Effects of flash glucose-sensing technology-based continuous glucose monitoring on compliance of patients with type 2 diabetes mellitus:study protocol for a randomized controlled trial
2019-03-30JingChenNaLuoLiWangZhenWenZhangYanLiu
Jing Chen,Na Luo,Li Wang,Zhen-Wen Zhang,Yan Liu
Department of Endocrinology,Northern Jiangsu People's Hospital,Yangzhou University,Yangzhou,Jiangsu Province,China
Abstract
Key words:type 2 diabetes mellitus;multiple daily insulin injections;blood glucose monitoring;flash glucose-sensing technology;selfmonitoring behavior;self-management;compliance;randomized controlled trial
INTRODUCTION
Importance of intensive insulin treatment for patients with diabetes mellitus
Chronic complications of diabetes mellitus such as blindness,renal failure,stroke,myocardial infarction,amputation,and other serious consequences,have become a heavy medical burden and social problem in China.1However,there is evidence that 36.1% of diabetes patients aware this disease,33.4% of diabetes patients receive treatment,and 30.6% of diabetes are controlled.2-4Type 2 diabetes is one kind of progressive diseases.5,6The continuous decline in islet beta-cell function leads to the gradual deterioration of glycemic control in type 2 diabetes patients.7Positive intervention measures such as intensive insulin therapy can improve the function of islet beta cells which can delay the progression of hyperglycemia.Multicenter randomized controlled trials showed that intensive insulin therapy (multiple daily injections and insulin pump therapy) was superior to oral hypoglycemic agents in improving islet beta-cell function in patients with newly diagnosed diabetes mellitus and a large number of patients receiving intensive insulin therapy have more than 1 year of glycemic remission.8For patients with non-newly diagnosed type 2 diabetes mellitus,short term intensive insulin therapy should also be given when the disease progresses and blood glucose is poorly controlled.Among 91 patients with type 2 diabetes mellitus with an average diabetic history of 7.2 ± 6.9 years who receive an average of 53.6 days of intensive insulin treatment,34.4% of them achieved a remission of 13.6 months.9Similar findings were obtained in other studies.10,11Therefore,intensive insulin therapy can be used in all stages of the diabetes course.Intensive insulin therapy can significantly delay the decline of islet function caused by high blood glucose and induce long time remission.However,the benefits would be weakened or even counteracted by severe hypoglycemia.12,13Sufficient glucose monitoring during the intensive insulin therapy is of important significance for preventing severe hypoglycemia.14For patients who need to continue multiple injections per day outside the hospital,self-glycemic monitoring is even more important.
The side effects of severe hypoglycemia and reality of the compliance of blood glucose monitoring and selfmanagement in Chinese diabetic patients
Severe hypoglycemia in intensive insulin therapy which is not discovered and treated in time would increase medical costs and cause heavy economic burden.15In particular in diabetes patients with serious complications,hypoglycemia can increase the risks of cardiovascular and cerebrovascular accidents.16Self-glycemic monitoring is regarded as one important method for good glycemic control.17A successful intensive insulin treatment requires close self-monitoring of blood glucose.Previous work recommends that patients with diabetes should monitor their blood glucose intensively no matter receiving multiple daily injections of insulin or not.18,19However,reality of self-glycemic monitoring in diabetic patients in China is a concern.A survey in China shows that less than 4% of diabetic patients monitor their blood glucose every day after discharge.20The multiple daily skin pricks and the pain often made patients get frustrated and refuse self-glycemic monitoring.21The factors that affect the frequency of blood glucose monitoring in patients with diabetes include age,family economic status,educational level,and psychological problems.22,23Poor compliance of self-glycemic monitoring increases the risk of severe hypoglycemia during the intensive insulin therapy.The reality of the compliance of blood glucose monitoring and self-management in Chinese patients with diabetes mellitus makes both patients and clinicians hesitated to carry out intensive insulin therapy.Anxiety and fear of hypoglycemia prevent clinicians and patients from receiving necessary intensive insulin treatment and mentally resist intensive glycemic control.
