Role of Mediterranean diet in preventing platinum based gastrointestinal toxicity in gynecolocological malignancies: A single lnstitution experience
2019-03-21EleonoraGhisoniValentinaCasaloneGaiaGiannoneGloriaMitticaValentinaTuninettiGiorgioValabrega
Eleonora Ghisoni, Valentina Casalone, Gaia Giannone, Gloria Mittica, Valentina Tuninetti, Giorgio Valabrega
Abstract BACKGROUND Gynecological malignancies represent a major cause of death in women and are often treated with platinum-based regimens. Patients undergoing chemotherapy suffer from alterations in nutritional status which may worsen gastrointestinal(GI) toxicities, quality of life and affect the overall prognosis. Indeed, assuring a good nutritional status and limiting toxicities during treatment are still major goals for clinicians.AIM To assess the role of Mediterranean Diet (MD) in reducing GI toxicities in patients with gynecological cancers treated with platinum-based regimens.METHODS We conducted an observational study on 22 patients with gynecological tumors treated with a platinum-based chemotherapy at Candiolo Cancer Institute FPO/IRCCS between January 2018 and June 2018. The food and frequency (FFQ)and the Patient-Reported Outcomes Common Terminology Criteria For Adverse Events (PRO-CTCAE) questionnaires were administered at baseline and at every Day 1 of each cycle. To evaluate the differences in GI toxicities the study population was divided in two groups according to the currently validated Mediterranean Diet Serving Score (MDSS) at baseline.RESULTS Patients with high MDSS reported a trend toward lower GI toxicities according to PRO-CTCAE at each timepoint (first evaluation: P = 0.7; second: P = 0.52; third: P= 0.01). In particular, difference in nausea frequency and gravity (P < 0.001),stomach pain frequency and gravity (P = 0.01 and P = 0.02), abdomen bloating frequency and gravity (P = 0.02 and P = 0.03), and interference with daily activities (P = 0.02) were highly statistically significant at the end of treatment.More than 60% of patients changed their food habits during chemotherapy mainly because of GI toxicities. A higher reduction of food intake, both in terms of caloric (P = 0.29) and of single nutrients emerged in the group experiencing higher toxicity.CONCLUSION Our results show that adherence to MD possibly reduces GI toxicity and prevents nutritional status impairment during chemotherapy treatment. Bigger studies are needed to confirm our results.
Key words: Mediterranean diet; Gynecological malignancies; Gastrointestinal toxicities;Platinum-based chemotherapy; Nutritional status
INTRODUCTION
In 2017 gynecological cancers (endometrium, cervix, and ovarian) accounted for approximately 12% (94990 out of 810320) of all new cancer diagnoses in women in the United States. Ovarian cancer (OC) and cervical cancers represent 1.3% and 0.7% of new cancer cases, respectively, in United States with OC ranks fifth in cancer deaths among women, accounting for more deaths than any other gynecological cancer[1,2].
Current standard treatment for gynecological cancers is represented by a multimodal approach including surgery, chemotherapy, radiotherapy (RT) and brachytherapy. Platinum agents (cisplatin and carboplatin) are the most often used drugs in these malignancies, both alone or in combination with other agents (i.e.,carboplatin-taxol in OC and endometrial cancer, in association with bevacizumab in cervical cancer) or radiotherapy (i.e. weekly cisplatin + RT in locally advanced cervical cancer)[3-7]. Expected toxicities from platinum based chemotherapy are mainly hematological and gastrointestinal (GI) (nausea, vomiting, diarrhea, or constipation)[8,9].
GI system does of course play a central role in nutritional modulation, but due to its direct exposure to diet intakes and its rapid cellular turnover and plasticity it is also affected by chemotherapy-induced toxicities and subject to nutritional stimuli. Food substances could therefore be able to modulate chemotherapy-induced GI toxicities,as suggested by recent studies[10,11].
