Current methods for the maturation of induced pluripotent stem cellderived cardiomyocytes
2019-02-20PranavMachirajuStevenGreenway
Pranav Machiraju, Steven C Greenway
Abstract Induced pluripotent stem cells (iPSCs) were first generated by Yamanaka and colleagues over a decade ago. Since then, iPSCs have been successfully differentiated into many distinct cell types, enabling tissue-, disease-, and patientspecific in vitro modelling. Cardiovascular disease is the greatest cause of mortality worldwide but encompasses rarer disorders of conduction and myocardial function for which a cellular model of study is ideal. Although methods to differentiate iPSCs into beating cardiomyocytes (iPSC-CMs) have recently been adequately optimized and commercialized, the resulting cells remain largely immature with regards to their structure and function,demonstrating fetal gene expression, disorganized morphology, reliance on predominantly glycolytic metabolism and contractile characteristics that differ from those of adult cardiomyocytes. As such, disease modelling using iPSC-CMs may be inaccurate and of limited utility. However, this limitation is widely recognized, and numerous groups have made substantial progress in addressing this problem. This review highlights successful methods that have been developed for the maturation of human iPSC-CMs using small molecules,environmental manipulation and 3-dimensional (3D) growth approaches.
Key words: Induced pluripotent stem cells; Induced pluripotent stem cell-derived cardiomyocytes; Regenerative medicine; Stem cell biology; Translational research
INTRODUCTION
The discovery of induced pluripotent stem cells (iPSCs) by Takahashi et al[1]launched a novel field of medicine. The ability to differentiate human iPSCs (hiPSCs) into various cell types allows for the generation of patient-, disease- and tissue-specific cells. These cells enable precise disease modelling, in vitro drug testing, and clinical regenerative medicine approaches[1,2]. After a decade of research, iPSCs can now be successfully differentiated into hepatocytes[3], cardiomyocytes[4,5], neural cells[6,7],adipocytes[8]and many other cell types[9].
Cardiovascular disease is the greatest cause of mortality worldwide[10]. As such,modelling these diseases in vitro is of paramount importance to advance our understanding of disease and allow the development of new drug therapies.Cardiomyocytes derived from human iPSCs (hiPSC-CMs) enable the creation of a patient-, heart-, and disease-specific in vitro model[5,11]. This is potentially most useful for the study of very rare cardiac disorders, including metabolic cardiomyopathies[12].These hiPSC-CMs are remarkably powerful as they replicate the genome of the patient donor and allow characterization of various diseases and drugs in a noninvasive manner[2]. In addition, their ability to contract allows for characterization of contractility and can thus serve as an accurate and translatable cardiac drug model[13].Recent studies have also showcased hiPSC-CMs’ ability to successfully engraft in a host organism[14,15]. One published study used macaque monkeys as a model for cardiomyocyte transplant outcomes. Transplanting human embryonic pluripotent stem cell derived-cardiomyocytes (hEPSC-CMs) through an intra-myocardial injection allowed the cells to graft with the host. Once attached, these cells showed crucial electromechanical coupling with the host as demonstrated by echocardiography[15].Similar regenerative medicine studies have also been performed using small (guinea pigs) and large (pigs) animal models[16,17].
Over the past few years, the efficiency of hiPSC-CM generation has been significantly improved. Methods involving modulation of the GSK and Wnt pathways using small-molecule inhibitors have been widely used[5,18]. In addition, use of BMP and Activin A, along with the Matrigel sandwich method have proven successful[18].Commercial kits such as those from STEMCELL Technologies (Vancouver, BC,Canada) and ThermoFisher Scientific (Carlsbad, CA, United States) have also entered the market and provide researchers with increased reproducibility and the ease of simplified protocols (Figure 1). Traditionally, hiPSC-CM generation has been characterised through flow cytometry staining for Troponin T (TNNT2), a cardiacspecific protein, in addition to visual qualification of spontaneously beating cell clusters. Current protocols allow for the production of > 80%-90% TNNT2-positive hiPSC-CMs. This showcases the field’s success in achieving high-purity cardiomyocyte differentiation[2]. Through the use of lactate metabolic selection, > 99%TNNT2-positive cells have been successfully derived[19]. The derivation of highlypurified cardiomyocyte populations represented an important step forward for the field of cardiac regenerative medicine.