Anxiety of patients during insulin treatment and new glucose monitoring technology
Conventional blood glucose monitoring should be very frequent during the intensive inulin treatment which brought the patients pain and discomfort.It is difficult to follow up the diabetic patients who receive multiple daily injections of insulin.However,if the frequency of blood glucose monitoring is insufficient,judging insulin treatment is difficult,in particular in those patients who receive multiple daily injections of insulin.Long time of inulin injection and highly frequent conventional blood glucose monitoring worsen anxiety in some patients.24Flash glucose-sensing technology provides continuous measurement of interstitial glucose levels.25Compared with conventional self-glycemic monitoring method,flash glucose-sensing technology-based continuous glucose monitoring (CGM) can provide more comprehensive blood glucose information,display the trend of blood glucose fluctuation,and discover a variety of blood glucose which is not easily detected by conventional monitoring method.26Flash glucose-sensing technology available in China is a new form of real-time CGM.A previous study confirmed the efficacy and safety of flash glucose-sensing technology compared with the conventional blood glucose monitoring method in clinical practice,27which makes it possible for obtaining real-time glucose level by scanning the sensor with the reader.Most studies have focused on anxiety-related syndromes associated with fear of hypoglycemia,in particular phobia of needles (i.e.,needle anxiety).28-30However,more studies about anxiety related to new and sophisticated diabetes technologies (e.g.,continuous glucose monitoring,continuous subcutaneous infusion therapy) are greatly needed.31
Objectives
The objectives of this study are to investigate whether flash glucose-sensing technology can improve the compliance of blood glucose monitoring and self-management of diabetic patients who receive multiple daily injections of insulin,shorten the time of reaching standard blood glucose level,reduce blood glucose fluctuation,and alleviate anxiety compared with conventional blood glucose monitoring methods.
SUBJECTS AND METHODS
Trial design
In this prospective single-canter,open labelled,randomized clinical trial,we will investigate the effects of glucose monitoring methods in patients receiving multiple insulin injections (Figure 1).Forty patients with type 2 diabetes mellitus who receive multiple daily injections of insulin at the Department of Endocrinology,Northern Jiangsu People's Hospital between August 2018 and August 2019 are included in this study.
The changes of self-monitoring behavior and self-management will be estimated by Self-Monitoring Behavior Scale (SMBSCA),the Summary of Diabetes Self-Care Activities (SDSCA) scale and Hamilton Anxiety Scale (HAMA).The primary outcome measure of this study is the score of SMBSCA.
Study population
Type 2 diabetes patients between 18 and 60 years old who were judged by specialists for multiple daily insulin injections were selected as study population.All included patients met the diagnostic criteria of type 2 diabetes formulated by the World Health Organization in 1999.32To complete the observation,the included patients should be able to complete the forms and use the smart mobile phone to contact with the researchers.
