mamajjaka Ghanavati in the treatment of type 2 diabetes mellitus:a prospective open label multi-center clinical study
2019-03-30MalalurNageshShubhashreeBikartanDasGopalChandraNandaGaddamKumaraSwamySaradaOtaShashidharDoddamaniMedaMruthyumjayaRaoVimalTewariBaghwanSahaiSharmaShrutiKhanduriRakeshRanaRichaSinghalAdarshKumarNarayanamSrikanth
Malalur Nagesh Shubhashree,Bikartan Das,Gopal Chandra Nanda,Gaddam Kumara Swamy,Sarada Ota ,Shashidhar Doddamani,Meda Mruthyumjaya Rao,Vimal Tewari,Baghwan Sahai SharmaShruti KhanduriRakesh RanaRicha SinghalAdarsh KumarNarayanam Srikanth
1 Technical Department,Regional Ayurveda Research Institute for Metabolic Disorders (RARIMD),Bengaluru,Karnataka,India
2 Technical Department,Central Ayurveda Research Institute for Hepatobiliary Disorders (CARIHD),Bhubneshwar,India
3 Technical Department,Dr.AchantaLakshmipati Research Centre for Ayurveda (DALRCA),Chennai,India
4 Technical Department,Central Ayurveda Research Institute for Neuromuscular &Muscular-Skeletal Disorders (CARINMSD),Cheruthuruthy,Kerala,India
5 Technical Department,Central Council for Research in Ayurvedic Sciences,New Delhi,India
6 Technical Department,Central Ayurveda Research Institute for Drug Development (CARIDD),Kolkata,West Bengal,India
7 Technical Department,Regional Ayurveda Research Institute for Infectious Diseases(RARIID),Patna,Bihar,India
Abstract
Key words:Mamajjaka Ghanavati;type 2 diabetes mellitus;Madhumeha;quality of life;blood glucose;efficacy;safety;single-arm clinical trial
INTRODUCTION
Diabetes mellitus (DM) has been vividly described in the context of Madhumeha in classical Ayurveda with striking resemblance of its Ayurvedic concepts with latest knowledge on diabetes mellitus.1Diabetes is a group of metabolic diseases characterized by hyperglycemia resulting from defects in insulin secretion,insulin action,or both.The chronic hyperglycemia of diabetes is associated with long-term damage,dysfunction,and failure of different organs,especially the eyes,kidneys,nerves,heart,and blood vessels.DM has become one of the largest global health emergencies during the 21stcentury and leads to life-threatening health problems.2According to International Diabetes Federation estimates,around 415 million people had DM in 2015 and this number is estimated to rise to 642 million by 2040.3India is home to 69.1 million people with DM and is estimated to have the second highest number of cases of DM in the world after China in 2015.Indians are also believed to have a greater degree of insulin resistance and a stronger genetic predisposition to diabetes.4As evident by the trends diabetes would be the seventh-leading cause of mortality by the year 2030.World health organization has emphasized to control Diabetes through its current theme “Beat Diabetes”.5
Ayurveda considers diabetes as Kaphaja disease in which Medas (adipose factor) and Ojas (Biostrength/immunity) have been described as the main factors which are responsible for the disease diasthesis.6Mamajjaka (Enicostemma littorale auct.non Bl) is a familiar herb used in Madhumeha particularly in the state of Gujarat,Madhya Pradesh and Rajasthan which is said to possess anti-diabetic properties7&antioxidant property.8It contains the Mamajjaka extract (8 parts),Mamajjaka churna (2 parts),Katuki (Picrorrhiza kurroa;2 parts),Pippali (Piper longum;2 parts),and Ativisha (Aconitum heterophyllum;2 parts).9Mamajjaka was described in Lakshmanadi Varga of Shodhala Nighantu.10It is therapeutically indicated in Madhumeha (diabetes) and Medoroga (obesity).It has Tikta rasa (bitter taste),Laghu guna,Ushna veerya (hot in potency),Katu Vipak and mentioned as Kaphapittashamak (subsided all the three humors) and it is effective for Prameha (urinary disorders),Madhumeha (diabetes),and other disorders.11With this perspective,the present study was performed to clinically evaluate efficacy and satefy of Mamajjaka ghanavati in the treatment of Madhumeha (type 2 DM;T2DM).