Cancer patients are often subjected to alterations in nutritional status due to both disease and treatment-related toxicities; indeed impairment in nutritional status could worse patients’ quality of life (QoL), lead to treatments’ doses modifications and schedule delays and finally affect the prognosis. Proposed nutritional interventions should have the following aims: Prevention and early treatment of symptoms due to chemotherapy, QoL improvement and avoidance of complications such as overweight on one side and cachexia on the other[12,13].
It is estimated that half of all patients with cancer eventually develop a cachectic syndrome during treatment and 70% of terminal patients suffer from this condition,which is the recognized cause of the 20% of all cancer deaths[14]. Cancer cachexia is characterized by systemic inflammation, negative protein and energy balance, and an involuntary loss of body mass. Recent therapies for the cachectic syndrome involve a multidisciplinary approach combining palliative therapy with orexigenic appetite stimulants and diet modification and/or exercise[15,16]. More recently the specific evaluation of food intake has been proposed as an important tool to prevent the onset of nutritional impairments[17].
It is well recognized that processed meat promote inflammation due to the presence of nitrites[18,19]and patients with high intake of “inflammatory” foods were found with greater levels of PCR, sICAM, IL-6, E-selectin, and homocysteine[20]. On the opposite, lower values of the same markers were found for those consuming higher intake of wholegrain cereals, nuts, vegetables, fruit and tea[21,22]. As if the above reported studies could suggest a diet with low content of animal proteins, it should be considered that a vegan diet could easily determine nutritional deficiencies, and should be particularly avoided in cancer patients considering the high risk of malnutrition[23].
In this context a valid alternative model represented by the Mediterranean Diet(MD) has already showed to assure well-balanced food intake and potentially play an anti-inflammatory role[24-26]. Figure 1 shows recommended daily nutritional intakes according to the MD model by the Mediterran Diet Foundation[27].
Numerous studies have already demonstrated a relationship between MD adherence and the prevention of cardiovascular diseases and diabetes. Moreover,Monteagudo et al[28]have validated the Mediterranean Diet Serving Score (MDSS) as an easy, valid, and accurate instrument to assess MD adherence based on the consumption of foods and food groups per meal, day, and week (range 0 to 24 points).
However, despite the recognized importance of prevent early onset of nutritional impairments in cancer patients assuring the maintenance of a good QoL and the relevance of gynecological cancers, few studies have explored the role of MD in preventing chemotherapy toxicities.
We aim to conduct an observational study to assess the role of MD in reducing GI toxicities in patients with gynecological cancers treated with chemotherapeutic platinum-based regimens according to their adherence to the MDSS.
MATERIALS AND METHODS
Ethic statement
We conducted an observational study on 24 patients with gynecological tumors treated with a platinum-based chemotherapy at Candiolo Cancer Institute (FPOIRCCS) between January 2018 and June 2018. Patients affected by intestinal chronic disease or any other chronic condition which could impact on GI toxicities or who required parenteral nutrition were excluded from the study. All recruited patients signed the written informed consent for observational study and the institutional review board of our Institutions provided approval.
Patients
For each selected patient, the following clinico-histopathological data were recorded:(1) Age; (2) Type of gynecological cancer (cervical, endometrial, ovarian); (3) Weight;(4) Body mass index (BMI); (5) Smoke and alcohol habits; (6) Chemotherapy treatment scheme; (7) Number of previous lines; and (8) Nutritional requirements [basal energy expenditure (BEE) and total energy expenditure (TEE)].
At baseline, patients were interviewed by the dietician through the food frequency questionnaire (FFQ) to evaluate food habits and intakes before the beginning of the chemotherapeutic treatment. FFQ was then administered each two cycles (up to three times for patients receiving 6 platinum-based cycles). Subsequent FFQs included two questions about food intakes’ differences compared to baseline and their causes.
