Role of endoscopy in acute gastrointestinal bleeding in real clinical practice: An evidence-based review
2019-02-27KyoungwonJungWonMoon
Kyoungwon Jung, Won Moon
Abstract Although upper gastrointestinal bleeding is usually segregated from lower gastrointestinal bleeding, and guidelines for gastrointestinal bleeding are divided into two separate sections, they may not be distinguished from each other in clinical practice. Most patients are first observed with signs of bleeding such as hematemesis, melena, and hematochezia. When a patient with these symptoms presents to the emergency room, endoscopic diagnosis and treatment are considered together with appropriate initial resuscitation. Especially, in cases of variceal bleeding, it is important for the prognosis that the endoscopy is performed immediately after the patient stabilizes. In cases of suspected lower gastrointestinal bleeding, full colonoscopy after bowel preparation is effective in distinguishing the cause of the bleeding and treating with hemostasis. The therapeutic aspect of endoscopy, using the mechanical method alone or injection with a certain modality rather than injection alone, can increase the success rate of bleeding control. Therefore, it is important to consider the origin of bleeding and how to approach it. In this article, we aim to review the role of endoscopy in diagnosis, treatment, and prognosis in patients with acute gastrointestinal bleeding in a real clinical setting.
Key words: Endoscopy; Gastrointestinal bleeding; Endoscopic bleeding control;Emergency bowel preparation; Bedside endoscopy; Second-look endoscopy
INTRODUCTION
Along with abdominal pain, gastrointestinal bleeding (GIB) is one of the most common conditions in the emergency department. Upper GIB (UGIB) is a major problem that has been declining over the past 20 years but still has a mortality rate of 2.1%[1]. Lower GIB (LGIB) has a mortality rate less than 5%, but it is common in older patients and those with intestinal ischemia and comorbidity[2].
GIB usually manifests as hematemesis (vomiting of blood or coffee-ground-like material), melena (black or tarry stools), and hematochezia. UGIB appears as hematemesis in 40%-50%, and as melena or hematochezia in 90%-98%, especially hematochezia in massive UGIB[3]. However, patients with LGIB typically present with hematochezia, but right-sided colonic bleeding or small bowel bleeding may show as melena[4]. Therefore, it is frequently difficult to distinguish between UGIB and LGIB based on only the initial symptoms of the patient[5]. In real clinical practice, it is necessary to approach the patient with melena and hematochezia based on the main symptom rather than UGIB or LGIB.
Early resuscitation of acute GIB is usually performed by the physician in the emergency room, but highly skilled endoscopists are needed to determine the cause and location of bleeding. In many cases, endoscopic treatment should be performed to stop bleeding and prevent recurrence[6]. Here, we will review the diagnostic,therapeutic, and prognostic roles of endoscopy in patients with clinical signs and symptoms of GIB.
ROLE OF ENDOSCOPY IN THE DIAGNOSTIC APPROACH
Timing of upper endoscopy for patients with hematemesis or melena
There is still a debate as to when endoscopy should be undertaken in patients with suspicious acute GIB. In particular, it is difficult to clearly distinguish the cause of UGIB from clinical symptoms alone. The causes of UGIB were summarized by frequency in Table 1.
When variceal bleeding is strongly suspected, endoscopy should be performed just after proper resuscitation when the patient is hemodynamically stable[7]. The guidelines of American Association for the Study of Liver Diseases recommend that endoscopy should be performed within 12 h for acute variceal bleeding[8]. Because gastroesophageal varix can be observed in more than 50% of patients with liver cirrhosis, and the 6-wk mortality of varix bleeding is about 20%, urgent endoscopy should be considered in patients with hematemesis and liver cirrhosis[7,9].
In the case of non-variceal UGIB, a previous Asia-Pacific Working Group consensus recommended endoscopic intervention be taken within 24 h of onset of bleeding in patients at high risk of pre-endoscopic assessment (e.g., Glasgow-Blatchford Score(GBS) ≥ 12; The GBS is a composite score of blood urea, hemoglobin, systolic blood pressure, pulse, history, and comorbidities)[10]. This consensus is similar to a recent cascade guideline of European Society of Gastrointestinal Endoscopy in 2018, which reported that endoscopy should be performed within 24 h after adequate initial management[11]. Therefore, it has been pointed out that, in patients who are hemodynamically unstable and presenting with massive hematemesis, endoscopy should not be done until after the patient is stabilized by resuscitation[12].
Several studies have investigated the role of emergency endoscopy within 12 h in non-variceal UGIB. A retrospective study of 361 patients found that patients undergoing emergency endoscopy had a 5-fold increase in the risk of adverse events including death, rebleeding, surgery, radiological intervention, or repeatedendoscopic management. In a subgroup analysis of that study, time to endoscopy was not significant as a predictor of worse outcome and was a weaker prognostic factor in patients with a high GBS score (≥ 12 points) than in patients with a low score[13]. Highrisk patients are more likely to undergo fluid therapy and rapid proton pump inhibitor (PPI) therapy before endoscopy because initial bleeding symptoms are more severe. This emergency medical therapy may be the most important factor to prevent a poor outcome from UGIB regardless of time of endoscopy.
