Multifocal G1-G2 gastric neuroendocrine tumors:Differentiating between Type I,II and III,a clinicopathologic review
2019-03-14KhaledAlgashaamyMonicaGarciaBuitrago
Khaled Algashaamy,Monica Garcia-Buitrago
Abstract
Key words: Gastric neuroendocrine tumors;Enterochromaffin like cells;Histopathological features;Immunohistochemistry;Diagnosis;Treatment
INTRODUCTION
Neuroendocrine tumors (NETs) of the gastrointestinal (GI) and pancreaticobiliary tracts are a heterogeneous group of tumors that have a diverse biologic and clinical profile that varies according to the primary tumor site of origin,and its histopathologic features[1-4].Gastric neuroendocrine tumors (gNETs),which are the product of the neoplastic transformation of the enterochromaffin like cells (ECL) of the gastric mucosa,are a rare and indolent category of neuroendocrine tumors that has witnessed a rise in incidence due to the widespread use of upper digestive endoscopy and the technical refinement of endoscopists[5].In this article we will review the histopathological features,immunohistochemistry,differential diagnosis,treatment,prognosis and outcome of these lesions.
HISTOPATHOLOGICAL FEATURES
The ECL cells play a fundamental role in the regulation of acid secretion,which is essential to understand the gNET classification into subtypes,each with distinct management and prognosis.Following food intake,the G cells of the antrum secrete gastrin which in turn stimulates the ECL cells along with the histamine-producing parietal cells to secrete hydrochloric acid (HCL),this phase is inhibited through a negative feedback mechanism that comes from the D cells,which through HCL acid stimulation secrete somatostatin reducing the secretion of gastrin[6].
Gastric neuroendocrine tumors are classified based on their location,syndromic association,clinical behavior and demographic profile,as follows.(Table 1).
Type I
These lesions correspond to the majority of gNETs found in the stomach (70%-80%),they are usually multiple small nodules/polyps in the gastric body and are limited to the mucosa and submucosa.These lesions are usually found in association with autoimmune chronic atrophic gastritis.The presence of anti-parietal cell or antiintrinsic factor antibodies leads to the destruction of the parietal cells reducing the level of hydrochloric acid (achlorhydria),which in turn eliminates the negative feedback inhibition of gastrin production by G cells leading to hypergastrinemia,this excess hormone production favors the appearance of multiple small lesions that are generally indolent and may regress.Lymph node metastases are very rare and occur only when the tumors are large (greater than 2 cm) and infiltrate the muscularis propria.These characteristics give these tumors a little aggressive behavior;however,they have an overall good prognosis[7].They are clinically associated with pernicious or megaloblastic anemia due to the body’s inability to absorb vitamin B12 secondary to a decrease in intrinsic factor[8].The findings on upper GI endoscopy include soft yellowish and transparent vasculature seen in the antral mucosa,contrasting with the smooth,erythematous looking mucosa of normal areas.The neuroendocrine tumors are seen as minute (less than 1-2 cm) and frequently multiple polyps (Figure 1).Histologic examination shows atrophied gastric oxyntic mucosa with occasional intestinal,pseudopyloric or pancreatic acinar metaplasia with nodules that are composed of sheets or nests of neuroendocrine cells.When these nodules measure less than 5 mm in greatest linear dimension,they should be named neuroendocrine microadenomas,rather than neuroendocrine tumors.These tumors usually exhibit nil mitotic activity and a low Ki67 proliferation index of less than 3%,compatible with well differentiated neuroendocrine tumors,grade 1 (G1) (Figure 1 and Figure 2).