Patients also received at each cycle the patients-reported outcome common terminology criteria for adverse events (PRO-CTCAE) questionnaire for toxicities assessment[29]. For this study, we decided to select only GI toxicities because of the relevant association with nutritional aspects. Particularly, key elements evaluated were the following: dry mouth feeling, swallowing difficulty, oral sores, cracks in mouth’s corners, difficulty of tasting food and beverages’ flavor, loss of appetite,nausea, vomiting, stomachache, intestinal gas, abdominal bloating, constipation,diarrhea, and abdomen ache.
Statistical analysis

Figure 1 The recommended daily nutritional intakes according to the Mediterranean diet model by the Mediterranean Diet Foundation.
Anthropometric and nutritional values were estimated using standard formula such as BMI and Harris-Benedict formula {i.e., for female 655 + [9.56 × body weight (kg)] +[1.85 × height (cm)] - [4.67 × age (years)]}. To understand patients’ TEE, the obtained value was multiplied for 1.2-1.5, depending on the individual status (underweight,overweight exc.). BEE was estimated on the basis of actual body weight, except for obese patients for whom BEE was adjusted to a “correct body weight” using the following formula: (Actual body weight × 0.25) + ideal body weight. Average food composition values, derived from CREA and IEO databases, were used to assess individual food intake detected by FFQ[30,31]. Adherence to MD was evaluated using MDSS (range 0-24), as reported above[28]. Patients were then divided in two groups according to the different adherence: Low (from 0 to 12 points) and high (from 13 to 24).
We therefore compared FFQ and PRO-CTCAE results to assess diet variations and toxicities profile during treatment in the two groups according to MDSS. A P < 0.05 was considered statistically significant. All analyses were performed using the SPSS statistical software program, version 22.0 (IBM SPSS Inc., Chicago, IL, United States).
RESULTS
Baseline evaluation
Twenty-four patients were enrolled in our observational study following the inclusion criteria. There were 2 drop-out due to premature interruption of the treatment.Therefore, only 22 patients completed all questionnaires’ time-points and were included in our analysis. Complete baseline patients’ clinical data are reported in Table 1.
According to the first FFQ, the majority of patients (59.10%) declared to have not received any nutritional advice from any specialist before treatment beginning. About half of them has independently searched information about nutrition and anti-cancer treatments: in the 83.3% patients utilized websites for their search. Table 2 shows anthropometric patients’ characteristics in the two groups of patients according to MDSS. Groups were well balanced as there was no statistically significant difference in median age (P = 0.11), weight (P = 0.34) and BMI (P = 0.6).
FFQ analysis
The analysis of the second FFQ showed that the majority of patients has changed food habits from the beginning of chemotherapy treatment. For the 64% of the sample the change was partial, for the 9% it was total. The presence of one or more GI symptoms has been the cause of the change for about 70% of them. Loss of appetite was present in 100% of them, followed by nausea (about 60%). Analysis of data showed a decrease in average MDSS of both groups. Average coverage of the TEE has also decreased: It is more than 100% in only 12 on 22 patients with a reduction in the group with higher MSSD compared to the baseline. Table 3 shows nutritional features emerged from this analysis. Questions in the third FFQ shows that about 60% of the sample has modified food habits again compared with the previous evaluation. Between them, about 70%has attributed the change to the presence of one or more GI symptoms. Even in this case, loss of appetite has been reported by all of them. Nausea, dysgeusia and oral sores are little decreased comparing the previous analysis, but on the oppositestomachache and vomiting are increased. Overall a comparative analysis of nutritional intake from the beginning to the end of the evaluations underlined a general reduction of all intakes and consequently of TEE coverage. Group with higher MSSD suffered from a higher reduction of all intakes.

Table 1 Clinical and nutritional characteristics of the sample at baseline
PRO-CTCAE analysis
Data from the PRO-CTCAE after the first chemotherapy cycle showed no statistically significant differences between the two groups, except for “abdominal pain frequency and gravity” which was higher in patients with MDSS < 13 (P = 0.04). Nevertheless,group with higher MDSS showed a tendency to suffer from less toxicities. This difference emerged more clearly when looked at the total GI data where the mean value differed of 1.7 points 13.4 vs 15.1).