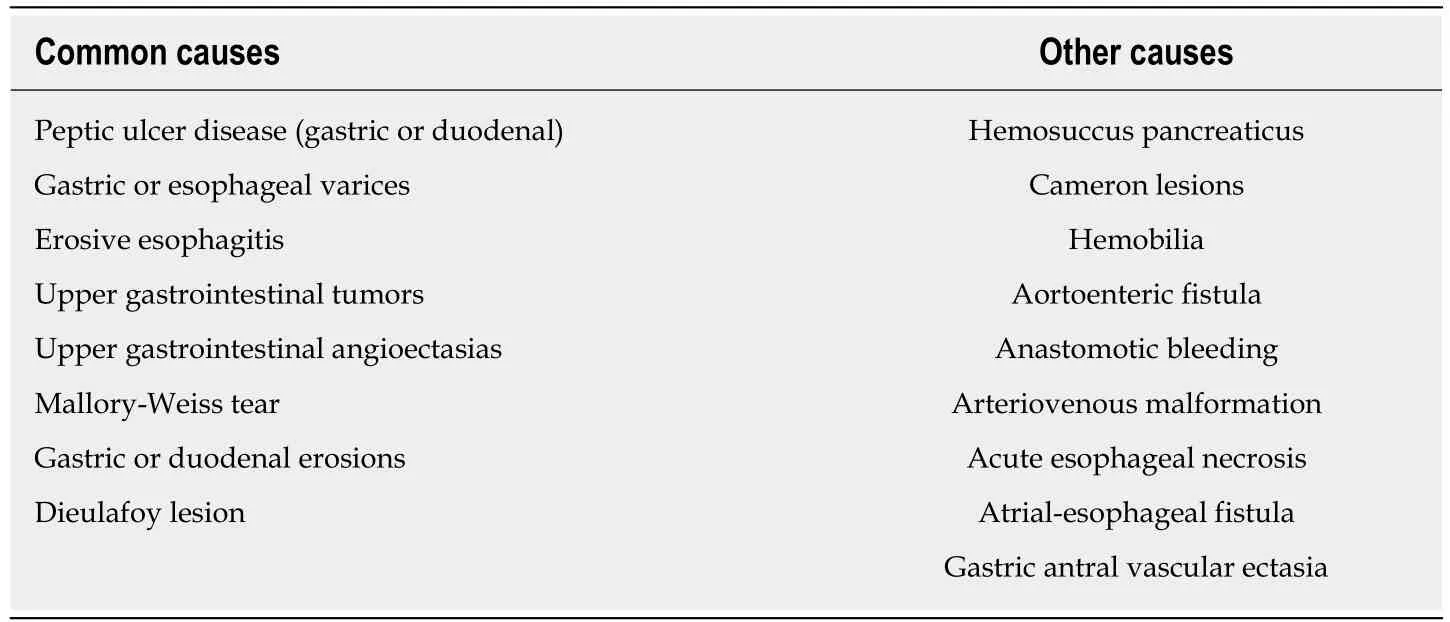
Table 1 Causes of upper gastrointestinal bleeding
A nation-wide cohort study by Laursen et al[14], which included 12601 peptic ulcer patients, suggested that patients with an American Society of Anesthesiology score of 3-5 points or who were hemodynamically unstable had a reduced rate of hospital mortality if they received an endoscopic intervention within 6 h-24 h after admission[14]. However, the exact timing within the 24 h is not yet clear. Another national survey conducted in the United Kingdom, which included 4478 patients,suggested an early endoscopy of fewer than 12 h after admission and, compared to endoscopy provided within 24 h, showed no association with lower mortality rate or need for surgery[15]. According to a cohort study in Singapore, the timing of endoscopy in high-risk UGIB patients with GBS > 12 is the most important factor related to all-cause mortality in hospitals[16]. The cut-off time of endoscopy that improved the survival rate was within 13 h from the onset of symptoms. Active hemorrhagic lesions requiring endoscopic hemostasis were frequent in patients who received endoscopy within 6 h, but it did not help in prevention of rebleeding,mortality, transfusion rate, or duration of hospitalization. Therefore, emergency endoscopy is not required within 6 h for all non-variceal UGIB.
In summary, recent guidelines and recent studies suggest that emergency endoscopy should be performed within 12 h if variceal bleeding is present or the patient is hemodynamically unstable[17]. In addition, endoscopy should be preceded by appropriate and prompt medical therapy, which includes fluid therapy and intravenous PPIs.
What is the best option for patients with hematochezia: Sigmoidoscopy or colonoscopy
Unlike UGIB, which is mostly divided into variceal bleeding and non-variceal bleeding, the causes of LGIB are variable, involving various clinical manifestations.Table 2 summarizes the causes of LGIB by category. Therefore, what should be done for patients with hematochezia? In cases of hemodynamic instability, emergency upper endoscopy should be considered while performing initial resuscitation because of the possibility of UGIB as mentioned above. However, if vital signs are stable, LGIB should be considered first.
In patients with hematochezia who are less than 50 years of age, anorectal disease predominates in about 90%, whereas the prevalence of colorectal cancer increases with age. Some researchers believe that total colonoscopy is not necessary for patients with hematochezia at age 50 or younger, and sigmoidoscopy alone is sufficient[18]. On the other hand, some authors suggest that colonoscopy be performed in all patients with hematochezia because it can help to diagnose associated diseases such as colorectal cancer and polyps[19]. In addition, patients who have undergone sigmoidoscopy might believe that full colonoscopy will reveal more dangerous and fatal diseases such as cancer. However, full colonoscopy can be required complete preparation and long observation time with pain. In some situations, anesthetics and long-term hospitalization are necessary[20].
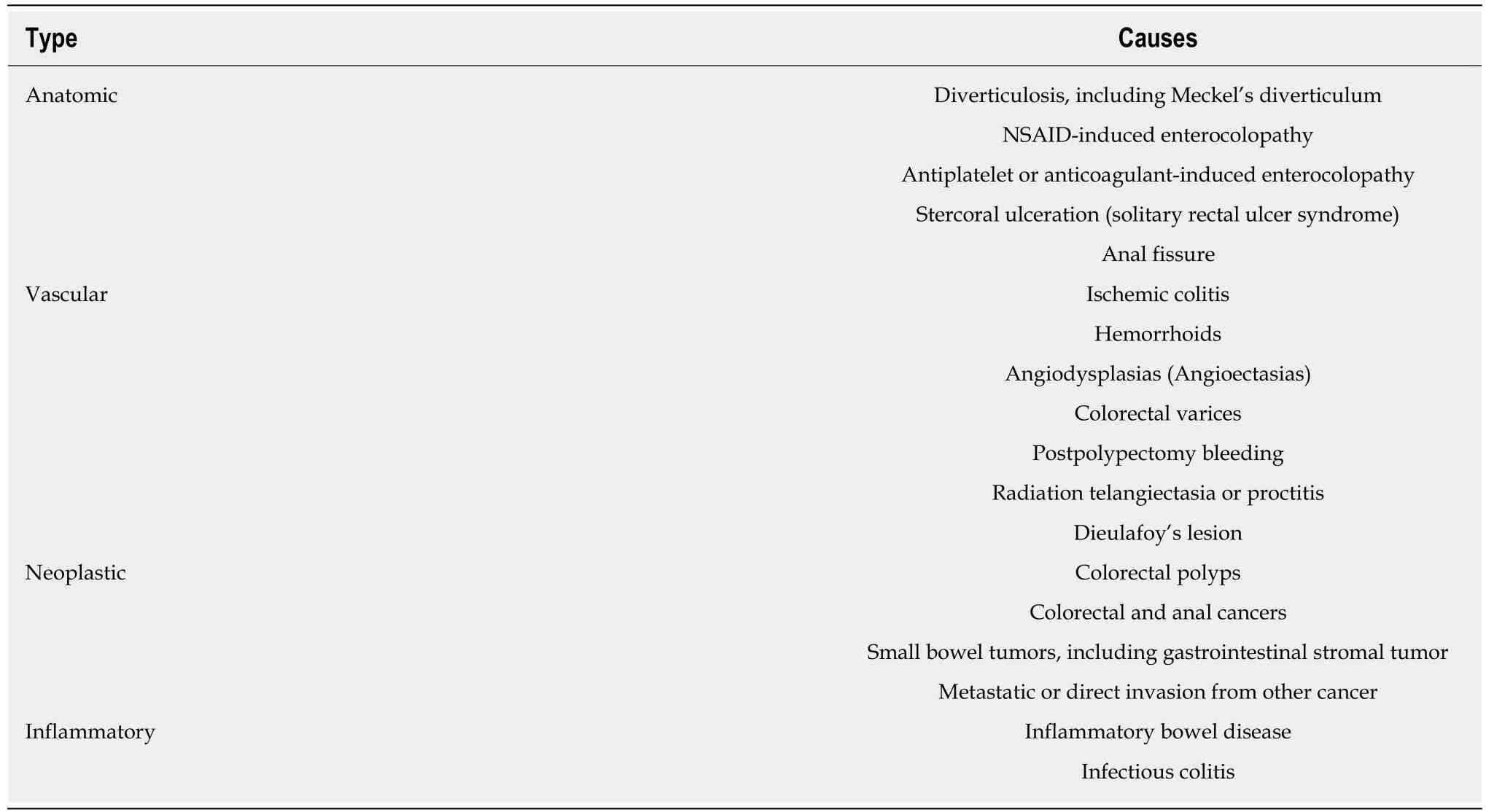
Table 2 Causes of acute small bowel and lower gastrointestinal bleeding by category
Currently, there is no clear consensus on whether patients with hematochezia should undergo full colonoscopy or sigmoidoscopy alone. To summarize the opinions of several researchers, sigmoidoscopy without bowel preparation can be performed to rule out bleeding from anorectal disease. However, to investigate the combined presence of colon polyp or cancer, we suggest that full colonoscopy be performed for the entire colon investigation, regardless of the presence of bleeding and the success or failure of hemostasis in the anorectal area.
In patients with suspected lower gastrointestinal bleeding, when is the best time to perform endoscopy?