Figure 1 Human induced pluripotent stem cell-derived cardiomyocytes.
Although hiPSC-CMs are now being produced with high efficiency, an important problem remains. The hiPSC-CMs generated with current protocols and commercial kits are qualitatively and quantitatively immature[2,20,21]. For example, in addition to immature calcium handling, hiPSC-CMs display immature ultrastructural and electrophysiological features, low expression of key maturation markers, and rely on glycolysis for their metabolism as opposed to fatty acid metabolism[2,20,22]. Immature cardiomyocytes have important differences when compared to adult cardiomyocytes and these differences may cause inaccurate disease modeling or drug testing and lead to unsuccessful clinical translation. For example, the effect of cardiac drugs on contractile characteristics may be inaccurate when using an immature model.However, given that hiPSC-CMs are being derived from pluripotent cells, it is not unexpected that the initial differentiated cells generated will be immature or fetal in their characteristics. It is therefore reasonable to expect that an additional maturation protocol (Figure 2) will be necessary to generate cells that truly reflect the in vivo tissue. As such, many research groups are currently focussing on methods to promote the maturation of hiPSC-CMs so that they are suitable for accurate disease-modeling and clinical applications. Methods evaluated to date include electrical stimulation,mechanical stimulation, modulation of carbon source, growth on various substrates,and the development of 3D culture conditions or organoids. Studies have also shown the positive effect of prolonged culture time on hiPSC-CMs[23,24]; however, culturing hiPSC-CMs for >90 d is neither time- nor cost-efficient and, given that these cells are usually cultured without antibiotics, remains a fraught enterprise. Therefore, other approaches must be used to create adult-like hiPSC-CMs within a reasonable time frame. Current protocols for hiPSC-CM production have failed to mature these cells due to a lack of knowledge regarding the mechanisms of heart maturation in vivo. At present, the field of cardiac regenerative medicine does not know the correct secretory factors, environmental cues, and external stimulation necessary to achieve proper adult-like cardiomyocytes. Maturing hiPSC-CMs is key to fully realizing the potential of these cells. Without proper maturation, hiPSC-CMs could cease to be clinically relevant. This review will examine the current methods for the maturation of iPSCCM and suggest a way forward for the field.
ADULT CARDIOMYOCYTES VS hiPSC-CMs
Typically, adult cardiomyocytes differ from hiPSC-CMs in 4 important ways: (1) the expression of specific genes; (2) differing structural features; (3) altered metabolism;and (4) contractile function (Table 1)[22].
Gene expression
Adult cardiomyocytes express high levels of important structural genes such as MYH7 (myosin heavy chain 7), N2B (cardiac titin), cTNi (troponin I), and SERCA2(sarcoplasmic reticulum ATPase)[22]. Adult cardiac heart tissues express high levels of genes such as ITPR3 (inositol-1,4,5-triphosphate), KCNH2 (potassium voltage-gated channel), CAV3 (caveolin 3), and RYR2 (ryanodine receptor 2)[2]. The importance of CASQ2 (calsequestrin 2), COX6A2 (cytochrome oxidase), S100A1 (calcium binding protein 1), SCN5A (sodium voltage-gated channel alpha subunit 5) and MYOM2/3(myomesin-2/3) as markers of maturation has also been demonstrated[25]. In contrast,hiPSC-CMs display high levels of MYH6 (myosin heavy chain 6) as opposed to MYH7, predominantly display the N2A isoform of cardiac titin instead of N2B[22]and have lower expression of other genes that are highly expressed in adult cardiomyocytes[26,27].