Not surprisingly, when evaluating the last PRO-CTCAE several statistical differences were reported between the two groups. Particularly, the group with higher MDSS reported lower values of the following toxicities: Nausea frequency andgravity (P < 0.001), stomachache frequency and gravity (P = 0.01 and P = 0.02),abdomen bloating frequency and gravity (P = 0.02 and P =0.03) and interference with daily activities (P = 0.02). Difference in total average GI toxicities also became statistically significant between groups at this last evaluation (P = 0.01), but overall a trend to lower GI toxicities according to CTCAE was observed at each timepoint (first evaluation P = 0.7; second: P = 0, 52; and third: P =0.01). Tables 4 and 5 reported completed and extensive data from the first and last PRO-CTCAE assessment respectively.

Table 2 Anthropometric and nutritional characteristics of the two groups at the baseline according to the Mediterranean diet adherence score
DISCUSSION
The Italian Association of Medical Oncology (AIOM) has recently highlighted the importance for cancer patients to receive information from skilled professionals (i.e.,dieticians, nutritionists and medical oncologists), regarding the nutritional status, its possible changes during chemotherapy due to toxicities and the negative consequences[32]. Proposed nutritional interventions should aim to prevent and/or limit treatments’ side effects, assuring well-balanced nutritional status and QoL[33,34].However, our study shows that about 50% of the patients do not receive sufficient information and nutritional advice before treatment.
First and second assessments combining FFQ and PRO-CTCAE did not show any statistically significant differences between the two groups. However, a trend toward lower GI toxicities was seen in patients with high MDSS at each timepoint (first evaluation P = 0.7; second: P = 0.52; and third: P = 0.01). Difference in nausea frequency and gravity (P < 0.001), stomachache frequency and gravity (P = 0.01 and P= 0.02), abdomen bloating frequency and gravity (P = 0.02 and P = 0.03) and interference with daily activities (P = 0.02) became highly statistically significant at the end of treatment. Moreover, more than 60% of patients declared to have changed their food habits during chemotherapy mainly because of GI toxicities. A higher reduction of food intake, both in terms of caloric (P = 0.29) and of single nutrients emerged in the group experienced higher toxicity. All together these results suggest a protective role of MD in preventing cumulative GI chemotherapy induced toxic effects and supporting patients nutritional wellness during chemotherapy[35,36]. Of note, no significant changes in body weight and BMI were observed in our study population during treatment.
We recognize that our study has the several weaknesses: Although all patients were treated with a platinum-based chemotherapy and were affected by a gynecologicmalignancy, it is undoubtable that size and heterogeneity of our population (in terms of cancer type, line of treatment, and chemotherapy schedule), which affect the statistical power, are important limitations. However, to our knowledge, this is the first observational study investigating the possible role of MD in preventing GI toxicities. Further studies with larger cohorts of patients homogeneous for type of disease and treatments, might help to elucidate if and how MD could impact on treatment related GI toxicities.