The primary objective of this study is to assess the clinical efficacy of Mamajjaka Ghanavati in the treatment of T2DM.The secondary objectives are to assess the clinical safety and changes in the quality of life (QOL) in patients with T2DM.
SUBJECTS AND METHODS
Study design and setting
This prospective,open-label,multi-centre,single-arm study was conducted at three peripheral institutes of Central Council for Research in Ayurvedic Sciences,that is Regional Ayurveda Research Institute for Metabolic Disorders (RARIMD),Bengaluru,Central Ayurvedic Research Institute for Hepatobiliary Disorders,Bhubaneswar and Regional Ayurvedic Research Institute for Eye Diseases,Lucknow under the Intra Mural Clinical research (IMR) program.The trial drug was administered for 84 days among 177 patients with clinically diagnosed T2DM.Written consents of these participants were obtained before their enrolment (Additional file 1).The trial drug was prepared and standardized as per Standard Operative Procedures described in the Ayurvedic Pharmacopoeia of India.12The study protocol was approved by the Institutional Ethics Committee of each participating institute (approval number:CTRI Reg No Ref/2016/06/011468) on March 14,2016 and performed in accordance with theDeclaration of Helsinki.The clinical trial was registered in the Clinical Trial Registry of India (CTRI/2017/09/009887) on September 22,2017.
Eligibility criteria
Inclusion criteria
Subjects presenting with all of the following conditions were considered for inclusion in this study:suffering from T2DM,either sex,age between 35 and 65 years,fasting blood sugar (FBS) level between 6.99-11.1 mM,postprandial blood sugar (PPBS) level 11.1-13.75 mM,13,14glycated hemoglobin (HbA1c) 4.8-7.5%,15willing to participate in this study for 12 weeks.
Exclusion criteria
Subjects presenting with any of the following were excluded from this study:those who used insulin for glucose control or had type 1 DM or those who concurrently used Metformin to control blood glucose levels;those suffering from the complications of DM,including diabetic neuropathy,diabetic nephropathy,diabetic retinopathy;those who had a past history of atrial fibrillation,acute coronary syndrome,myocardial infarction,stroke or severe arrhythmia in the last 6 months as well as hypertension (>140/90 mmHg) or clinical evidence of heart failure.Further,subjects with concurrent serious hepatic dysfunction (defined as aspartate transaminase and/or alanine transaminase>3 times of the upper normal limit) or renal dysfunction (defined as serum creatinine>106.8 μM),uncontrolled pulmonary dysfunction (asthmatic and chronic obstructive pulmonary disease patients) or other concurrent severe disease,alcoholics and/or drug abusers as well as subjects on steroids,oral contraceptive pills or estrogen replacement therapy were also excluded from the study.Subjects who had completed participation in any other clinical trial during the past 6 months,pregnant/lactating women as well as evidence of malignancy,those suffering from major systemic illness necessitating long term drug treatment (rheumatoid arthritis,psycho-neuro-endocrinal disorders) and those with a history of sensitivity to any of the trial drugs or their ingredients or any other condition which may jeopardize the study were excluded.
Withdrawal criteria
All the subjects were free to withdraw from the study at any time on their own.The effort was made to collect the data of such subjects by making phone calls.The investigator withdrew the subjects from the study if the FBS level rose to>13.75 mM or postprandial/random blood glucose level increased to>16.5 mM and was controllable within 1 month.Or if any serious complication developed which required urgent treatment with any other drug/therapy (not related to diabetes mellitus).
Interventions
Mamajjaka Ghanavati was procured from Pharmacy of Gujarat Ayurveda University,Jamnagar,Gujarat,India.It was administered orally in a dose of one gram (two tablets of 500 mg each) twice daily after food with water for 84 days.The drug was prepared according to the Standard Objective Procedures for the preparation of Ghanavati and standards mentioned in the Ayurvedic Pharmacopoeia of India (API).16The Kwath churna (Mesh no.44) of dried Mammajak was taken in vessel and soaked overnight in potable water.On the next day,the content was heated to maintain temperature around 100°C without covering the mouth of the container and stirring the content continuously.The content was filtered through a piece of clean muslin cloth to reduce to ¼thpart (Mamajjak kwath).This kwath was again heated to 90-95°C with continuous stirring till the attainment of semisolid consistency (Mamajjak Ghana).The safety parameters,i.e.,content of heavy metals,aflotoxin and microbial load were within the permissible limit as per the guidelines of API.