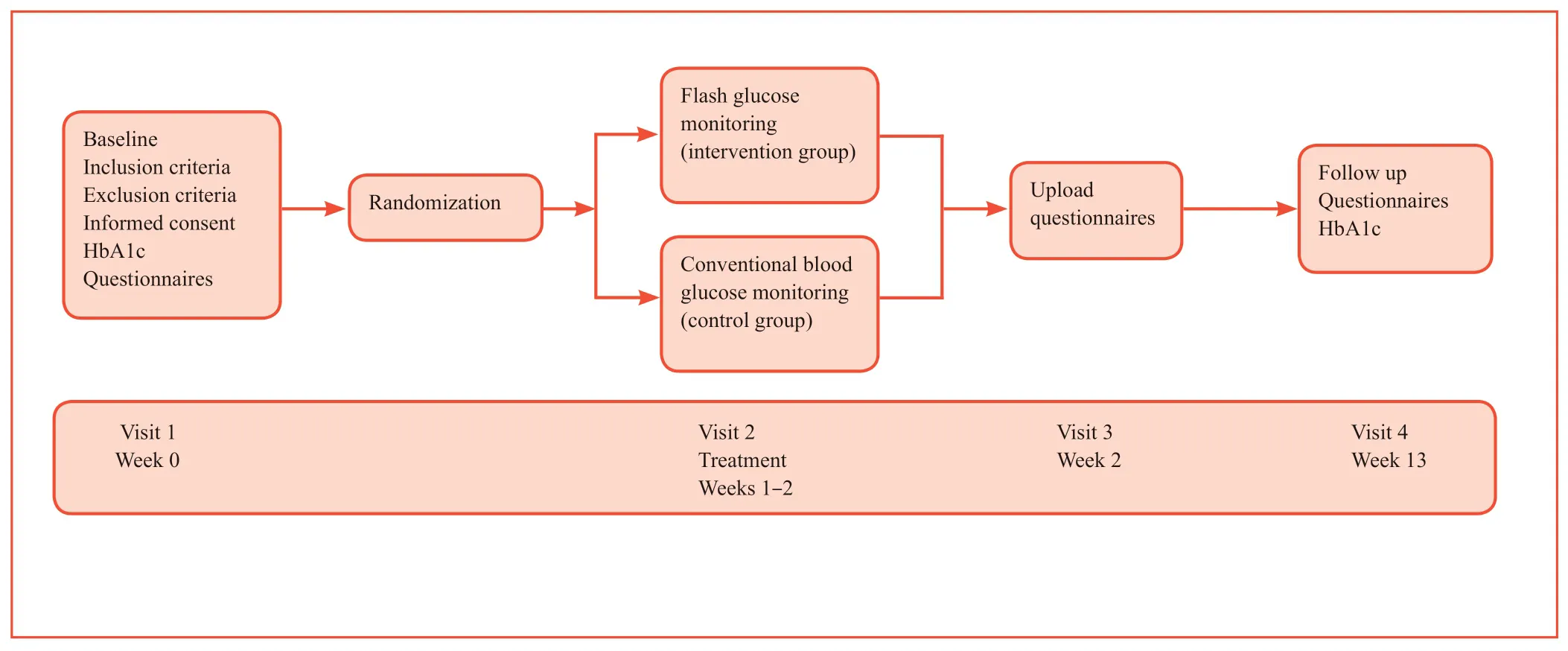
Figure 1:Study flow chart.
Inclusion criteria
Participants meeting all of the following conditions are considered for inclusion in this study:
-Age ≥ 18 years old and ≤ 65 years old27
-Meeting the diagnostic criteria of type 2 diabetes formulated by the World Health Organization in 199932
-Judgment by specialists for multiple daily injections of insulin
-Being able to complete the forms and use smart mobile phone to contact with the researchers
Exclusion criteria
Participants meeting any of the following conditions are excluded from this study:
-Age<18 years old,or>65 years old
-Type 1 diabetes or gestational diabetes mellitus or other types of diabetes
-Other situations the researches judge not suitable to attend the study
Withdrawal criteria
Participants are withdrawn from the study if any of the following occurs:
-The subjects cannot complete the experiment as required
-Severe adverse events,complications,and special physiological changes occurred (including pregnancy)
-Patients who quit due to various reasons
Randomization
A doctor arranges the numbers of two groups of patients in sequence,and then uses EXCEL to generate 40 random numbers.The serial numbers assigned to each patient are preserved in opaque sealed envelopes.Participants are randomized in a 1:1 ratio to undergo flash glucose monitoring-sensing technologybased CGM (intervention group) or self-monitoring of blood glucose (control group).
Blinding
With the difficulty of blinding in instruments,our design is open-labeled.Participants,investigators,and study staff are not blinded to group allocation.
Baseline collection
Patients' basic information:age,identifier number,gender,occupation,history of type 2 diabetes,native place,marriage,family history,complications of diabetes,hypoglycemia frequency during the past three months,and skin sensibility.
Physical anthropometry indexes:height,body weight,blood pressure,waist-hip ratio,body mass index,and abdominal circumference.
