Synthesis,Crystal Structure and Thermal Stability of Copper Complex of 1,3-Propane Diamine
2019-02-20MEIJieTIANHuixiangXIAYuyanWUYewenZHOUHong
MEI JieTIAN HuixiangXIA YuyanWU YewenZHOU Hong
1.School of International Education,Wuhan Institute of Technology,Wuhan 430074,China;
2.School of Chemical and Environmental Engineering,Wuhan Institute of Technology,Wuhan 430205,China
Abstract:An unexpected complex with the maximum crystal size of about 7 mm×3 mm×5 mm was prepared by a reaction of CuCl2with 1,3-propane diamine(pn)in methanol.The characterization results with infrared spectroscopy,elemental analysis and X-ray diffraction show that the complex exhibits three dimensional network structure,which is contributed to the hydrogen bonding and electrostatic interactions between coordination ion[CuCl(pn)2]+and its counter anion Cl-,where CH3OH also participates the hydrogen bonding interactions.In addition,thermogravimetric analysis and differential scanning calorimetry techniques show that the thermal stability of synergistic interactions increases in the order of electrostatic,coordination and hydrogen bond.The synergistic interactions are in favor of the formation of bigger size crystals of the complex.
Keywords:1,3-propane diamine;crystal structure;copper(II)polymer;chlorinum-containing-hydrogen bond
The nitrogen-containing ligandshave strong coordination ability with most of metal ions and are capable to form variety ofcomplexes[1-6].The structures of these complexes vary with various kinds of factors such as ligands,metal ions as well as the anions introduced by metalsalts.Vezzosiand coworkers[7]found that 1,3-propane diamine (pn)reacting with CuCl2·2H2O with mole ratio of Cu(II):pn=1∶2 produced a dinucelar copper(II)complex with a pn as a bridged group.However,the same reaction with the mole ratio of1∶2 led to amononuclear copper(II)complex[8].Besides the coordination interactions in the complexes,there are also others such as hydrogen bond and p-p stacking,which are attributed to the formation of final space configuration.In the reported complexes,Cl-is found to exhibit multifunction.Llobet and coworkers[9]synthesized a chloro-brideged dimer([Cu2Cl2(pn)2]Cl2)by the reaction of pn and CuCl2with mole ratio of 1∶1 in methanol,where Cl-exhibited strong coordination ability, hydrogen bonding and electrostatic interactions. The researches on the molecular structure will provide useful information not only to understand the constitution of the substances but also to reveal reasons for the existence of some unknown ones. Although many polyamine copper (II)complexes have been reported[10-11],the complex with formula of[CuCl(pn)2]Cl·CH3OH has not been reported yet.In this work,this complex with unexpected maximum crystal size of about 7 mm×3 mm×5 mm was synthesized.The structure and the thermal stability of the complex have been thoroughly studied by infrared spectrum,X-ray diffraction,elementary and thermogravimetric analyses.
1 Experimental
1.1 Materials
Methanol was purified to anhydrous one by the general method,and other solvents and chemicals we used were of analytical grade and used as received.1,3-propanediaminewaspurchasedfrom Aladdin(Shanghai,China)and used as received.
1.2 Synthesis of complex 1
The solution of CuCl2(1.709 9 g,10 mmol)was added to methanol(20 mL).Then,30 mL methanol solution containing pn(1.487 4 g,20 mmol)was added dropwise under magnetic stirring. The resulting solution was stirred at room temperature for 24 h and filtered.The blue-black crystals suitable for the X-ray diffraction analysis were obtained by the diffusion of ethyl acetate into the filtration for a month.Yield:56%.Anal Calcd for C7H24Cl2CuN4O(%):C,26.71;H,7.69;N,17.80.Found(%):C,26.34;H,7.26;N,17.58.FTIR(KBr:cm-1):ν(C-H)2 943 cm-1,ν(N-H)3 446 cm-1,3 371 cm-1,ν(C-N)1 168 cm-1.
1.3 Physical measurements
A vector 22 FI-IR spectrophotometer using KBr disc was used to record on IR spectra.And a Vario EL III CHNOS elemental analyzer was used to perform on the Elemental.Thermogravimetric analysis(TGA)was performed on a SDT Q600Q50 TGA(USA).The obtained samples(20 mg)were placed in a platinum crucible,and heated from 25℃to 800℃in Ar atmosphere at a heating rate of 20 ℃·min-1.