Structural features
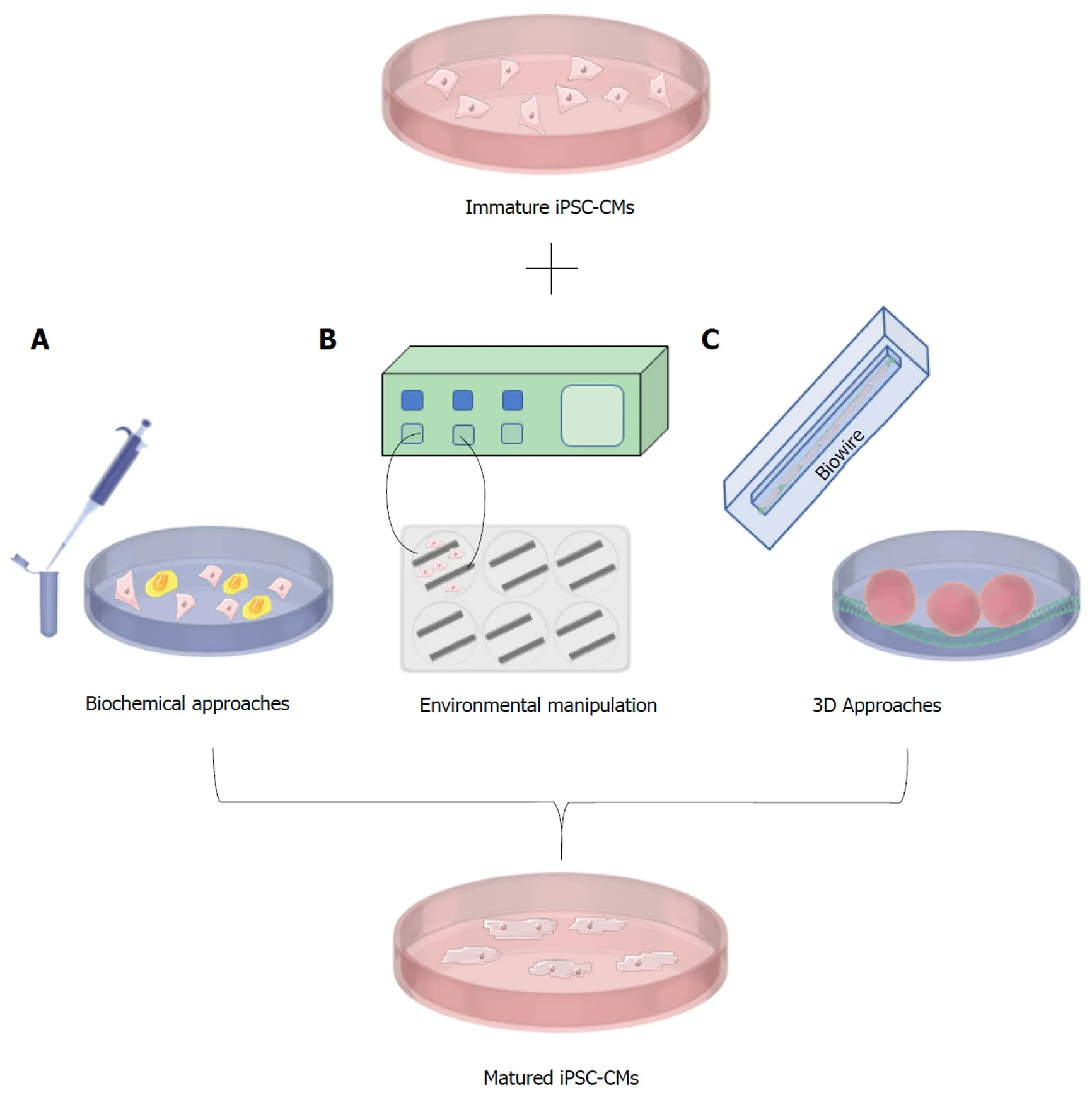
Figure 2 Methods for the maturation of induced pluripotent stem cell-derived cardiomyocytes.