Table 3 Nutritional-anthropometric features derived from the analysis of the second food frequency questionnaire
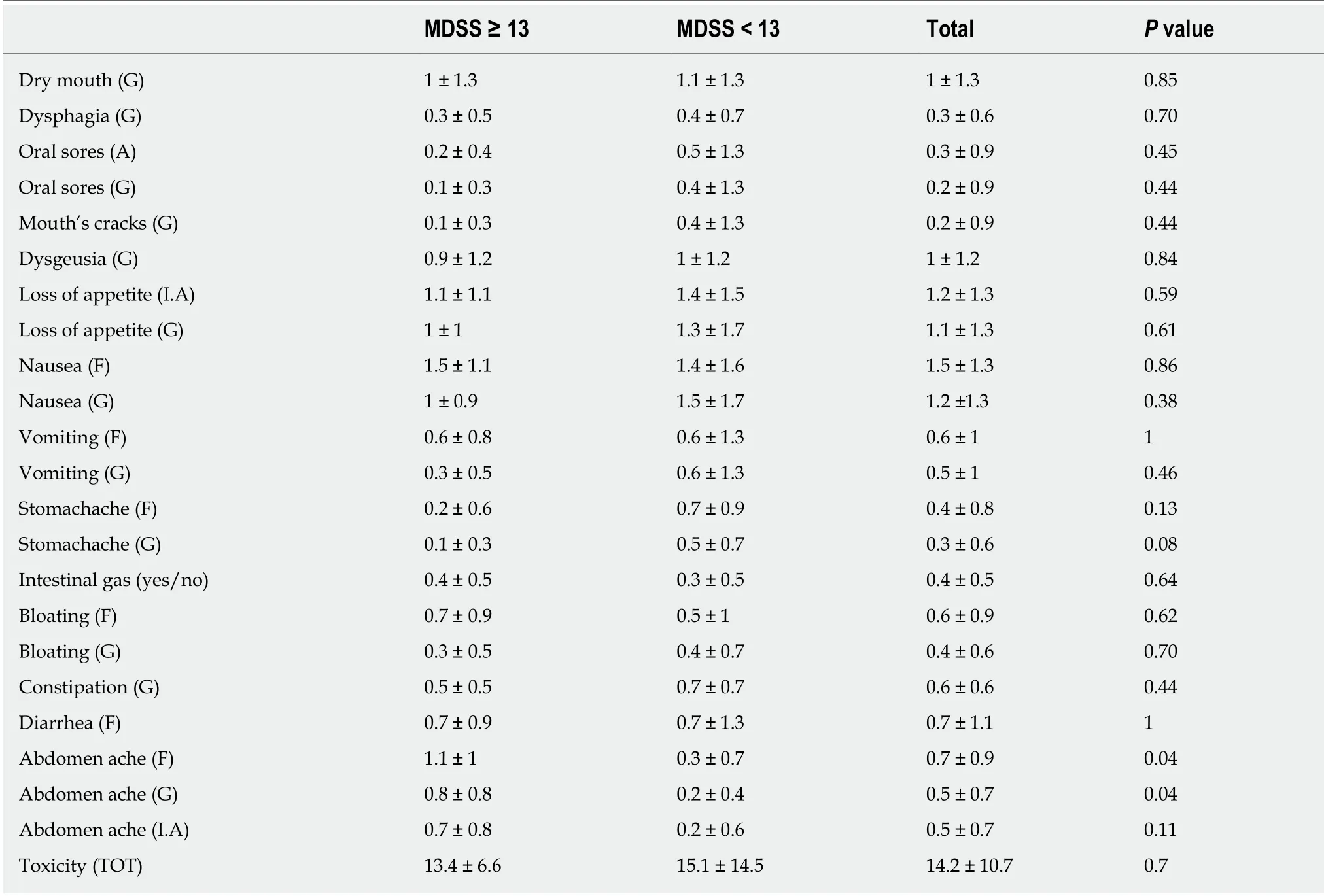
Table 4 Gastrointestinal toxicities' score after first chemotherapy cycle in the whole study population and in the two study's groups according to the Mediterranean diet serving score