The optimal timing of colonoscopy for acute LGIB is controversial. Three recent systematic reviews with meta-analysis have attempted to address the question of whether urgent colonoscopy improves the outcome of LGIB. Seth et al[21]evaluated six studies including two randomized controlled trials (RCTs) and four observational studies involving 23419 patients who received urgent or elective colonoscopy. Urgent colonoscopy is one that is performed within 8 h to 24 h of observing LGIB. The use of urgent colonoscopy increased the detection rate of stigmata for recent bleeding but did not reduce rebleeding, mortality, or surgical necessity[21]. Kouanda et al[22]evaluated 10172 patients with urgent colonoscopy and 14224 patients with elective colonoscopy in an analysis of 12 studies. Urgent colonoscopy was associated with increased use of endoscopic therapeutic intervention. The reason for the difference in the use of endoscopic therapy is that hemorrhoid band ligation was included as an intervention. However, there was no significant difference in localization of bleeding source, adverse event rates, rebleeding rates, transfusion requirement, or mortality.Moreover, a limitation was that the definition of urgent colonoscopy varies widely in every study from within 8 h to within 24 h[22]. The third meta-analysis was performed by Sengupta et al[23]and involved 422 patients in the early colonoscopy group (< 24 h)and 479 patients in the delayed colonoscopy group (> 24 h). There was no statistically significant difference in blood transfusion requirement, need for surgery, or inhospital mortality. Early colonoscopy showed a high correlation with detection rate of definite bleeding focus and endoscopic intervention[23].
The efficacy of early colonoscopy for reducing hospitalization, transfusion, and need for surgery is not clear. A timely colonoscopy is basically recommended as an initial diagnostic procedure for patients with acute LGIB because most guideline colonoscopy is highly accurate in detecting and treating the major causes of LGIB[4].
Is bowel preparation helpful before colonoscopy?
Bowel preparation is closely related to timing of colonoscopy. In urgent colonoscopy,there is no time to perform proper bowel preparation. However, if vital signs in patients with hematochezia suggest a low probability of UGIB, bowel preparation is recommended. Proper bowel preparation is associated with successful and safe colonoscopy insertion and the ability to detect and treat lesions and to prevent rebleeding of high-risk lesions such as diverticular bleeding or angiodysplastic bleeding[4]. Generally, urgent colonoscopy of acute LGIB is done with “rapid purge”bowel preparation of a high-dose (4 L-6 L) polyethylene glycol (PEG) formulation administered over 3 h to 4 h[4,24]. Stools should be checked frequently during preparation, and PEG should be provided until the patient is properly prepared. To facilitate rapid purge[25], a nasogastric tube for PEG administration could be used for patients with low risk of aspiration[4].
Prospective trials evaluating urgent colonoscopy with PEG preparations appear to achieve adequate visualization of approximately 90% of the colon with 4 L-6 L of PEG over 3 h-4 h. In an RCT by Laine et al[26], the patients received 4 L of PEG solution over 3 h for urgent colonoscopy within 12 h of presentation, and no colonic preparation quality was reported; only 7% of patients required repeat colonoscopy because of inappropriate preparation. Another RCT by Green et al[27]reported only 8% of poor preparation cases in an urgent colonoscopy group using a similar preparation method. Because of the good efficacy of the urgent colonoscopy preparation method,the American College of Gastroenterology recommends not performing non-prepared colonoscopy in patients with acute LGIB[4]. Repaka et al[28]assessed the possibility of unprepared hydroflush colonoscopy in patients with severe LGIB. Only 13 procedures were included in the study, and the patients were treated with tap water enema and immediate colonoscopy without oral bowel preparation. The rate of cecal intubation was as low as 69.2%. A definite source of bleeding was found in only 38.5%of the patients, and 25% of the patients had repeated bleeding during the same hospitalization[28].
Although there are not many studies on the safety of bowel preparation in acute LGIB patients, overall bowel preparation is considered safe. Niikura et al[29]evaluated the safety of preparation and performance of colonoscopy in 161 patients admitted with acute LGIB compared to controls without bleeding. There was no significant difference in adverse events between the bleeding group and control group. None of the patients had volume overload, aspiration pneumonia, or loss of mental status[29].In the guideline of the American College of Gastroenterology, precaution for aspiration is recommended for patients of old age and debilitation[4].