Structurally, adult cardiomyocytes display a high length-to-width ratio, may be binucleated, and form sophisticated structures such as T-tubules and the sarcoplasmic reticulum within the sarcomere’s Z-line[22]. T-tubules are significant due to their role in contraction propagation[28]. Absent or disrupted T-tubules have been implicated in heart failure in animal models[29]. Adult cardiomyocytes display Z-discs, I-, H-, A- and M- bands. In addition, adult cardiomyocytes have sarcomeres that are long (2.2 μm)and highly organized[22]. These cells also possess large numbers of mitochondria due to the heart’s ceaseless energetic demands. Myocardial mitochondria tend to be evenly distributed throughout the cell and account for 20%-40% of cell size[22]. In contrast, immature hiPSC-CMs tend to be round, usually mono-nucleated, and the sarcomere is disorganized and shorter (1.6 μm). These cells also do not possess T-tubules and only have Z-discs and I- bands[22].
Metabolism
Metabolically, adult cardiomyocytes rely primarily on fatty acid oxidation as opposed to glycolysis for efficient energy production and have high levels of oxidative phosphorylation. In contrast, hiPSC-CMs mainly rely on glucose and lactate but do possess some capacity to metabolize fatty acids[2,22,27].
Contractile function
Adult cardiomyocytes tend to be more quiescent in terms of beating but generate greater force, upstroke and conduction velocities when stimulated[2,22]. In contrast,hiPSC-CMs have lower conduction and upstroke velocities but, due to an increase in the pacemaker current If, are still able to beat spontaneously[2,22].
BIOCHEMICAL APPROACHES FOR MATURATION
One approach for the maturation of hiPSC-CMs involves the manipulation of growth conditions through the addition of small molecules or changes in culture medium.Tri-iodo-L-thyronine (T3), has shown promise in stimulating the maturation of hiPSCCMs[30]. One study noted larger cell sizes and increased sarcomere length, in addition to higher contractile force and increased mitochondrial respiration capacity post-T3 treatment. Treated hiPSC-CMs also exhibited lower proliferative activity and improved calcium handling properties[30]. The authors of this study treated hiPSCCMs with 20 ng/ml of T3 and noticed key morphological differences. Upon treatment, hiPSC-CMs became significantly less round and more elongated[30]. In addition, they found that cell size also increased post-T3 treatment. T3 treatment also resulted in higher expression of key maturation markers. The authors also showed that treated hiPSC-CMs exhibited increased contractile force as quantified through micropost arrays. Treated hiPSC-CMs exhibited a contractile force of 12.3 ± 0.7 nmol/L/cell while control cells were significantly lower at 7.5 ± 0.4 nmol/L/cell[30].The mechanism of T3 in hiPSC-CM maturation is not completely understood;however, T3 has been shown to have an important role in cardiomyocyte differentiation through transcriptional regulation. Interestingly, blocking the action of T3 results in lower cardiomyocyte yield. It is hypothesized that downstream effects of T3 signaling may be responsible[30]. Studies suggest T3 by itself is insufficient in achieving maturation of hiPSC-CMs to an adult-like state. However, combining hormonal approaches with other strategies may be more successful. For example,treatment of hiPSC-CMs with both T3 and dexamethasone has shown success in furthering the maturational state of hiPSC-CMs[31]. When hiPSC-CMs were cultured with both chemicals for 15 days, an extensive T-tubule network was generated, a key indicator of adult-like cardiomyocytes as the extensions are crucial in contractility[31].Many heart diseases result in defective T-tubule structures and therefore impaired contractility[29].