Testing items:fasting plasma glucose,postprandial blood glucose,glycosylated hemoglobin.
Questionnaires:SMBSCA,SDSCA,and HAMA scales.
Intervention
Specialists give weekly insulin dosage guidance to both groups of patients.Specialized nurses give weekly diet and exercise guidance to both groups of patients.The guidance is the same in both groups.The patients in the intervention group are requested to sense glucose every 6 hours per day and those in the control group are requested to test blood glucose at least four times a day within a week.
Outcome measures
Primary outcome measure
The primary outcome is the patients' compliance of blood glucose monitoring in two groups.The SMBSCA33is mainly used to measure the recent implementation of blood glucose monitoring in diabetic patients at home.Cronbach's alpha coefficient is 0.98.A total of 10 items are scored.The higher the total score,the better the behavior of self-monitoring of blood glucose in diabetic patients.
Secondary outcome measures
Secondary outcomes include medical and psychosocial measurements covering the time taken for reaching the goal of glycemic control;HbA1c;self-management scores;hypoglycemia times;change of anxiety scores.
Hypoglycemia is categorized as follows:
·Symptomatic hypoglycemia is defined as symptoms of hypoglycemia responding to ingestion of carbohydrate or an episode associated with a blood glucose level of ≤ 3.9 mM.
·Asymptomatic hypoglycemia is defined as a measured blood glucose level of ≤ 3.9 mM and is not associated with clinical symptoms.
·Confirmed hypoglycemia is defined as a measured blood glucose level of ≤ 3.9 mM with or without symptoms of hypoglycaemia.
·Nocturnal hypoglycemia is defined as hypoglycemia occurring while the subject was asleep responding to ingestion of carbohydrate or associated with blood glucose level ≤ 3.9 mM.
·A severe hypoglycemia is defined as an event with clinical symptoms that are considered to result from hypoglycemia in which the patient required the assistance of another person because the patient cannot treat her/himself due to acute neurological impairment directly resulting from the hypoglycemia (assistance by another person when the patient could have treated her/himself is not considered as requiring assistance)
Time schedule of this study is shown in Table 1.
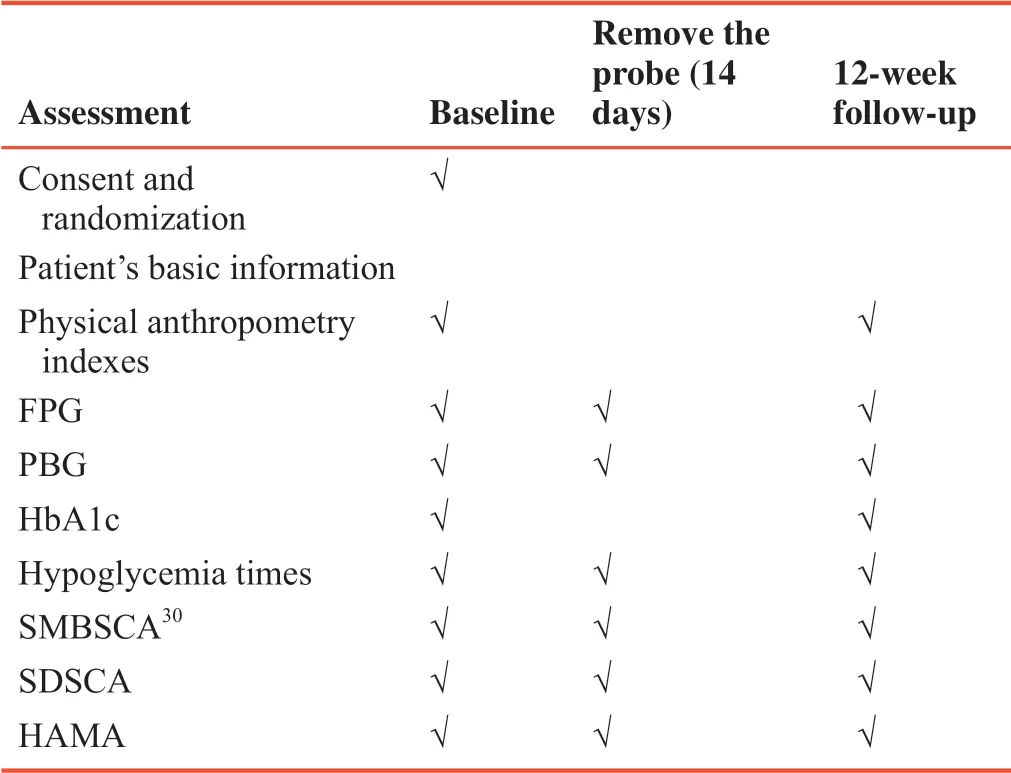
Table 1:Study assessment schedules
Sample size calculation
According to a previous study,29up to 26% of Chinese diabetic patients can monitor blood glucose following doctors' suggestion.The implementation rate is hoped to be 90% after intervention.The formula is as follows:N = {Zα/ 2 [(2P average) (1-P average) (Q1-1+Q2-1)] 0.5 + Zβ [P1Q1-1 (1-P1) + P2Q2-1 (1-P2)] 0.5}2 / (P1-P2) 2