Type II

Table1 Features of Types I,II and III gastric neuroendocrine tumors
These lesions account for 7% of gNETS,they are usually small,multiple tumors and have a bimodal age distribution affecting young adults with multiple endocrine neoplasia type 1 (MEN1),Zollinger-Ellison syndrome and older adults.MEN1is a tumor suppressor gene present at 11q13 locus,the transcription product ofMEN1gene is the Menin protein[9-13].Biallelic inactivation through a mutation in 1 allele of MEN1,coupled with the loss of the remaining wild type allele is identified in about 90% of gNETS of this type[14].Upper GI endoscopy reveals multiple tumors measuring less than 2 cm noted in the body or fundus of the stomach and an adjacent normal or hypertrophic gastric mucosa with clinical findings of hypergastrinemia and low gastric PH (hyperchlorhydria)[15].Serial measurements of serum gastrin levels following intravenous administration of secretin can be performed and show an increase in gastrin levels for patients with gastrinoma,while a decrease in gastrin levels is seen in healthy individuals.Histopathologic examination demonstrates a gastric oxyntic mucosa with increased oxyntic cell mass due to uninhibited gastrin stimulation along with nodules of neuroendocrine tumor nests that have negligible mitotic activity,absent necrosis and typically a low Ki67 proliferation index (less than 3%) compatible with a well differentiated neuroendocrine tumor,G1 and rarely a G2 in the advent of a Ki67proliferation index between 3% to 20%.In contrast to type I tumors,10%-30% metastasize.Neuroendocrine microadenomatosis is a common feature ofMEN1.Tumors greater than 2 cm that invade the muscularis propria and exhibit vascular invasion are more likely to metastasize.
Type III
These lesions are sporadic tumors and are the second most common gNET.They occur in the absence of ECL cell hyperplasia and are not associated with hypergastrinemia,chronic atrophic gastritis,MEN1or Zollinger- Ellison syndrome.Upper endoscopy reveals a single solitary lesion,greater than 2 cm,that can be located in any part of the stomach.Histopathologic examination shows cords and nests of tumor cells.Many cases show intermediate cytological atypia characterized by abundant amphophilic cytoplasm,enlarged nuclei with open chromatin and prominent nucleoli,single cell apoptosis or extensive necrosis can be identified along with a Ki67 proliferation index in excess of 3%,thereby making these tumors G2 or in rare occasions G3 when the Ki67 index exceeds the 20% threshold.Metastases are common and are associated with larger size,angioinvasion,and invasion of the muscularis propria.
IMMUNOHISTOCHEMISTRY
Endocrine cells of the GI and pancreaticobiliary tracts and NETs are labelled by neuroendocrine markers,includingCD56/NCAM1,leu7/B3GAT1,protein gene product 9.5 (PGP9.5),neuron specific enolase,synaptophysin and chromogranin A.It is considered that synaptophysin is the most sensitive,and chromogranin A is the most specific of the neuroendocrine markers.Therefore,in the majority of practices those two markers are used to determine neuroendocrine differentiation.The other stains mentioned are not commonly used,due to their low sensitivity and specificity[16].Grading of NETs is done using the Ki67 proliferation index,as recommended by the World Health Organization (WHO) classification of neuroendocrine tumors (2010 and most recently in 2017),the labelling index should be measured in 500-2000 cells in the most proliferative “hot spot” areas[17].Indices that are less than 3% are considered a G1 tumor,indices that are between 3% and 20% are considered G2 tumors and those that have a Ki67 proliferation index higher than 20%are considered to be G3 lesions,based on the latest (2017) WHO recommendation.The mitotic count is also used in the grading of these tumors,grade 1 tumors have less than 2/10 HPF,grade 2 tumors have 2-20/10 HPF,and grade 3 tumors have more than 20/10 HPF (based on 2017 WHO recommendation,Table 2).Cases with discrepancies between the Ki67 proliferation index and the mitotic count comprise one third of the cases;and in these cases,the higher grade should be selected[18].Combined,these two features have been shown to reflect the clinical behavior and prognosis of these tumors[19,20].
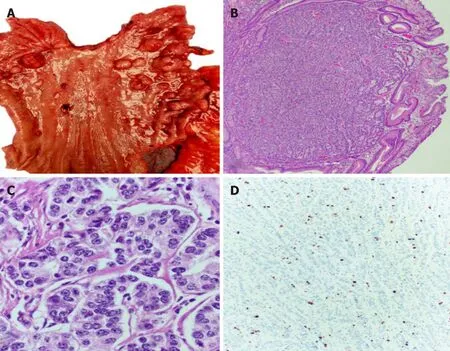
Figure1 The neuroendocrine tumors are seen as minute (less than 1-2 cm) and frequently multiple polyps.
PROGNOSIS AND PREDICTIVE FACTORS
The prognosis of patients with gNETs is highly variable.According to the WHO,these neoplasms are classified into different grades with distinct prognosis.Histologic features that correlate with a favorable prognosis include the following:(a) Growth within the mucosa-submucosa interface;(b) Bbsence of angioinvasion;(c) Lesion size less than 1 cm;(d) Absence of endocrine syndrome on clinical evaluation;and (e) Low mitotic activity[21,22].Tumor related deaths in Type I lesions are only observed in exceptional situations,while 1 out of 10 patients have died from Type II lesions.On the other hand,type III lesions carried a mortality rate of 27% with a mean survival of 28 mo[23].