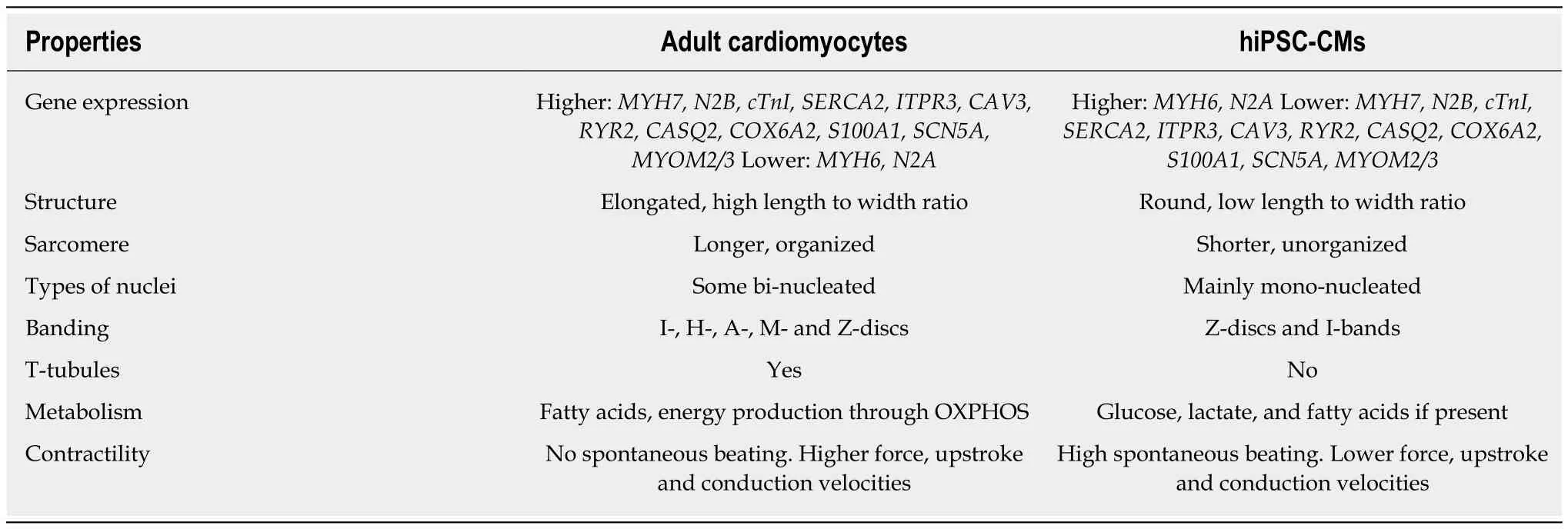
Table 1 Properties of adult cardiomyocytes vs currently generated Cardiomyocytes derived from human induced pluripotent stem cells
As current protocols for iPSC-CM generation result in cells that rely on glycolysis instead of fatty acid metabolism for energy, there has been recent emphasis on maturing hiPSC-CMs metabolically. One way of achieving this is through the use of glucose-free medium, forcing hiPSC-CMs to rely upon fatty acid metabolism. One study showcased how altering the metabolic state of hiPSC-CMs can induce increased maturation[32]. The authors exposed hiPSC-CMs to glucose-free medium containing insulin and fatty acids for three days. Doing so increased the sarcomere length significantly, showing the effect of altering metabolism on the structural features of the cell. In addition, this sarcomere length increase was correlated with improved electrophysiological characteristics[32]. Specifically, the upstroke velocity and duration of the action potential was increased in treated cells. Various maturation-related genes also displayed increased expression. A comparable study was carried out recently and displayed similar results. Correia et al[33]showed how the maturation state of hiPSCCMs can be altered through the addition of galactose and fatty acids, accompanied by removal of glucose from the medium. Their experiments improved the metabolic,structural, and electrophysiological state of hiPSC-CMs. Immature hiPSC-CMs show remarkable flexibility in adapting to growth conditions. As such, incubating these cells with fatty acid-rich, glucose-free medium seems to be altering the transcriptional signature of these cells towards a more mature phenotype. As the immature hiPSCCMs suddenly face glucose starvation, they may be pushed towards increasing transcription of genes key in metabolizing fatty acids in order to survive. As mentioned, fatty acid metabolism is characteristic of adult cardiomyocytes. Altering the carbon source of hiPSC-CMs is easy to implement and these studies have shown its relative efficacy in maturing hiPSC-CMs.