The ratio of the two samples is 0.5/0.5,so Q1 and Q2 are 0.5.
Zα/2:α = 0.05,Zα/2 = 1.960;Zβ:β = 0.10,Zβ = 1.282;Q1 and Q2 represent the ratio of samples in the intervention and control groups,respectively.In this study,Q1 = 0.5,Q2 = 0.5;P1 and P2 represent the rate obtained in pre-test in the intervention and control groups,respectively.In this case,the executive rate is P1 = 0.90 and P2 = 0.26;P average = P1Q1 + P2Q2 = 0.9 × 0.5 + 0.5 × 0.26 = 0.58;N is the total number of samples in the two groups (N = n1 + n2).
The total number of sampleNis approximately 34,taking into account the loss rate of 20%,a total of 40 patients were included.
Ethical approval and consent
This study protocol was approved by the Medical Ethics Committee of Northern Jiangsu People's Hospital,China (approval No.2018045) (Additional file 1) and registered with the Chinese Clinical Trial Registry (registration number:ChiCTR1800017456) on July 31,2018.Written informed consent will be provided by the patients (Additional file 2).The protocol adheres to the recommendations provided by the SPIRIT 201334(Additional file 3).
Data collection
Participant data to be collected over the treatment period include the following:Patients' basic information (such as sex,age,history of DM,diagnosis of DM,treatment,complications of DM and so on),physical anthropometry indexes (such as height,weight,blood pressure,the waist hip circumference ratio and so on),blood glucose monitoring compliance,HbA1c before and after the intensive insulin injection,self-management score,occurrence of hypoglycemia cases,anxiety score.All data will be collected using a case report form which was showed in brief as the flow chart (Figure 1).
Data analysis
Data is collected by a case report form and analyzed by the department of statistics,Yangzhou University Medical College,China.
Descriptive analysis:The data of the basic information such as age,body weight,and height are expressed as the mean ± standard deviation,while the measurement data in the basic data are analyzed with the independent-samplet-test,and the data of different stages are tested by analysis of variance.A level ofP<0.05 is considered statistically significant.
Intention-to-treat analysis:In both groups,all data collected from subjects who undergo randomization are analyzed using an intention to-treat approach.
Criteria for feasibility success
The trial successful criteria include the following:
·Over 50% of appropriate patients approached agree to participate in the randomized controlled trial and exit rate is below 10%.
·Over 80% of enrolled and eligible patients complete the 3-month observation.