Laboratory investigations
Laboratory investigations [such as FBS,PPBS,HbA1c,hemoglobin (Hb) %,total leucocyte count,and differential leucocyte count,erythrocyte sedimentation rate,blood urea (serum electrolytes-sodium,potassium,and chloride),serum uric acid,serum creatinine],liver function tests (aspartate transaminase,alanine transaminase,total protein,serum albumin,and serum globulin),lipid profile,and urine tests for glucose,protein,white blood cells,red blood cells were done at the commencement (baseline) and the end of the study.However,electrocardiogram and micro albuminuria measurement were done initially to rule out cardiac diseases and nephropathy.
Outcome measures
The primary outcome measure was the change in HbA1c on the 84thday relative to baseline data.The secondary outcome measures included change in blood sugar (fasting and postprandial) and change in symptoms assessed by Diabetes Symptoms Questionnaire (DSQ) at the end of the 28th,56th,and 84thdays relative to baseline data.
The ayurvedic parameters as described in classical ayurveda,i.e.,prabhootamutrata-excessive urination,avilamootrata (urine turbidity),madhvivamehati-passes urine similar to Madhu,Madhuryachatanoratah-patient's body starts yielding sweet smell and taste,Malina danta (tartar in teeth),Hasta pada daha (burning sensation of hands and feet),Deha chikkanata (excessively glossy/oily skin),Trishna (excessive thirst),Madhurya masya (feeling sweetness in mouth),Prabhuta mutrata (excessive urination),Avila mutrata (turbid urination),Madhu samana varna of mutra (urine presenting color of honey),Sweda (excessive perspiration),Anga gandha (bad body odour) Shithilangata (flaccidity of muscles),Shayana asana Swapna sukha (desire for sedentary life),Shitapriyatwa (desire for cold food &environment),Gala talu shosha (dryness of palate &throat)17were also assessed at baseline and at the 84thday of the treatment.Clinical safety of Mamajjaka Ghanavati and change in QOL were assessed by liver and renal function tests and using 36-Item Short Form Health Survey (SF-36),respectively on the 84thday relative to baseline.
Follow-up
Subjects were followed up on the 14th,28th,42nd,56th,70thand 84thdays for clinical assessment.Drug compliance was assessed in a special form which was issued to each subject for filling up to ensure the consumption of medicines.Subjects were also instructed to return the empty containers and sachets.The details of study schedule are presented in Figure 1.
Statistical analysis
All data were analyzed using Statistical Package for Social Sciences (SPSS) 15.0 (SPSS,Chicago,IL,USA).Comparison of clinical symptoms and laboratory parameters were subjected to repeated measures analysis of varince to elicit the withinsubjects and between-subjects effects at baseline and different time points of follow-up.Paired samplet-test was used to compare mean changes in the hematological and biochemical parameters,and DSQ.The percentage of relief was calculated by applying the formula (BT-AT) × 100/AT (BT:Before test;AT:after test).
RESULTS
Total 180 subjects were included in this study (n= 60 in each center),among them 162 subjects completed the trial and data of 15 subjects imputed as they had taken one month course of treatment.The last observation of these subjects were carry forward till the end of study.Finally,data of 177 subjects were analyzed and the details are shown in Figure 2.
Demographic profile
The detailed demographic profile of 177 subjects is presented in Table 1.
It is observed that 74% of patients had no addictions,76.3% of patients had normal sleep,87.6% had regular bowel habits,39.1% of patients were having Pitta Kapha prakriti followed by 26.3% with Vata-Pittaja Prakriti.