Approach to unknown origin gastrointestinal bleeding
Obscure GI bleeding (OBGIB) is defined as persistent or recurrent bleeding, despite of examination by esophagogastroduodenoscopy or colonoscopy[30]. OBGIB can be divided into overt bleeding with apparent gastrointestinal hemorrhage, such as hematochezia or melena, and occult bleeding, with repeated positive findings of fecal occult blood test or laboratory finding of iron deficiency anemia[30]. This OBGIB accounts for about 5% of all gastrointestinal bleeding, and it is known that more than 80% of these bleeding occur in the small intestine[31]. The development of capsule endoscopy has enabled the full examination of small intestine mucosa. The deviceassisted enteroscopy has enabled therapeutic endoscopy for these lesions[32]. Details of small bowel bleeding are not covered in this review, but only the common causes of small bowel bleeding are summarized in the Table 2 together with LGIB.
Is it helpful to perform video capsule endoscopy in the emergency room?
The first attempt to use video capsule endoscopy (VCE) in evaluating UGIB patients was performed in a multicenter study after nasogastric tube and conventional endoscopy[33]. Although bloody materials were detected significantly more often by VCE than by nasogastric tube aspiration, there was no difference in the identification of inflammatory lesions between VCE and sequential conventional endoscopy. VCE may be feasible and safe method in patients with acute UGIB.
Following promising initial results, a prospective RCT was performed. Seventy-one UGIB patients were randomly assigned to receive standard care including early endoscopic evaluation within 24 h or VCE using PillCam ESO 2 in the emergency room. The need for hospitalization was determined by the findings of VCE[34]. This study showed a greater than 70% reduction rate without serious adverse events in the VCE group. Comparing the VCE results with the initial GBS, hospital admissions were significantly reduced for patients recruited to receive the VCE. Based on these results, the authors considered VCE in the emergency room to be an appropriate screening tool to distinguish patients who do not need hospitalization.
Although the initial data seem to be promising, it would be premature to use VCE as a screening tool for decisions to hospitalize. To date, there is only one small RCT that supports using VCE as a tool for patient classification. In addition to an inappropriate duodenal visualization, the possibility of missing lesions in the fundus and other less accessible areas is a limitation of VCE. Moreover, it is difficult to train emergency doctors or specialists in for interpretation and set up of VCE in the emergency room[17].
RISK STRATIFICATION AND PRE-ENDOSCOPIC ASSESSMENT FOR GASTROINTESTINAL BLEEDING
In patients with suspected upper gastrointestinal bleeding including hematemesis or melena, treatment may be different according to the etiology of the bleeding.However, the evaluation of vital signs, hemodynamic status and appropriate fluid treatment are important in all patients[35]. If there are hypovolemic shock, rapid pulse rate, high blood urea nitrogen level, decreased urine volume at the time of presentation or previous history of acute bleeding, more aggressive initial monitoring,fluid treatment, and blood transfusion treatment are needed. However, if there is suspicion of massive bleeding, careful observation and follow up are necessary because early level of hemoglobin in acute bleeding may be normal[36].
The scoring system used when referring to the emergency department due to upper gastrointestinal bleeding can be divided into two types, one that includes endoscopic findings and the other that does not. The most commonly used scoring system is the Rockall score (RS) published by Rockall et al[37]In 1996. This scoring system predicts the likelihood of death within 30 d by using the five factors: Patient age,accompanying shock, co-morbidities such as heart, liver, and kidney, causative diseases of bleeding, and endoscopic bleeding stigmata. However, since there is a disadvantage that the endoscopic findings must be known, in practice, the preendoscopic RS that can be calculated with the three findings except the etiology of the bleeding and endoscopic findings is used. This is useful for predicting rebleeding and mortality risk[38].
In addition, the Glasgow-Blatchford score (GBS) developed in 1882, which was calculated from patient's symptoms, blood test, physical examination, and accompanying diseases before endoscopy, is widely used to predict the need for transfusion, endoscopic treatment, rebleeding rate and prognosis[39]. In particular, this scoring system has the advantage of being able to quickly and simply measure in the emergency room due to blood urea, hemoglobin, systolic blood pressure, pulse rate,presence of melena or syncope, liver disease, and heart failure.
Recently, AIMS65, a simpler scoring system, has also been proposed, including albumin, prothrombin time, mental state, systolic blood pressure and age over 65 years. It is easy to memorize, and it can be calculated objectively and easily[40]. In one study, mortality from AIMS65 scores ranged from 0.3% to 32%[41].
The initial treatment of patients with GI bleeding is to restore the stability of the hemodynamic circulation. In order to maintain blood vessel volume and hemodynamic stability, it is important to secure a large vein, and it is important to check whether it is accompanied by heart, kidney, and liver disease[35].
Although the nasogastric tube insertion is controversial, it can detect the need for immediate endoscopic hemostasis if blood is seen from the upper gastrointestinal hemorrhage to the nasogastric tube. However, it should be remembered that there may be a false negative due to duodenal hemorrhage[35]. One dose of antibiotic erythromycin administered 30 min-120 min before endoscopy is not recommended on a routine basis, but it is recommended to improve endoscopic visualization, reduce the need for transfusion and endoscopy, and reduce the length of hospital stay[42,43]
THERAPEUTIC ROLE OF ENDOSCOPY
In recent years, endoscopic techniques have improved the management of GIB,including peptic ulcer, variceal, diverticular, and angiodysplastic bleeding. Moreover,an increase in accessible and technologically advanced, well-trained, endoscopy center-related specialists has led to early diagnosis through endoscopic intervention[44].
Methods of endoscopic hemostasis for acute UGIB and LGIB include injection(usually diluted epinephrine or a special sclerosing agent), contact and non-contact thermal devices (unipolar or bipolar electrocoagulation, heater probes, and argon plasma coagulation), and mechanical devices (endoscopic clips and band ligation)[45].Diluted epinephrine injections of 1:10000 to 1:20000 dilution facilitate primary hemostasis of active bleeding; to reduce the risk of rebleeding, mechanical or thermal therapy to obtain definite hemostasis should follow immediately as a secondary method[4,46]. Randomized trials are insufficient in assessing the endoscopic hemostatic effects on acute GIB. The choice of a hemostasis method is generally determined by the cause and location of GIB, the ability to access the site, and the experience of the endoscopist.
In the following, we will discuss the most clinically relevant methods of endoscopic treatment for the four major types of GIB and describe the most effective procedure.