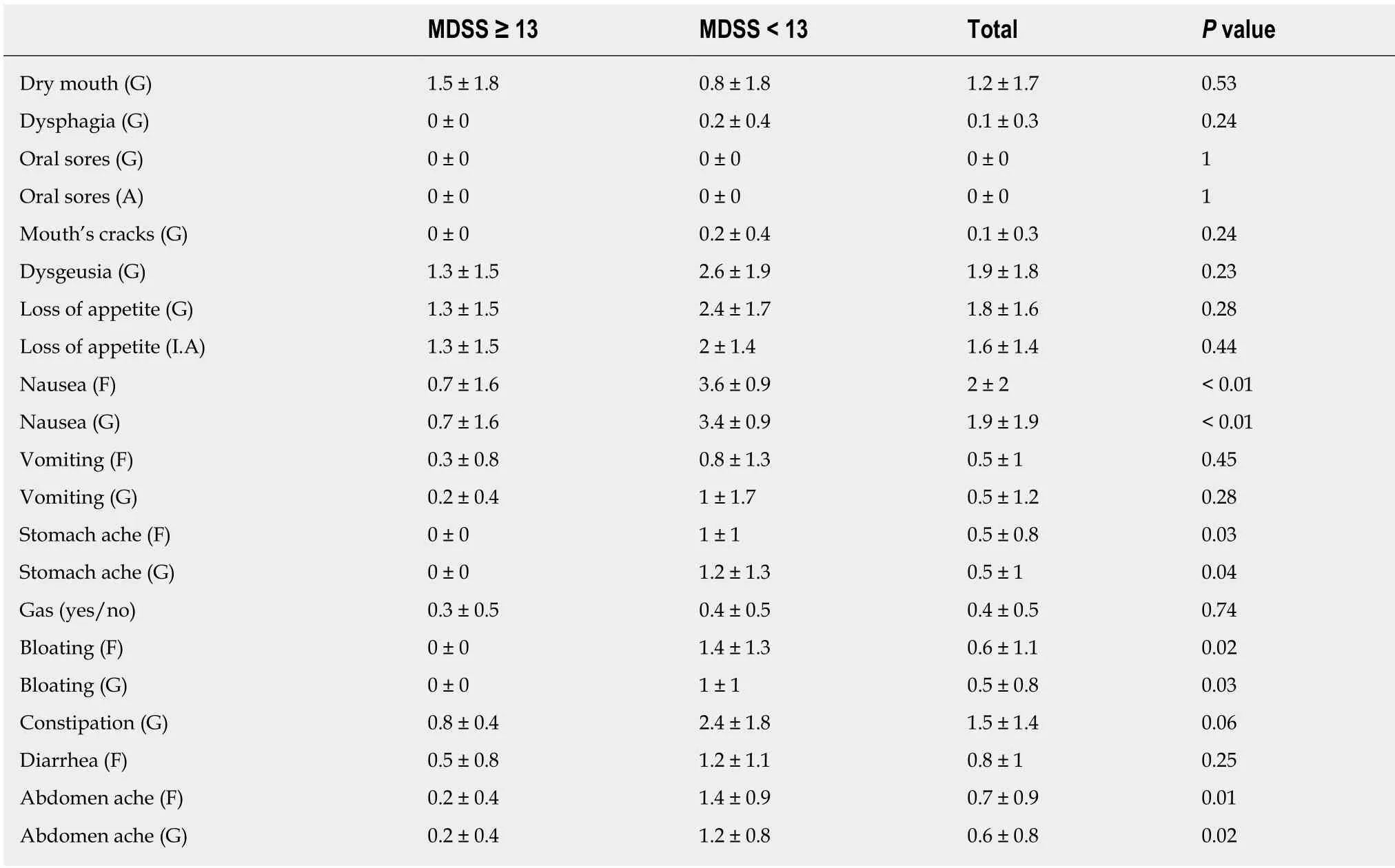
Table 5 Gastrointestinal toxicities' score after last chemotherapy cycle in the whole study population and in the two study's groups according to the Mediterranean diet serving score

I.A: Interference with daily activities; MDSS: Mediterranean diet serving score; G: Gravity; F: Frequency; TOT: Time of treatment; GI: Gastrointestinal.