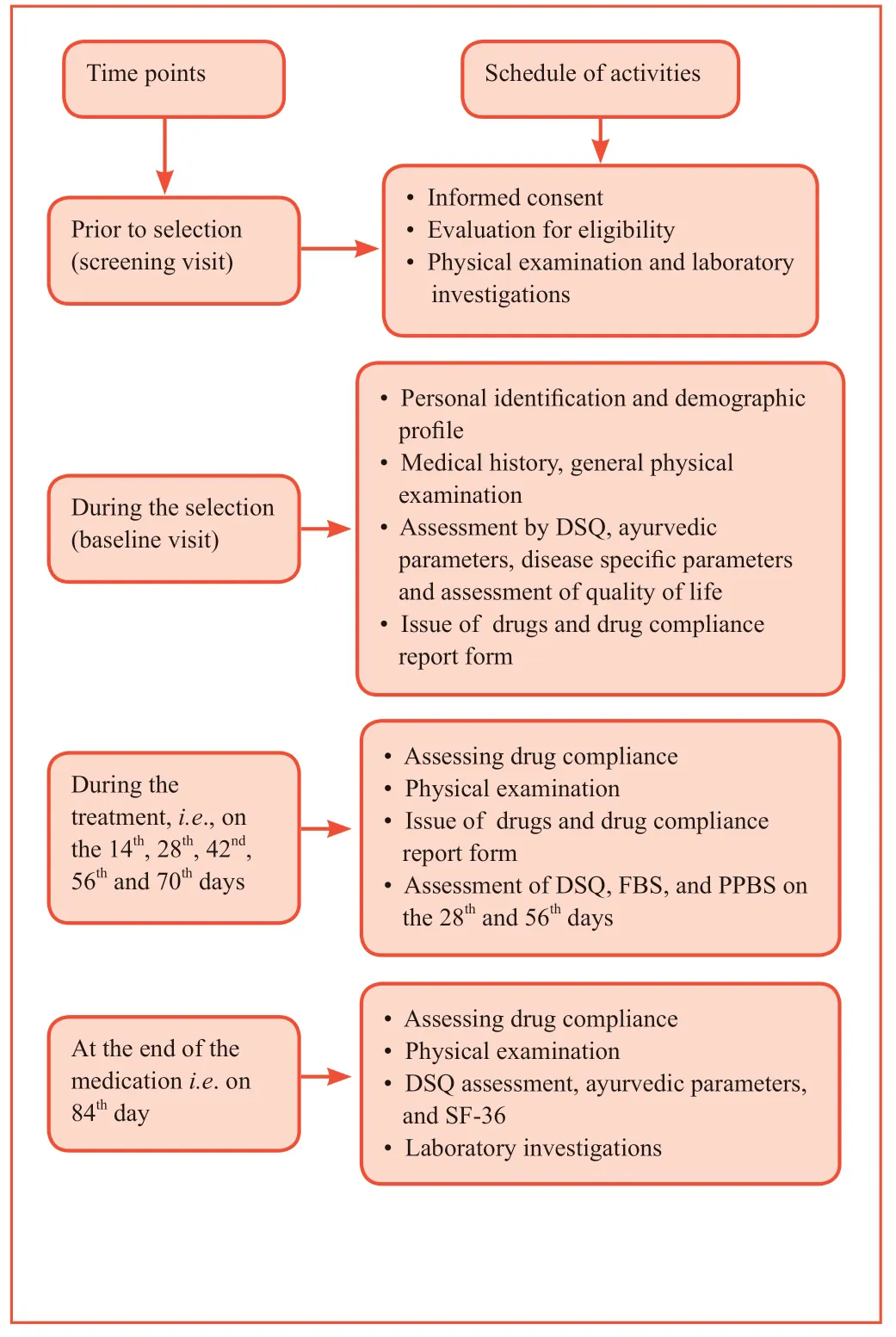
Figure 1:Study schedule.