Peptic ulcer bleeding
Peptic ulcer bleeding, which accounts for 30%-60% of UGIB, has been the most studied. Although the classifications for peptic ulcer bleeding were created a very long time ago, an endoscopic classification called Forrest classification is widely used as a standard for endoscopic treatment. In most cases in the Forrest classification, Ia to IIa lesions have a rebleeding rate greater than 50%, in most of which active endoscopic treatment should be performed[47,48].
However, the Forrest classification is over 40 years old; recently, de Groot et al[49]evaluated whether this classification is useful in predicting the rebleeding and mortality of peptic ulcer bleeding and conducted a study to assess whether it could be simplified. They have simplified the Forrest classification as high risk (Forrest Ia),increased risk (Forrest Ib-IIc), and low risk (Forrest III). The rate of rebleeding in a total of 397 patients was highest (59%) in Forrest Ia peptic ulcers, but the rebleeding rates in Ib and IIc were similar. In subgroup analysis, prediction of rebleeding using the Forrest classification is more reliable for gastric ulcers than for duodenal ulcers.Simplifying this classification can reduce interobserver variability in classifying lesions but requires confirmation studies[49].
Traditionally, three endoscopic treatment methods of peptic ulcer bleeding have been used: Injection therapy, thermal therapy, and mechanical therapy. The question of whether monotherapy or combined modality therapy is more effective has been the subject of several trials and meta-analyses. The Cochrane review in 2014 evaluated 19 RCTs with 2033 patients and concluded that the second bleeding control method significantly reduced the risk of rebleeding and emergency surgery compared to epinephrine injection therapy alone[50]. Mortality was also reduced but was not statistically significant. Similar results were shown in other meta-analyses[51,52]. In a study published in 2016[52], involving 2888 patients, hemoclips alone or injection therapy combined with thermal therapy were more effective than injection therapy alone. Thus, it was concluded that epinephrine injection therapy should not be used as a monotherapy but in conjunction with a secondary therapy. After endoscopic treatment, adverse outcomes including perforation and therapy-induced bleeding can occur. They may be more common in endoscopic therapy than in medical therapy alone, but a meta-analysis showed no statistically significant difference (0.8% vs 0.1%)[53].
After endoscopic treatment for spurting bleeding or exposed vessel lesion, which is known to be highly rebleeding, high dose PPI is known to be an important medication to prevent rebleeding[54]. However, according to recent study, risk of rebleeding associated with Forrest Ib is very less compared Forrest IIa and IIb and may not require high dose IV PPI after successful endotherapy[55].
Variceal bleeding
Variceal bleeding is a common and very serious complication of portal hypertension.In previous studies, variceal bleeding in patients with liver cirrhosis has been reported to result in a mortality rate of up to 50%[56]. The use of vasoactive drugs,endoscopic management, and prophylactic antibiotics has improved mortality, but esophageal varix bleeding is still associated with 20% mortality within 6 wk[9]. It is important to stabilize patients prior to endoscopic treatment for variceal bleeding and to maintain an intravenous line for hemodynamic stability and a hemoglobin level of at least 7-8 g/dL through blood volume resuscitation[57]. Administration of prophylactic antibiotics such as intravenous quinolone or ceftriaxone is also necessary and could lower systemic bacterial infection and reduce mortality[58]. Vasoactive drugs such as octreotide, somatostatin, and terlipressin are recommended to be administered as soon as possible[56].
Endoscopic variceal ligation (EVL) is the treatment of choice for esophageal variceal bleeding and secondary prevention. The diagnosis of variceal bleeding in the setting of active bleeding is based on the appearance of bleeding varices, stigmata of recent bleeding including an adherent clot over varix or platelet plug called by white nipple marks, or presence of varices without definite active bleeding focus[59]. In a recent meta-analysis of 1236 cases in 14 studies reported by Dai et al[60], EVL is better in terms of major outcome including rebleeding, variceal eradication, and complication rate compared with endoscopic injection sclerotherapy but not in mortality. Therefore,EVL is the most effective first choice for esophageal varix bleeding. After acute esophageal variceal bleeding, repeated endoscopy with EVL until varix eradication is recommended, usually requiring 2 to 4 sessions of therapy[61]. The optimal interval of each EVL for secondary prevention has been undefined and usually ranges from 2 wk to 8 wk in studies evaluating repeated EVLs for secondary prevention.
Post-EVL band-induced ulcer bleeding may occur as a complication of EVL.Sinclair et al[62]reported that the incidence was just 2.8%, but was significantly associated with mortality. A high MELD score (MELD is an abbreviation for Model for End-stage Liver Disease, which is calculated using serum bilirubin, prothrombin time, and serum creatinine) was associated with more frequent development of bandinduced ulcer bleeding[62]. Transjugular intrahepatic portosystemic shunt (TIPS) or sclerotherapy can be considered as a treatment for band-induced ulcer bleeding, and pantoprazole for 10 d can reduce the ulcer size[63]. Moreover, rebleeding from band ulcers can be treated by hemostatic power or spray that used in management of peptic ulcer bleeding[64,65]. Recently, a study by Ibrahim et al[66]showed that immediate application of hemostatic powder is effective for early clinical course and endoscopic hemostasis in patients with acute initial variceal bleeding.
In addition, we could consider another management including esophageal balloon tamponade in patients of recurrent or refractory variceal hemorrhage despite of the most effective EVL treatment. The esophageal stent, which was mainly used for luminal GI stenosis, has been used in place of balloon in refractory variceal bleeding,showing statistically significant rate of treatment success and bleeding control[67]. As mentioned previously, TIPS treatment is used for recurrent and refractory variceal bleeding in patient with high-risk criteria (Child-Pugh B plus active bleeding at endoscopy or Child-Pugh C). However, early TIPS in a Child-Pugh B patient for recurrent variceal bleeding could be accelerating the development of acute-on-chronic liver failure and/or death. The careful decision of patients for TIPS is essential and other parameters should be considered, such as systemic inflammation, non-selective beta blocker-non-response and portal vein thrombosis[68].
Diverticular bleeding
The prevalence of colonic diverticula increases with aging, up to 30% at 50 years and 70% by 80 years[69,70]. Clinically significant bleeding occurs in 3%-15% of patients with colon diverticulosis, usually as a result of traumatic injury to the vasa recta at the neck or dome of the diverticulum. Nonsteroidal anti-inflammatory drugs (NSAIDs),aspirin, hypertension, and anticoagulants are known to be associated with diverticular bleeding[71]. Diverticular bleeding, which accounts for 20%-65% of LGIB,is known to stop spontaneously in about 75% of patients[72], and 25%-40% experience rebleeding within four years. Therefore, endoscopic treatment is required in patients with stigmata[73]. Only conservative therapy can be used in patients with a very high rate of rebleeding, and active endoscopic treatment is recommended and effective for long-term follow-up[24].