1.4 Crystal structure determination
A single crystal with dimensions of 3.2 mm×2.7 mm×2.5 mm was mounted on a SMART-CCD area-detector diffractometer equipped with a graphitemonochromatic MoΚα radiation(λ=0.710 73 nm).The SMART and SAINT programs were used to reduce date and refine cell,respectively.The structure was solved by direct methods(Bruker SHELXTL) and refined on F2by full-matrix least-squares(Bruker SHELXTL)using all unique data. The non-H atoms were considered as anisotropic thermalparameters.Hydrogen atoms were located geometrically and refined in a riding mode.
2 Results and discussion
2.1 Crystal structure
The complex was obtained by one-pot reaction at room temperature.The result of the elemental analysis for the complex matched well with the complex'scomposition.As shown in Fig.1,the band at 2 942 cm-1can be assigned to the stretching vibration of CH2.The absorption peaks at 3 446 cm-1and 3 371 cm-1are due to the existence of NH.And C-N stretching vibration is found in 1 168 cm-1.These results are in agreement with the structure of the complex.The morphology of the complex is shown in Fig.2(a).They were black block with the maximum crystal size of about 7 mm×3 mm×5 mm.The crystallographic data and details about the data collection are presented in Table 1.And selected bond lengths(nm)and angles(°)relevant to the metal coordination spheres of the complex are listed in Table 2.Some hydrogen bond date are shown in Table 3.
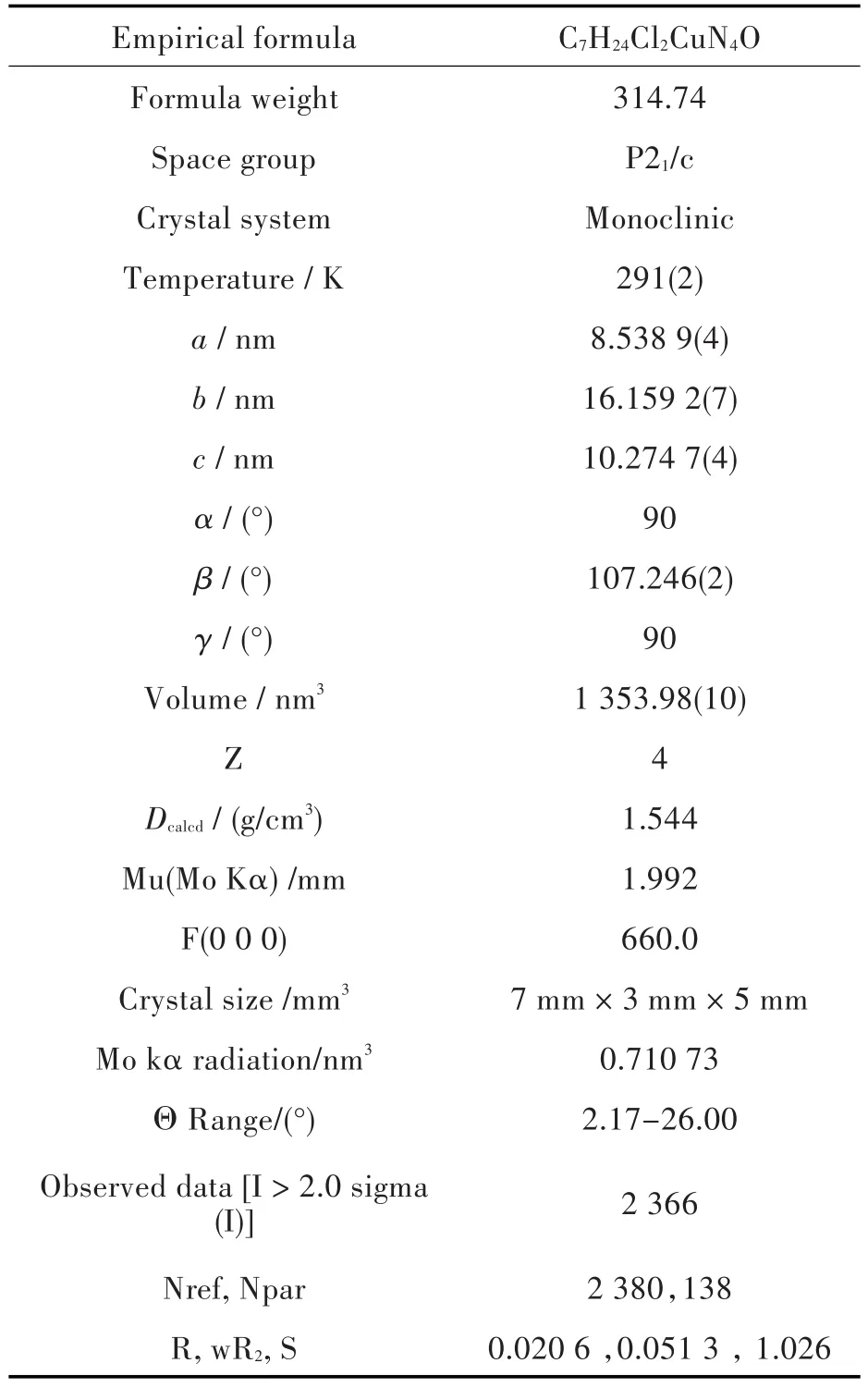
Tab.1 Crystal data and structure refinement for the complex
Themolecularstructureofthecomplexis shown in Fig.2(b).The complex crystallizes in monoclinic space group.It contains one coordination ion [Cu(pn)2Cl]+,a counter anion Cl-and one CH3OHmolecule.Each Cu(II)in the[Cu(pn)2Cl]+unit coordinates with four nitrogen atoms from two pn molecules and one Cl-. The coordination configuration of Cu(II) can be approximately described as a square pyramid as ascertained by Reedijk'st factor of 0.30[12-15].The four nitrogen atoms from two pn units are nearly coplanar constituting the base plane of the pyramid.The distances of Cu-N(1),Cu-N(2),Cu-N(3)and Cu-N(4)are 2.025,2.038,2.030 and 2.027 nm,respectively.As shown in the Fig.2(b),Cl(1)occupies the apical position with the distance of Cu-Cl(1)of 2.717 nm,and Cu-Cl(1)is almost perpendicular to the base plane with the angle of 88.0°.In the crystal structure,one coordination unit[Cu(pn)2Cl]+connects with three Cl(2)anions,two Cl(1)anions and one CH3OH through Cl-containing hydrogen bonding interactions,shown in Fig.2(c).The uncoordinated Cl(2)resides in the voids of