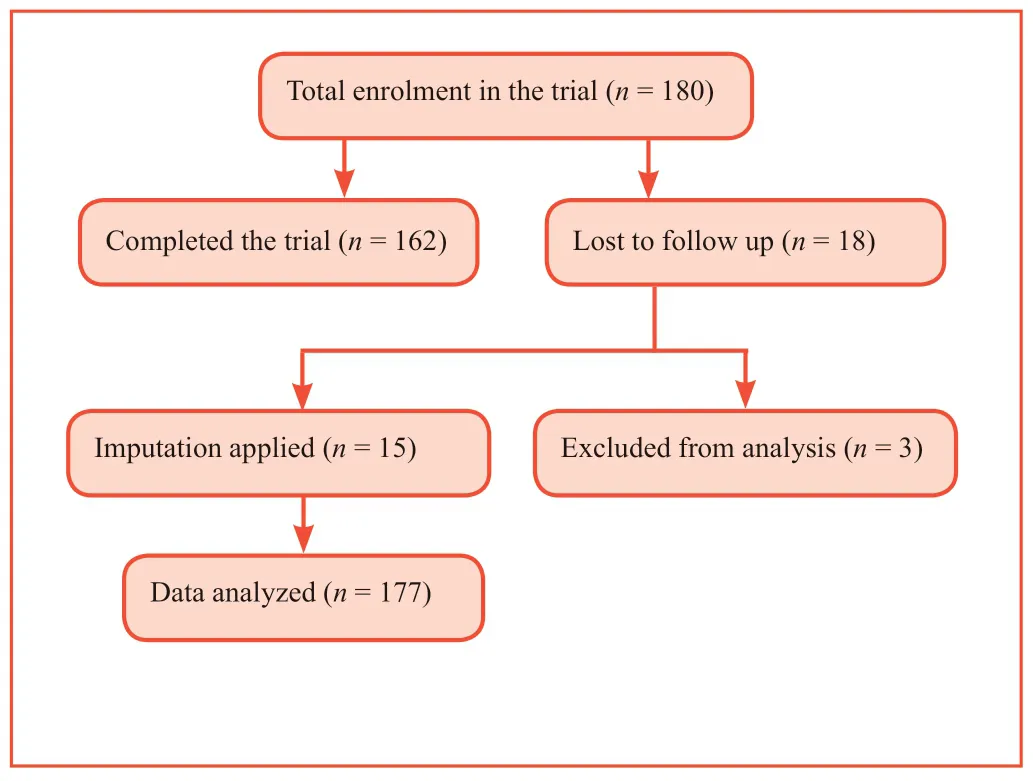
Figure 2:Quantitative analysis of study participants.
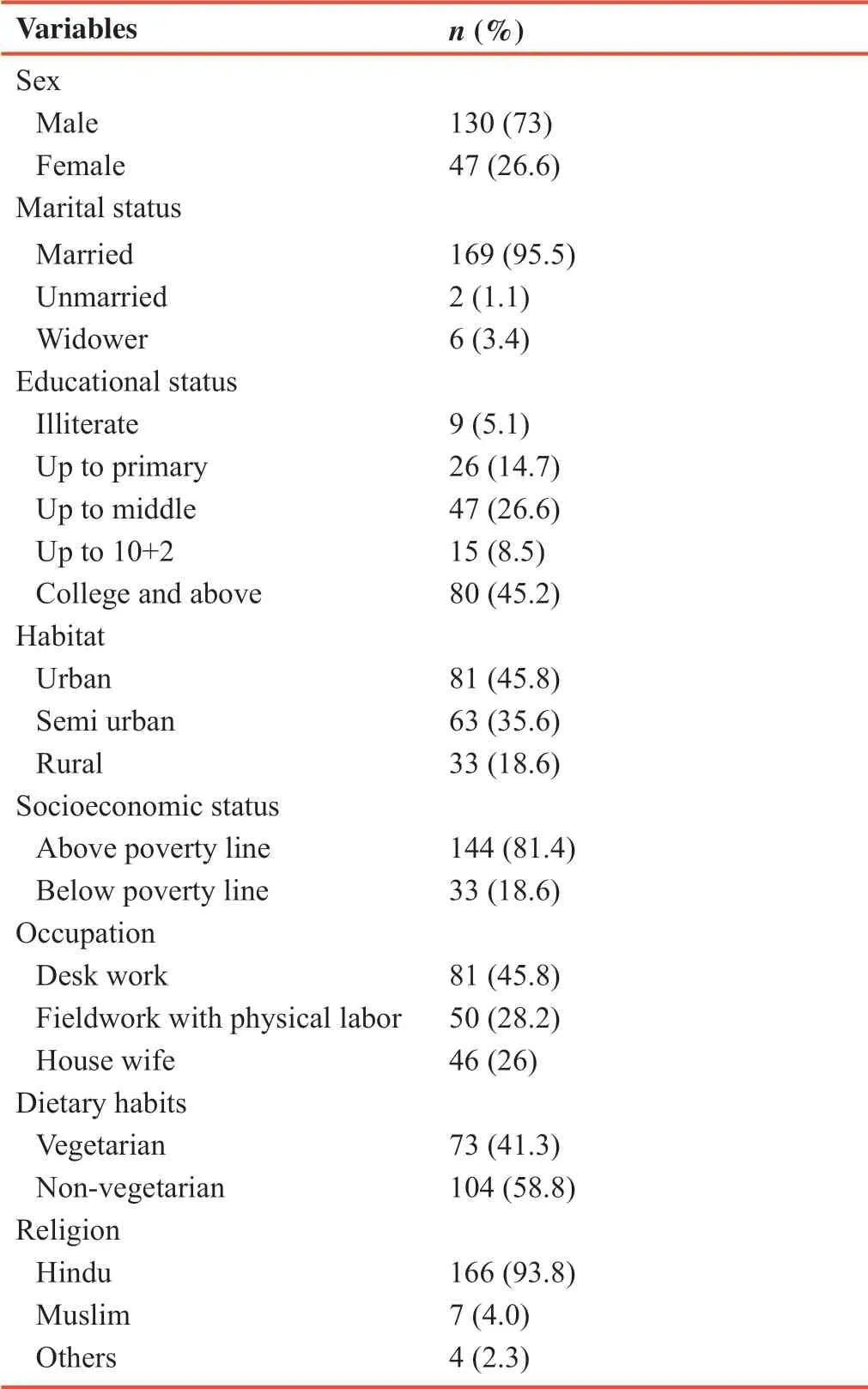
Table 1:Demographic and baseline characteristics of 177 included participants
Effect of Mamajjaka Ghanavati on signs &symptoms mentioned in classical ayurveda
Effect of Mamajjaka Ghanavati on the clinical signs and symptoms specifically mentioned for Madhumeha in classical ayurveda was assessed at baseline and at the end of the treatment.The effect of the drug on each parameter is shown in Figure 3.
Mamajjaka Ghanavati had significant effects on each parameter at the end of the treatment relative to the baseline,i.e.,70% of subjects got relief from the symptom Madhuryamasya (sweetness in the mouth),78% of subjects got relief from Hastapadadaha (burning sensation in the palm and feet) and 75% of subjects from Avila-mutrata (morbidity in the urine).Further,more than 60-65% of subjects got relief from Dehachikkanata (developed oiliness in the skin) and Trishna (excessive thirst).