Various methods of endoscopic treatment have been used to treat colonic diverticular bleeding, including bipolar coagulation, epinephrine injection, clipping including the over-the-scope clip, and ligation such as endoscopic band ligation and endoscopic detachable snare ligation[4,22,74]. Due to lack of muscle layer in the colonic diverticulum, bipolar electrocoagulation is not recommended because of the risk of perforation. Epinephrine injection monotherapy has a high risk of rebleeding (20%)[74].A very useful technique is to directly attach the clip to the neck of the diverticulum containing the bleeding stigmata[75]. Band ligation is a familiar method for endoscopists. Recently, many studies have reported band ligation as a safe and effective hemostatic method for diverticular bleeding[76]. Ishii et al[76]have reported that simple band ligation of diverticular bleeding can be performed by both endoscopic expert and trainee with similar safety, efficacy, and procedure time.
In a meta-analysis recently conducted by Ishii et al[74]to confirm the efficacy of endoscopic treatment for colonic diverticular bleeding, 16 studies of 384 colonic diverticular bleeding patients were analyzed. Ligation therapy was found to be more effective than clipping in terms of avoiding trans-arterial embolization. Surgery,coagulation, clipping, and ligation were equivocal in terms of effectiveness for initial hemostasis and early recurrent bleeding. However, most studies in meta-analysis have been retrospective in design and small in sample size, making them susceptible to recall and selection biases. A recent prospective study of recurrent diverticular bleeding showed that 1-year rebleeding was higher in clipping (37%) than in ligation(11.5%), suggesting that band ligation is superior to clipping for treatment and elimination of colonic diverticular bleeding[77]. However, ligation may be limited due to inadequate suction in the presence of a small orifice or a large dome, and these cases have reported a high prevalence of rebleeding. Therefore, further studies are required for its application in real clinical practice.
Angiodysplastic bleeding
The prevalence of colonic angiodysplasias have various clinical presentations; for example, 1%-2% in screening colonoscopy and 40%-50% in patients with hematochezia[78]. Studies have shown that angiodysplasias account for 3%-15% of LGIB. The incidence of angiodysplasias increases with age, and more than two-thirds of lesions are observed in patients > 70 years[46]. The cause of angiodysplasias is degenerative changes of submucosal vessels with chronic intermittent low-grade occlusion[79]. These vessels are mainly located in the right colon including the cecum and ascending colon. Colonoscopy can reveal multiple angiodysplasias, which appear as flat, red lesions ranging in size from 2 mm to a few centimeters with tree-like morphology from a central feeding vessel.
Risk factors for angiodysplasia bleeding include older age, combined morbidities,presence of multiple angiodysplastic lesions, and use of anticoagulants or antiplatelet agents[80,81]. Clinical presentation may occasionally have intermittent hematochezia,melena, or occult bleeding with anemia. The detection rate of angiodysplasias by colonoscopy is 80% based on sensitivity[82]. However, with the use of sedatives,mucosal blood flow could be reduced, and colonoscopy can make it difficult to detect these lesions[83].
Contact and non-contact thermal coagulation with argon plasma are useful for endoscopic treatment of angiodysplasias. Argon plasma coagulation can be a preferred technique due to its ease of application, the therapeutic potential of a large surface area, and predictable penetration depth of the colon wall[84]. A low power setting of 30 W to 45 W and an argon flow rate of 1 L/min are used to reduce the risk of perforation to the thin wall of the right colon. The probe should preferably be 1 mm to 3 mm from the mucosal surface and should be applied at 1-2 s[25]. In follow-up data of 100 patients with angiodysplastic bleeding (including 31% colon lesions) for a median of 16 mo, argon plasma coagulation led to significantly improved hemoglobin level and reduced blood transfusion requirement without adverse events[84].
Recent developments for endotherapy in patients with acute GI bleeding
Various endoscopic therapies have been attempted in cases of failure of hemostasis due to general endoscopic treatment. The OTSC (Over-the-scope clip, Ovesco AG,Tübingen, Germany) system, which is inserted at the upper end of the endoscope, has been widely used in fistulas and perforations. However, it can be used in cases of continuous hemorrhage due to local injection or clipping[85]. In addition, the hemostasis of the bleeding site through the nano powder (Hemospray, Cook Medical,Winston-Salem, NC, USA) or starch (EndoClot Plus Inc., Santa Clara, CA, USA ) can be used when other hemostasis is not treated. Previously, it was difficult to distribute the powder or starch materials to the hemorrhagic lesion, but in recent years,disposable powder roots have been developed and can be used more easily[86,87].
ENDOSCOPY FOR CRITICALLY ILL PATIENTS WITH SUSPECTED GIB
Bedside endoscopy for intensive care unit patients
Patients in the intensive care unit (ICU) often have GIB from a variety of reasons. GIB in the ICU is an important event with serious complications that increase morbidity and mortality. Management of GIB in the ICU is difficult because most patients have complex poor prognostic factors; in most cases, they cannot be transferred to the endoscopy center. Therefore, bedside endoscopy is a good option for these patients[88,89].
In a recent study by Kim et al[88], 253 cases of bedside endoscopy and 69 cases of bedside colonoscopy were analyzed. The most common causes of UGIB were peptic ulcer and acute gastric mucosal lesion, and the causes of LGIB were ischemic colitis and rectal ulcer. The detection and treatment rate of bleeding focus were significantly increased in patients who underwent early upper endoscopy within 24 h. However, in patients with LGIB, early colonoscopy led to lower detection and hemostatic rate because of poor bowel preparation and bloody stool materials. Therefore, early upper endoscopy could be effective when UGIB is suspected in ICU patients, while in cases of colonoscopy, appropriate bowel preparation may first be necessary.
Prophylactic endotracheal intubation before upper GI endoscopy
Patients with UGIB have a particularly high risk of cardiopulmonary complications due to aspiration of blood and gastric contents and the presence of underlying comorbidities[90,91]. In a study in 2003 by Rudolph et al[92], high-risk patients with UGIB requiring ICU admission had 33.6% overall cardiopulmonary morbidity during hospitalization and 13.6% mortality. Upper GI endoscopy-related cardiopulmonary complications were common (4.1%), and new development of pulmonary infiltration was found in 14.1% of patients[92].