ARTICLE HIGHLIGHTS
Research background
Gynecological cancers still account for approximately 12% of all new cancer diagnoses in women and are often treated with platinum-based chemotherapy scheme. Cancer patients are often subjected to alterations in nutritional status due to both disease and treatment-related toxicities,especially gastrointestinal (GI) ones. Indeed impairment in nutritional status could worse patients’ quality of life (QoL), lead to treatments’ doses modifications and schedule delays and finally affect the overall prognosis.
Research motivation
The Mediterranean Diet (MD) model has already showed to assure a well-balanced food intake and potentially play an anti-inflammatory role. Moreover, several studies have already demonstrated a relationship between MD adherence and the prevention of cardiovascular and metabolic diseases and diabetes. More recently, Monteagudo and colleagues have validated the Mediterranean Diet Serving Score (MDSS) as an easy, valid, and accurate instrument to assess MD adherence[28]. Despite the recognized importance of prevent early onset of nutritional impairments in cancer patients assuring the maintenance of a good QoL and the relevance of gynecological cancers, few studies have explored the role of MD in preventing chemotherapy toxicities.
Research objectives
We aim to conduct an observational study to assess the role of MD in reducing GI toxicities in patients affected by gynecological cancers treated with chemotherapeutic platinum-based regimens according to their adherence to the MDSS.
Research methods
We conducted an observational study on 24 patients with gynecological tumors treated with a platinum-based chemotherapy at Candiolo Cancer Institute (FPO-IRCCS) between January 2018 and June 2018. Patients affected by intestinal chronic disease or any other chronic condition,which could impact on GI toxicities were excluded from the study. Patients were interviewed at baseline by the food frequency questionnaire (FFQ) to evaluate food habits and intakes before the beginning of the chemotherapeutic treatment. FFQ was then administered each two cycles(up to three times for patients receiving 6 platinum-based cycles). Patients also received at each cycle the patients-reported outcome common terminology criteria for adverse events (PROCTCAE) questionnaire for GI toxicities assessment[29]. Furthermore, anthropometric assessments[weight; body mass index (BMI); basal energy expenditure; and total energy expenditure] were measured at each cycle.
Research results
Our study showed a trend toward lower GI toxicities in patients with high MDSS at each timepoint (first evaluation: P = 0.7; second: P = 0, 52; and third: P =0.01). Difference in nausea frequency and gravity (P < 0.001), stomachache frequency and gravity (P = 0.01 and P = 0.02),abdomen bloating frequency and gravity (P = 0.02 an P = 0.03), and interference with daily activities (P = 0.02) became highly statistically significant at the end of treatment. A higher reduction of food intake, both in terms of caloric (P = 0.29) and of single nutrients emerged in the group experienced higher toxicity. Of note, no significant changes in body weight and BMI were observed in our study population during treatment, even if more than 60% of patients declared to have changed their food habits during chemotherapy mainly because of GI toxicities.
Research conclusions
Both FFQ and PRO-CTCAE results in our series suggest a protective role of MD in preventing cumulative GI chemotherapy induced toxic effects and supporting patients nutritional wellness during chemotherapy. However, our study also showed that about 50% of the patients declare to not receive sufficient information and nutritional advice before treatment, pawing the way for a better effort to assure patients high-quality comprehensive care.
Research perspectives
This is the first observational study investigating the possible role of MD in preventing GI toxicities in gynecological cancer patients. Further studies with larger cohorts of patients might help to confirm if and how MD could impact on treatment related GI toxicities. The Italian Association of Medical Oncology (AIOM) has recently highlighted the importance for cancer patients to receive information from skilled professionals (i.e., dieticians, nutritionists, and medical oncologists), regarding the nutritional status, its possible changes during chemotherapy due to toxicities and the negative consequences. Future nutritional interventions should aim to prevent treatments’ side effects, assuring well-balanced nutritional status and QoL.
ACKNOWLEDGEMENTS
The work was partially funded by Italian Ministry of Health, Ricerca Corrente 2019.