STAGING AND TREATMENT
CT scan is recommended for type I and type II lesions that are larger than 2 cm and for all type III lesions.The use of magnetic resonance imaging of the abdomen,octreotide scintigraphy and PET-CT is limited for specific cases[24].According to the AJCC (8th edition),lymph node metastases (N1) are detected in about 5% of type I,30% of type II and 71% of type III,while distant (liver) metastases (M1) are found in 2.5%,10% and 69% of cases of type I,II and III,respectively[25].Therefore,the majority of ECL cell NETs of type I and II are considered to be stage I,i.e.,T1 N0 M0,and only a selected few would be stage IIa or T2 N0 M0.On the other hand,most type III lesions fit stages IIa,IIb (T3 N0 M0),IIIa (T4 N0 M0),IIIb (T1 N1 M0) or even IV (any T,any N,M0)[26].

Figure2 Tumors usually exhibit nil mitotic activity and a low Ki67 proliferation index of less than 3%,compatible with well differentiated neuroendocrine tumors,grade
Treatment of gNETs is a multifaceted approach,depending on the clinical type,disease extent,degree of differentiation of the lesion and the presence or absence of poor prognostic indicators.Type I lesions are treated by endoscopic mucosal resection as most of these lesions are small,well differentiated and exhibits excellent prognosis[27].Supplementing Vit B12 is also recommended.Surgical treatment of type I gNETs is reserved for cases in which endoscopic resection is not feasible or when poor prognostic indicators are present.The choice of the best surgery (i.e.,antrectomy,subtotal or total gastrectomy) for these lesions is also controversial[28].Antrectomy has been proposed to remove the gastrin producing G cells;however,failure may occur due to improper removal of those cells or because the ECL cells have become autonomous.This led to the suggestion of subtotal gastrectomy to allow adequate removal of the G cells while reserving total gastrectomy for those cases with substantial disease in the gastric fundus[29].Clinical management for type I gNETs while available,is hardly an effective option for long term management.The use of somatostatin analogue (octreotide) has been attempted by some authors;however,following discontinuation of the treatment it was noted that follow-up serum gastrin levels increased,and the tumor progressed[30,31].Type II lesions are usually treated by localizing and resecting the gastrinoma.Type III lesions are treated more aggressively with treatments including but not limited to subtotal,or total gastrectomies(depending on the location) with associated lymphadenectomy.The presence of metastatic disease in the liver is treated by resection if present in a resectable location otherwise arterial embolization or radioablation have a success rate of 50%[32].In cases of type III lesions with extrahepatic metastasis or recurrent disease,systemic cytotoxic chemotherapy or molecular targeted agents can be introduced.
CONCLUSION
gNETs are a group of tumors that are witnessing a rise in incidence of diagnosis due to an increase in upper GI endoscopy,and refinement of the endoscopic techniques.Those lesions while arise from the same group of cells,are different in etiology andtherefore differ in the natural disease progression.Identifying the type of gNETs is a collective effort of clinical and pathologic correlation,the correct grading and staging of these lesions is of paramount significance,due its impact on patient management and prognosis.Finally,it is worth noting that this area of digestive pathology is an active area of research and studies that will clarify the disease biology and improve its management.

Table2 Grading system of well-differentiated neuroendocrine tumors according to the 2017 World Health Organization classification
杂志排行
World Journal of Clinical Cases的其它文章
- Attention deficit hyperactivity disorder and comorbidity:A review of literature
- Dietary manipulation and testosterone replacement therapy may explain changes in body composition after spinal cord injury:A retrospective case report
- Risk factors,clinical features,and short-term prognosis of spontaneous fungal peritonitis in cirrhosis:A matched case-control study
- Incidence of portal vein thrombosis after splenectomy and its influence on transjugular intrahepatic portosystemic shunt stent patency
- Multiplex gene expression profile in inflamed mucosa of patients with Crohn’s disease ileal localization:A pilot study
- Analysis of the postoperative hemostatic profile of colorectal cancer patients subjected to liver metastasis resection surgery