Prophylactic endotracheal intubation is performed to protect the airway and prevent aspiration during upper GI endoscopy and severe UGIB. It is thought to be effective in prevention of aspiration pneumonia, but there have been few studies. In a retrospective study conducted in 2009, of 307 patients with UGIB who received upper endoscopy, 53 underwent prophylactic endotracheal intubation, but cumulative incidence of cardiopulmonary complications, ICU or hospital length of stay and mortality were similar to non-intubated patients[93]. However, in a study by Hayat et al[94]in 2017, 200 patients were divided into groups of 100 based on need for intubation or not. In the intubation group, unplanned cardiopulmonary events were significantly higher than in the non-intubation group, and this difference between groups did not change after adjustment for presence of esophageal varices. Therefore, prophylactic endotracheal intubation should be carefully considered before upper GI endoscopy,
THE ROLE OF ENDOSCOPY IN PROGNOSIS AFTER GIB
Is there a difference in prognosis between endoscopic and clinical findings?
Endoscopic findings of GIB are observed, and successful hemostasis can improve the prognosis and increase the survival rate. However, endoscopic hemostasis is not always successful. If we know the factors that are likely to fail in endoscopic hemostasis, decisions can be made to change the modality.
In a previous report[95], the success of endoscopic treatment of peptic ulcer bleeding was reported to be 94%, followed by that of permanent hemostasis without rebleeding(82.5%). There was failure of endoscopic treatment or rebleeding in 17.5% of endoscopic treatments. The patients had significantly higher rates of active bleeding at the time of diagnosis, shock at admission, or low hemoglobin level. However,medication history, old age, and location of gastric ulcer had no effect on the failure rate of endoscopic bleeding control. In another large study involving injection and thermal therapy of 3386 patients with peptic ulcer bleeding, 98.6% had successful initial hemostasis, but 8.2% had rebleeding. Therefore, the final failure rate of bleeding control was 9.6%. When blood pressure was low, hemoglobin was less than 10 g/dL, fresh blood was observed in the stomach, and large or active ulcers were seen, the failure rate of endoscopic bleeding control was significantly higher[96]. In a study by Thomopoulos et al[97], 427 patients were endoscopically treated, with a failure rate of 20.1%. Endoscopic findings of spurting bleeding and duodenal ulcer on the posterior side or anastomosis site showed significant treatment failure. In summary,signs of severe bleeding, including shock, decreased hemoglobin level, fresh blood at the time of initial presentation, or ulcer with a large surface area and spurting blood,could lead to failure of the endoscopic procedure.
Variceal bleeding is affected by portal hypertension. The hepatic venous pressure gradient, which reflects portal pressure, is most important. In addition, the severity of liver disease reflected by the Child-Pugh class or MELD, encephalopathy, platelet count, history of alcoholism, and presence of portal vein thrombosis were found to be independent factors for failure of endoscopic variceal bleeding control[98,99]. Therefore,in high-risk patients with a high probability of failure of bleeding control, a preemptive TIPS should be prepared, and tamponade ballooning should be performed temporarily after retrials of endoscopic hemostasis[100]. Unlike bleeding in other diseases, variceal bleeding should be considered to be more affected by the severity of liver cirrhosis than by endoscopic findings[101].
The determinant of LGIB recurrence is the pattern used to achieve primary hemostasis. Predisposing factors of recurrent LGIB are primary hemostatic modality[102]; use of antiplatelet, anticoagulant agent, and NSAIDs; presence of chronic kidney disease or liver cirrhosis; and etiology of initial bleeding[4]. It is not clear how the proportional influence of these individual characteristics will affect the incidence of recurrent bleeding. In a retrospective study with 171 severe LGIB cases[103], the three causes of bleeding were diverticular bleeding, which was the most common cause,anorectal diseases, and angiodysplasia. In particular, 15% of the subjects were treated previously with antiplatelet agents and 9% with anticoagulants. During the mean follow-up period of 11 years, about one-third of the participants had recurrent LGIB.As noted in previous studies, LGIB is more likely to rebleed and affects older patients taking several medications. One of the most important points in diverticular bleeding and angiodysplasias bleeding, which comprise a large part of LGIB, is that they can stop spontaneously and rebleed at the same site or in other lesions.
Is routine second-look endoscopy necessary?
Recurrent bleeding occurs in 8%-15% of patients with peptic ulcer bleeding and increases mortality by 2 to 5 times. The goal of routine second-look endoscopy performed within 24 h after initial endoscopic hemostasis is to treat stigmata of peptic ulcer bleeding preemptively before rebleeding symptoms develop[17].
A meta-analysis based on eight prospective RCTs was conducted to evaluate whether rebleeding can be reduced by second-look endoscopy in very high-risk patients without high dose PPI[104]. The pooled data showed that second-look endoscopy reduced the need for surgery but not mortality. Moreover, there was no benefit in second-look endoscopy by subgroup analysis after exclusion of two trials with bleeding in high-risk patients[104,105]. In a recent randomized trial comparing second-look endoscopy and intravenous PPI infusion after endoscopic hemostasis for peptic ulcer bleeding[105], there were no differences in recurrence bleeding, need for surgery, and mortality between the two treatment strategies. In addition, second-look endoscopy did not appear to be cost-effective when offered to all patients.
The remaining problem is whether we can identify high-risk patients and obtain the benefit from second-look endoscopy with repeated endoscopic treatment for stigmata by the next day. In a study of 699 patients in Korea, use of NSAIDs, a large volume of transfused blood, and failure of second-look endoscopy were risk factors for rebleeding after endoscopic intervention[106]. A study in Taiwan, involving 316 patients who received high dose PPI after endoscopy, attempted to formulate predictive scores using endoscopic monotherapy and serum albumin level[107]. This score indicated that the receiver operating characteristic curve would help predict the need for routine second-look endoscopy, but the results were insufficient. However, a recent multicenter prospective study showed that the success of initial hemostasis, the use of NSAIDs, and the large number of blood transfusions were independent risk factors for rebleeding. Therefore, scheduled second-look endoscopy could be helpful for patient with unsatisfactory initial endoscopic hemostasis, use of NSAIDs, large amounts of blood transfusions[108].
Therefore, the risk assessment method and preemptive endoscopic treatment for selecting high-risk patients who require second-look endoscopy are not clear.However, since many clinicians are practicing prophylactic second-look endoscopy for patients with a high risk of rebleeding, further studies on risk classification and selecting the method for routine second-look endoscopy are needed.