Effect of Mamajjaka Ghanavati on chief complaints
At baseline,94 (53%) subjects complained of polyurea,80 (45%) subjects complained of polyphagia,127 (71.8%) and 110 (62%) subjects complained of exhaustion/tiredness and body ache respectively.Other complaints like giddiness,visual disturbances,and polyneuritis were also present in some of the subjects.The effect of Mamajjaka Ghanavati after three months of treatment is shown in Figure 4.
The result shows that 99.9% of subjects got relief from the symptom polydipsia followed by 86.3% of subjects from giddiness,82.5% of subjects from polyphagia,81% of subjects from exhaustion/tiredness,79% of subjects from body ache and 78.5% of subjects from the symptom visual disturbances.
Effect of Mamajjaka Ghanavati on outcome measures
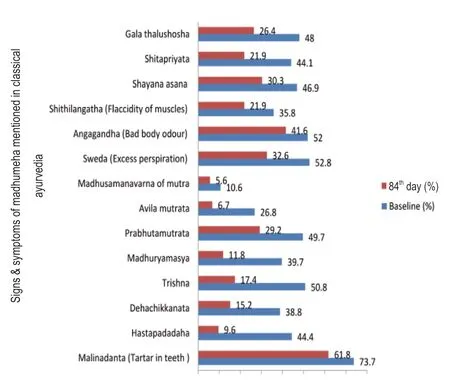
Figure 3:Effect of Mamajjaka Ghanavati on signs &symptoms mentioned in classical ayurvedic at baseline and after treatment (84th day).