Co-culturing hiPSC-CMs with other cell types has also been shown to further the maturation state of these cells[34]. Yoshida et al[34]describe matured hiPSC-CMs resulting from co-incubation with human mesenchymal stem cells hMSCs. As mesenchymal stem cells are reported to secrete factors key to the differentiation and electrical coupling of hiPSC-CMs, they sought to elucidate its effect on iPSC-CM maturity. The authors reported increased structural maturation through aligned A-,H-, and I- myofibrils, increased gap junction formation, increased energy production,and reduced reactive oxygen species production under stress[34]. This study then implicated VEGF, bFGF, SDF-1, and GM-CSF as secreted factors from hMSCs that are key in hiPSC-CM maturation. It is thought that secretion of these factors into hiPSCCM cultures is able to induce maturation through upregulation of crucial adult cardiomyocyte gene MYH7.
The approaches mentioned in this section have all reported an increase in the maturation state of hiPSC-CMs. These approaches are simple and practical to implement but there are some key disadvantages. First, no study to date has shown complete maturation of hiPSC-CMs using only these methods. Although these methods further the state of maturation in these cells structurally, metabolically and electrophysiologically, they still do not create cells that fully recapitulate adult cardiomyocytes. These approaches may need to be combined with other more complex techniques such as electrical and mechanical stimulation.
ENVIRONMENTAL MANIPULATION
A hallmark of immature hiPSC-CMs is their electrical immaturity. These cells often display low expression of IKspotassium and INasodium channels[2,22]. In addition,immature hiPSC-CMs spontaneously beat suggesting increased expression of the pacemaker current If[22]. To transition hiPSC-CMs into a mature electrical state, the use of electrical and/or mechanical stimulation is being extensively explored. Although potentially cost- and resource-prohibitive, these approaches may prove vital in the quest towards complete maturation of hiPSC-CMs. Cardiomyocytes can be subjected to various mechanical and electrical forces in an effort to mature them electrically.Previous studies elucidated the effects electrical pacing can have on cultured cardiomyocytes. In 2006, Brundel et al[35]showed the use of an in vitro system to model alterations in the contractility of cardiomyocytes by displaying how electrical pacing can induce tachycardia. Another study showed how stimulating canine cardiomyocytes through 24-h pacing can actually remodel the electrical features of the cells[36]. Furthermore, many studies have shown how electrical pacing can cause activation of L-type calcium channels, elevate intracellular Ca2+levels and therefore stimulate increased contractility[37-39]. In hiPSC-CMs, authors of one study subjected these cells to both mechanical static stress (through maintenance of a fixed construct length) and mechanical static stress with electrical pacing conditions for 2 wks postdifferentiation[40]. Their results were exciting as hiPSC-CMs exposed to both static stress and static stress with electrical conditioning experienced increased maturation.Both treatment groups experienced increased cell alignment, Frank-Starling forcelength relationships, increased contractility, tensile stiffness and cell size[40]. These results display the success of mechanical and electrical stress in enhancing the maturation state of hiPSC-CMs[40].
One recent study showed the ability of heart muscle engineered through hiPSCCMs to structurally and functionally mature through the use of passive stretch[41]. The authors created engineered tissue with the use of computational modelling and polydimethylsiloxane reservoirs to create passive stretch. They found that the tissue displaying a stretch of 7 mm resulted in the hiPSC-CMs showing increased expression of maturation genes involved in the troponin complexes along with potassium ion channels and T-tubule proteins[41]. This result suggests that passive stretch was able to induce both structural and functional changes in hiPSC-CMs. Other studies have also shown similar results suggesting that electrical and mechanical stimulation is an effective promoter of cardiomyocyte maturation[42-44].