CONCLUSION
The symptoms of bleeding in the GI tract that are encountered in real clinical practice are mainly melena, hematemesis, and hematochezia. When a patient with these symptoms presents to the emergency room, endoscopic diagnosis and treatment are considered together with appropriate initial resuscitation. For better prognosis in cases of suspected variceal bleeding, it is paramount that endoscopy is performed immediately after the patient is stabilized, and it would be sufficiently effective for endoscopy to be undertaken within 24 h from symptom development for non-variceal UGIB. In cases of suspected LGIB, sigmoidoscopy may be initially performed if there is a strong suspicion of anorectal bleeding. However, on the whole, full colonoscopy after bowel preparation is effective for distinguishing the cause and location of bleeding and treating with hemostasis.
There are three methods used to perform hemostasis by endoscopy: Injection,thermal, and mechanical therapy. Using a mechanical method or injection therapy combined with other modalities, rather than injection therapy alone, increases the success rate of bleeding control. In patients in the ICU, bedside endoscopy may be effective, but prophylactic intubation is still controversial. Proper endoscopic hemostasis can affect prognosis and prevent rebleeding. Routine second-look endoscopy does not affect the outcome of hemostasis, but it may be helpful in selected patients with a high risk of rebleeding. From the emergency room to discharge of the patient, the contents of this review are summarized in Figure 1.
In conclusion, the role of endoscopy in GIB is very important, and many guidelines have been developed about endoscopic treatment for specific bleeding diseases.However, there are still parts that have not been established. Especially, further studies on prophylactic intubated endoscopy, routine second-look endoscopy and emergency capsule endoscopy issues are needed.
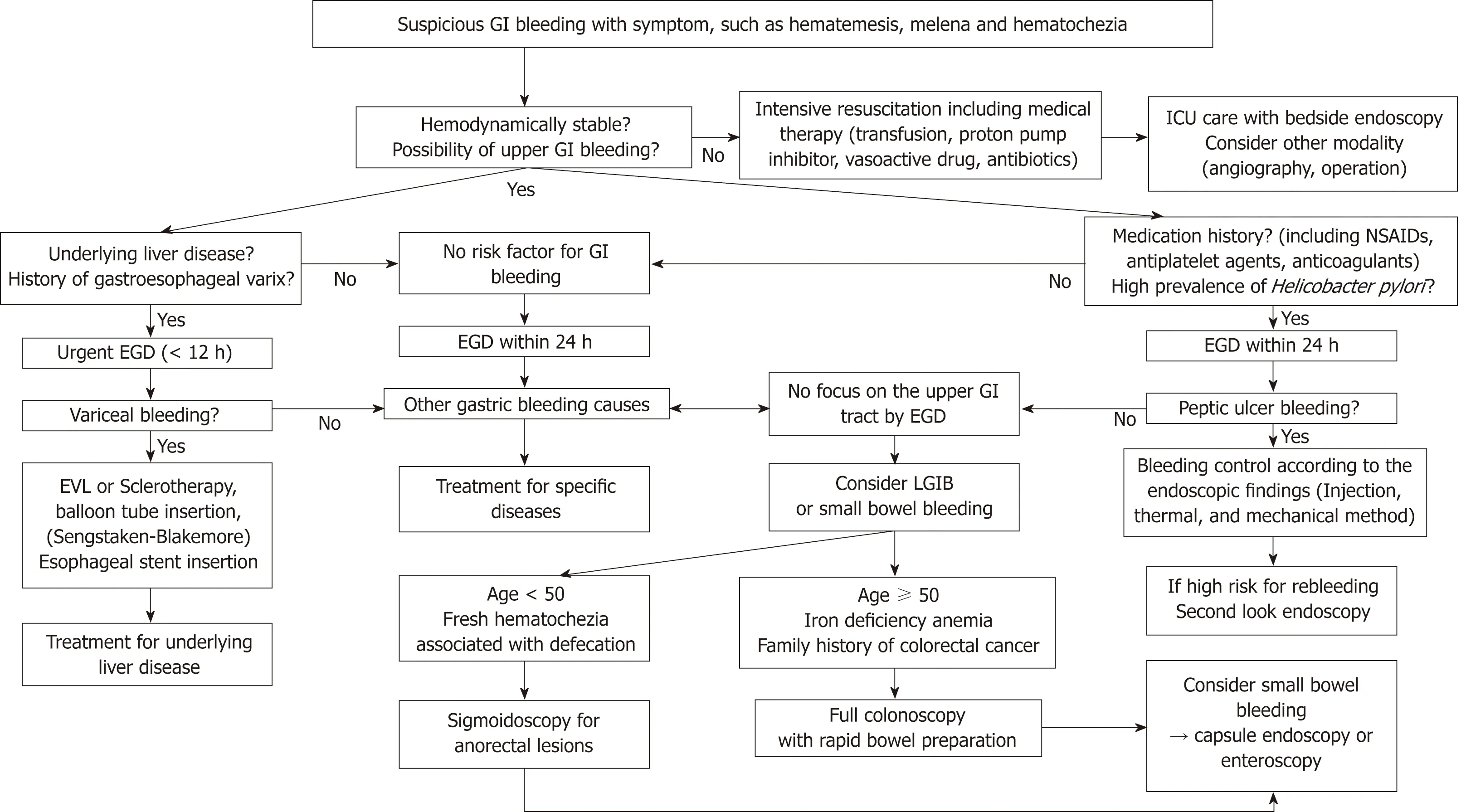
Figure 1 Flowchart of assessment and management of patients with suspicious gastrointestinal bleeding. GI: Gastrointestinal; EGD:Esophagogastroduodenoscopy; NSAIDs: Non-steroidal anti-inflammatory drugs; ICU: Intensive care unit; EVL: Endoscopic variceal ligation; UGIB: Upper gastrointestinal bleeding; LGIB: Lower gastrointestinal bleeding.
杂志排行
World Journal of Gastrointestinal Endoscopy的其它文章
- Role of endoscopy in the management of primary sclerosing cholangitis
- Radiofrequency and malignant biliary strictures: An update
- Endoscopic ultrasound-guided drainage of the biliary system:Techniques, indications and future perspectives
- Spectrum of gastrointestinal involvement in Stevens - Johnson syndrome
- No significant difference in clinically relevant findings between Pillcam® SB3 and Pillcam® SB2 capsules in a United States veteran population
- Age, socioeconomic features, and clinical factors predict receipt of endoscopic retrograde cholangiopancreatography in pancreatic cancer