DISCUSSION
Up to now,hypoglycemia cannot be completely avoided in the intensive insulin treatment.Successful intensive insulin treatment requires close self-monitoring of blood glucose,however,self-monitoring of blood glucose is very insufficient among most of Chinese diabetic patients,especially in patients with diabetes mellitus receiving multiple insulin injections every day.It has been shown that flash glucose monitoring has no significant effect on HbA1c,although it does reduce time spent in the hypoglycaemic glucose range.27We hypothesize that flash glucose monitoring can improve the outcome of patients' acceptance of intensive insulin injection and quality of life for the intensive control of blood glucose because it can relieve pain and inconvenience.At the same time,we want to obverse if flash glucose monitoring can shorten the time to target with less hypoglycaemia.If flash glucose monitoring is found to be beneficial in this program,more Chinese diabetic patients with intensive insulin treatment will benefit.
Real-time glucose monitoring based on flash glucose monitoring methods is an effective way to increase the safety of intensive insulin injection in diabetic patients.We wanted to know if real-time glucose monitoring based on flash glucose monitoring methods can change the monitoring behavior or anxiety about the self-glucose monitoring.What is more,we want to compare the time to target and times of hypoglycemia between the intervention and control groups at the same time.If the flash glucose monitoring method can reduce the anxiety of type 2 diabetes patients receiving intensive insulin injection and shorten the time to target will be the other outcomes we are concerned.Results from this study will provide evidence to help endocrinology specialists to improve the management of diabetic patients.
This study is limited by the small size of samples.Due to financial and ethical constraints,we designed a single-center experiment.
We anticipate this study will provide some evidence that flash glucose monitoring methods can increase patients' acceptance of intensive insulin injection and improve the quality of life for the intensive control of blood glucose.
TRIAL STATUS
This study is currently recruiting participants.Study enrollment is expected to be completed by June 2019.
Additional files
Additional file 1:Ethical approval documentation (Chinese).
Additional file 2:Model consent form (Chinese).
Additional file 3:SPIRIT checklist.
Acknowledgments
We are grateful for all researchers,patients,and others involved in the evaluation of flash glucose monitoring method.
Author contributions
Study concept and design:NL,YL;manuscript writing:JC,LW;providing weekly diet and exercise guidance for patients:LW;providing guidance and advices for study design:ZWZ;manuscript revision and approval for final publication:all authors.
Conflicts of interest
None declared.
Institutional review board statement
This study protocol approved by the Medical Ethics Committee of Northern Jiangsu People's Hospital of China (approval No.2018045).The study will be performed in accordance with theDeclaration of Helsinki,and informed consent will be collected from each patient prior to enrollment.
Declaration of patient consent
The authors certify that they will obtain all appropriate patient consent forms.In the forms,the patients will give their consent for their images and other clinical information to be reported in the journal.The patients will understand that their names and initials will not be published and due efforts will be made to conceal their identity,but anonymity cannot be guaranteed.
Reporting statement
This study followed the Standard Protocol Items:Recommendations for Interventional Trials (SPIRIT) guidance for protocol reporting.
Biostatistics statement
The statistical methods of this study were reviewed by the biostatistician of Northern Jiangsu People's Hospital,Yangzhou University,China.
Data sharing statement
All individual participant data will be available beginning 9 months and ending 36 months following article publication with researchers who provide a methodologically sound proposal for individual participant data meta-analysis.Individual participant data that underlie the results reported in this article,after deidentification (text,tables,figures,and appendices).Study protocol,statistical analysis plan,clinical study report and adverse effects reports will be available with the publication of papers and data will be available at http://www.chictr.org.cn.
Plagiarism check
Checked twice by iThenticate.
Peer review
Externally peer reviewed.
Open access statement
This is an open access journal,and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
杂志排行
Clinical Trials in Degenerative Diseases的其它文章
- Effects of exergames on motor coordination and balance in convalescent drug addicts:a feasibility study
- Effect of beraprost sodium on pulmonary hypertension due to left ventricular systolic dysfunction:protocol for a randomized controlled clinical trial
- Efficacy and safety of Mamajjaka Ghanavati in the treatment of type 2 diabetes mellitus:a prospective open label multi-center clinical study
- Information for Authors - Clinical Trials in Orthopedic Disorders