molecular architecture,acting as a counterion and a hydrogen acceptor to three pnwith N-H ××× Cl interaction and oneCH3OH with C-H ××× Cl interaction.The distances of N×××Cl are in the range of 3.207 nm-3.420 nm,and the angle of N-H×××Cl is of 111.0°-176.6°.CH3OH involving hydrogen bond parameters as well as the detailed information about the length and angle of N-H×××Cl are listed in Table 2.And the three dimensional network is shown in Fig.2(c).The 3D network frame of the complex ismainly contributed to the hydrogen bonding and electrostatic interactions between coordination ion[CuCl(pn)2]+and its counter anion Cl-.
2.2 Thermal stability analysis
The thermalstability ofthe complex was quantitatively determined by TGA.The plots of weight loss and relative heat flow of the complex verse temperature are exhibited in Fig.3.The weight loss between 25℃and 164℃could be assigned to the escape of methanol from the complex,the weight loss 10.2%matched very well with the content of onemolar CH3OH in the complex formula.The weight lose between 164℃and 230℃was corresponding to the dissociation of one pn,and the loss of the second pn happened in the range of 230-380℃,the relative weight losses were in agreement with the relative molecule's dissociation.From 380℃to 800℃,the weight loss could be attributed to the decomposition of CuCl2,where at 430℃,CuCl2changed into CuCl,then it gradually decomposed into Cu and Cl2..The content of residual sample was 22.87%at 789℃,which was slightly larger than the content(20.19%)of Cu in the formula of the complex.According to the above analysis,the thermal decomposition scheme for the complex can be proposed,and the scheme for the suggested procedure can be described as Scheme 1.Differential scanning calorimetry has exhibited three endothermic reactionsand two exothermic reactions.Methanol molecule produces a small sharp endothermic peak centered around 164℃,two pn molecules produce two endothermic peaks at 230℃and 330℃,respectively.And the decomposition of CuCl2exhibits two exothermic peaks at 397℃and 502℃,respectively.The results show that methanol molecule in lattice can be easily escaped from the complexbecauseof its weak hydrogen bonding interactions with the other components in the complex.The decomposition of two pn molecules at highertemperature range indicatesthatpn has strongercoordination interactionswith copper(II)ions.Cl-changed into Cl2and escaped from the sample at temperature range from about 400℃to 800℃,giving a strong support with the additional electrostatic interactionsbetween Cu(II)and Clbesides their coordination interactions.