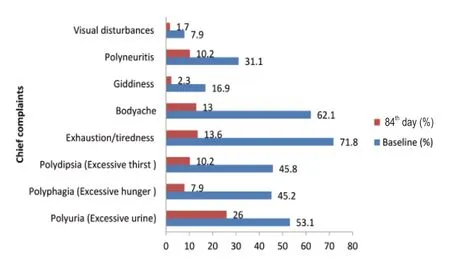
Figure 4:Effect of Mamajjaka Ghanavati on chief complaints of the subjects at baseline and after treatment (84th day).
Table 2 shows that DSQ results showed significant changes on the 84thday relative to baseline (P< 0.001).However,though there were no changes in other parameters like HbA1c,FBS and PPBS but these were static during the study period.There were no abnormal changes in the hematological parameters during the study.No significant changes in liver and renal function tests at the 84thday relative to baseline (P>0.05).There were no abnormal changes in safety parameters,i.e.,liver and kidney functions.
Figure 5 shows the effect of Mamajjaka Ghanavati on the QOL of the subjects,which was assessed by SF-36 consisting of eight domains.There were significant changes in the score of domains like energy/fatigue,pain emotional well-being,social functioning and general health (P<0.001).
DISCUSSION
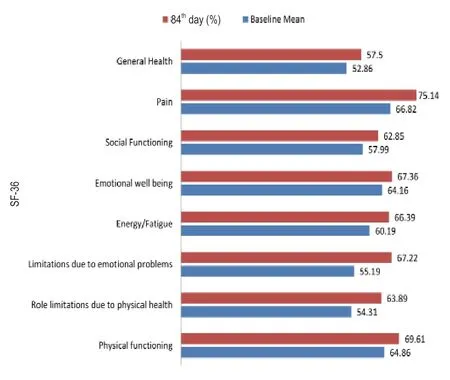
Figure 5:Effect of Mamajjaka Ghanavati on various domains of 36-ltem Short Form Health Survey (SF-36) at baseline and after treatment (84th day).
The present study on the role of Mamajjaka Ghanavati in the management of DM shows that this drug is effective in reducing the symptoms like Hastapadadaha (burning sensation in hands and feet) Prabhutamutrata &Avila-Mutrata (increased frequency/quantity of the urine and morbidity of urine),Trishna (feeling of thirst),Galatalushosha (dryness in oropharyngeal region),Sweda (excessive sweating) as observed in Figure 3.The disease specific ayurvedic parameters of Madhumeha mentioned above show the observational supremacy of Ayurveda as these symptoms predict the disease in its early stage and help to check its progression and complications.1As evident from Figure 4,there has been a marked improvement in the subjective parameters like Polyuria,Polyphagia,Polydipsia,Exhaustion,Bodyache,Giddiness,polyneuritis,and visual disturbances.Clinically,these findings are very relevant because diabetes management has largely focused on control of hyperglycemia,while the rising burden of this disease is mainly correlated to its vascular complications.18Reduction in the signs and symptoms implies that the disease is not progressing towards complications.
The mere presence of diabetes deteriorates a person's QOL.QOL is an important health outcome in its own right,representing the ultimate goal of all health interventions.19In patients with diabetes,one of the most important methods for evaluating the efficacy of treatment regimen and care is to assess their quality of life.20
In this study,subject's QOL was assessed by SF-36.SF-36 consists of 36 questions (items) measuring physical and mental health status in eight health domains.Among the eight health domains,the domains like pain,energy/fatigue,etcshowed significant changes after 84 days treatment with trial drug relative to baseline.

Table 2:Effect of Mamajjaka Ghanavati on outcome measures
Changes in the domain “social functioning”,“Pain” represent health status as absence of disability whereas the significant changes in the domain “General health and energy/fatigue” implies positive health state.The positive changes in the QOL suggest a new approach toward diabetes management.Probably,the trial drug has helped in maintaining the normal state of Agni and Ojas,i.e.,stabilizing metabolism and strengthening the immunity of diabetic patients.
On the other hand,diabetes has been known as an oxidative stress disorder caused by imbalance between free radical formation and the ability of the body's natural antioxidants.21There is considerable evidence that induction of oxidative stress is a key process in the onset of diabetic complications.There is evidence that antioxidants exhibit protective effects and they may be helpful in treating diabetes and its complications.22The antioxidant effect of Mamajjaka,23Pippali,24and Katuki25might have brought about the significant changes in energy/fatigue and SF-36.The combined antioxidant effect of Mamajjaka Ghanavati might have contributed to the improvement in QOL and reduction of symptoms.