Tab.2 Selected bond distances(nm)and bond angles(°)for complex
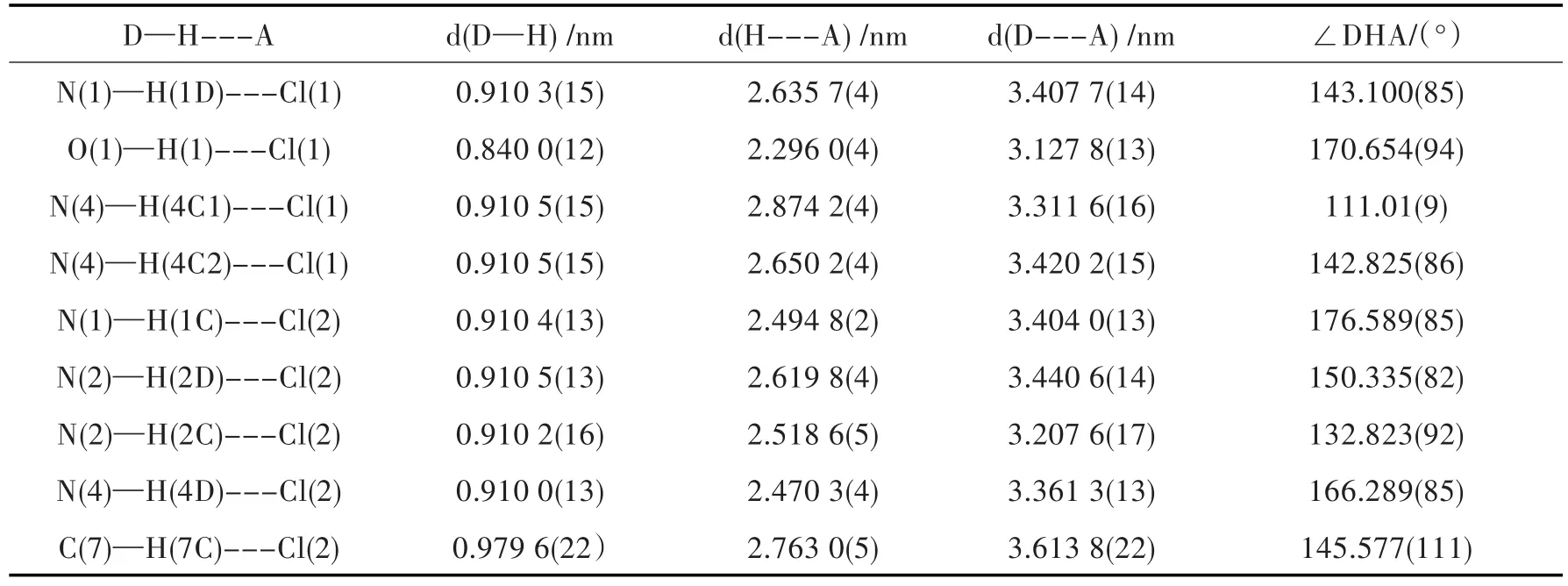
Tab.3 Hydrogen bond date
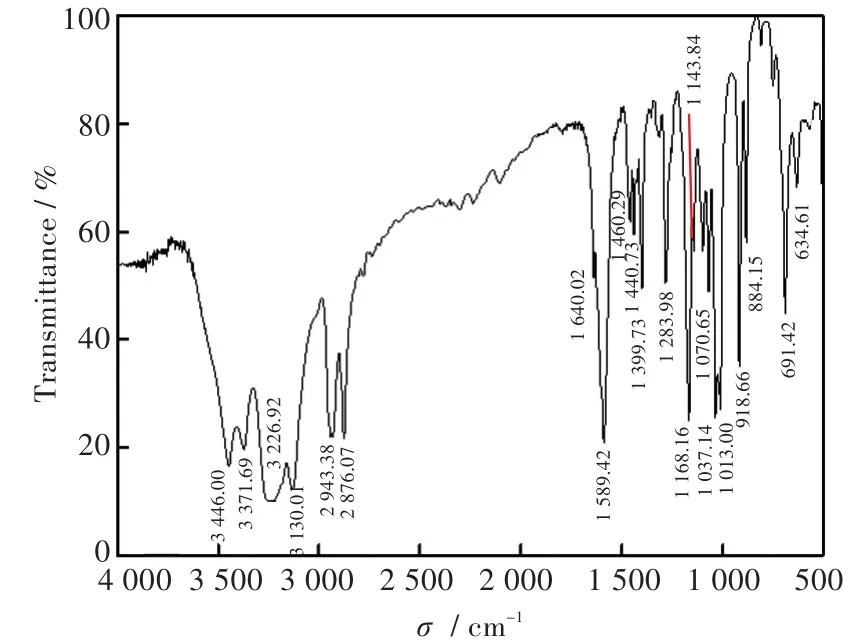
Fig.1 FT-IR spectrum of the complex
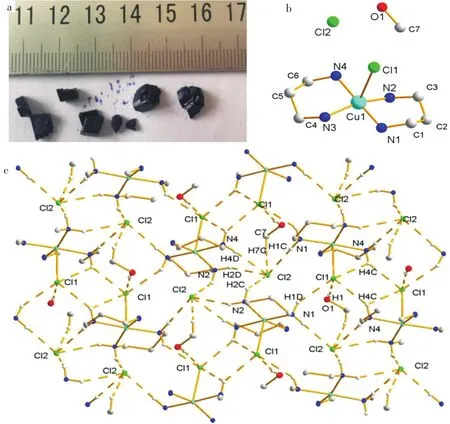
Fig.2 (a)The morphology of the complex,(b)the molecular structure of the complex(Symmetry code a:2-x,0.5+y,1.5-z),(c)three-dimensional network of the complex showing the hydrogen bond interactions.The atoms that have not participated in the hydrogen bond interactions have not been marked for clarity
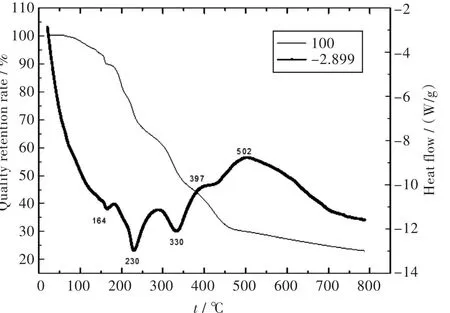
Fig.3 TGA and DSC curves of the complex
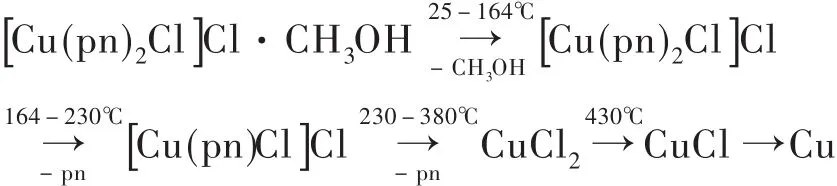
Scheme 1 The thermal decomposition scheme for the complex
3 Conclusion
A three-dimensional network complex derived from the reaction of 1,3-propane diamine and Cu(II)wassuccessfully obtained.The unitare joined together by the hydrogen bonding interaction in the form of N—H---Cl and electrostatic interactions.The resultsshow thatthe thermalstability is decreased in the order of electrostatic,coordination and hydrogen bond.The synergistic interactions contribute to the formation of bigger size crystals of the complexes.The study provides useful information to understand the constitution of the chloridion-containing substances.