In spite of replacement of habituated hypoglycemic drug like Metformin by trial drug,HbA1c value remains at 6.2% before and after the trial.As presented in Table 2,no much change is observed in FBS,PPBS from baseline to post-treatment and to post-trial,which implies that mild hypoglycemic action has been exerted,though it has not shown any marked changes.In contrast to these reports,the potent anti-diabetic properties of Mamajjaka and Mamajjaka Ghanavati lowering FBS and PPBS by Mamajjaka Ghanavati has been reported by many scientists.26-28
Experimental studies have also revealed the dose-dependent hypoglycemic effect of aqueous extract of Mamajjaka as well as the glucose-lowering effect of the aqueous extract of Mamajjaka.29,30Hence,it is recommended to carry out the study in a larger number of patients with minor alterations in the inclusion criteria (HbA1c 4.8-5.8%) so that mild hypoglycemic action can be elicited.
Limitation of the study
As this is an open study without any masking,a controlled study in comparison with standard drug may be undertaken with longer duration of treatment (6 months) and with a bigger sample size.
Conclusion
This clinical study was undertaken to evaluate the effect of Mamajjaka Ghanavati on T2DM.The above formulation has also brought in significant amelioration of chief complaints and improved QOL on SF-36 scale.In addition,there were no significant changes in hepatic and renal parameters.This may be safely used for the treatment of DM.
Additional files
Additional file 1:Model consent form.
Acknowledgments
The authors are highly indebted to the Director General,CCRAS,New Delhi for in time support.The authors are also thankful to the study participants.Thanks also conveyed to Dr.Aarti Sheetal,Senior Research Fellow (Ayurveda) for her technical assistance.
Author contributions
Concept and design of the study,interpretation of data,drafting and revision of the article:MNS,SO;data acquisition:MNS,BD,GCN,GKS,SD,MMR,VT;analysis of data:RR,RS;revising the article critically for important intellectual content:BSS,SK;approval of final article for publication:AK,NS.
Conflicts of interest
None declared.
Financial support
This work was supported by a grant from Central Council for Research in Ayurvedic Sciences,Ministry of AYUSH,New Delhi.The funding body played no role in the study design,in the collection,analysis and interpretation of data,in the writing of the article,and in the decision to submit the article for publication.
Institutional review board statement
This study was approved by the Medical Ethics Committee of Central Council for Research in Ayurvedic Sciences,New Delhi (approval number:CTRI Reg No Ref/2016/06/011468) on March 14,2016 and registered in the Clinical Trial Registry of India (CTRI/2017/09/009887) on September 22,2017.The study was performed following the guidelines of WHO_Good Clinical Practices (GCP) and GCP of India.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms.In the forms,the patients have given their consent for their clinical information to be reported in the journal.The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity.
Reporting statement
This study follows the Recommendations for the Conduct,Reporting,Editing and Publication of Scholarly Work in Medical Journals developed by the International Committee of Medical Journal Editors.
Biostatistics statement
The statistical methods of this study were reviewed by the biostatistician of Central Council of Research in Ayurvedic Sciences,Ministry of AYUSH,India.
Copyright license agreement
The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement
The data of individual subject will not be provided.However,the study protocol and report will be made available to the researchers beginning 9 months and ending 36 months following article publication.To access the same the researcher has to request Director General,CCRAS through e-mail dg-ccras@nic.in.
Plagiarism check
Checked twice by iThenticate.
Peer review
Externally peer reviewed.
Open access statement
This is an open access journal,and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
杂志排行
Clinical Trials in Degenerative Diseases的其它文章
- Effects of flash glucose-sensing technology-based continuous glucose monitoring on compliance of patients with type 2 diabetes mellitus:study protocol for a randomized controlled trial
- Effects of exergames on motor coordination and balance in convalescent drug addicts:a feasibility study
- Effect of beraprost sodium on pulmonary hypertension due to left ventricular systolic dysfunction:protocol for a randomized controlled clinical trial
- Information for Authors - Clinical Trials in Orthopedic Disorders