Another promising approach involves the addition of conductive materials to the cell substrate or matrix. For example, the addition of polypyrrole chitosan (PPC) to create a composite hydrogel has shown great promise as a biomaterial capable of improving the conduction between clusters of cardiomyocytes. The authors of one study used calcium imaging to show how PPC improved electrical signal propagation between isolated rat cardiomyocytes and synchronized their contraction[45]. Although the effect of this hydrogel is yet to be evaluated in hiPSC-CMs, previously mentioned literature has suggested that electrical stimulus is an important variable in achieving mature iPSC-CM populations.
As electrical and mechanical stimulation becomes more prevalent as a tool for hiPSC-CM maturation, private biotechnology companies have been developing electrical and mechanical devices commercially. One such device is C-Pace, a device created by IonOptix (Westwood, MA, United States). This device offers electrical stimulation and mechanical stretch through the use of a control interface and special plates outfitted with electrodes. The control interface allows for the manipulation of various current intensity and duration along with the force of mechanical stretch. This device can not only mature hiPSC-CMs functionally, but it can also induce arrhythmias and tachycardia for disease modelling. As mentioned, electrical and mechanical stimulation is not without its drawbacks. For one, it may prove cost prohibitive for many research groups. In addition, throughput is reduced as cells must be subjected to electrical pacing and/or mechanical stimulation for a certain period of time using specialized plates. However, these approaches seem to be important for the maturation of hiPSC-CMs and should be considered when developing a maturation protocol. The mechanism of hiPSC-CM maturation through the addition of mechanical and electrical cues is yet to be completely understood.However, it is hypothesized that conditional cues may upregulate the expression of key genes involved in establishing proper cardiomyocyte structure and contractility.For example, expression of calcium handling genes SERCA2 and RYR2 is increased following administration of static stress[40].
3D APPROACHES
Recently, 3D cardiomyocyte cultures, also known as organoids, have garnered great interest (Figure 3). The use of a multicellular 3D cultures potentially allow for higher accuracy in disease modelling and drug testing as 3D cardiomyocyte aggregates are closer to in vivo morphology[2,20,22]. Despite this, there have been significant challenges in developing 3D model systems. Some of these challenges include maintaining highly pure cardiomyocyte populations along with regulating clump/cluster size and providing adequate oxygen and nutrients. However, 3D culture also seems to improve the maturation state of hiPSC-CMs. In a recent study, 3D hiPSC-CMs had their transcriptome and metabolic status analysed[46]. The authors showed how 3D culture furthered the metabolic maturation of these cells and resulted in lower flux through glycolysis and increased oxidative phosphorylation[46]. Another study by the Radisic lab in Toronto described the development of a novel platform to mature hiPSC-CMs[47]. Through their Biowire device, the researchers were able to cultivate hiPSC-CMs in three dimensions. Differentiated hiPSC-CMs were seeded onto a wire substrate containing a template polydimethylsiloxane channel and collagen gels. After a week post-seeding, they noticed spontaneous contractions. They then exposed multiple cardiac wires to electrical stimuli and stimulated cells displayed improved calcium handling properties, increased myofibril organization, and higher conduction velocity[47]. Their results indicated the potential importance of an electrical stimulus combined with a 3D arrangement of hiPSC-CMs to induce maturation. In another study, a tissue-engineered cardiac patch was used to promote the maturation of hiPSC-CMs[48]. Differentiated cardiomyocytes became aligned through the use of passive tension and displayed higher conduction velocity and increased sarcomere length. Expression of various contractile genes such as SERCA2 and CASQ2 were also visibly increased[48]. Other 3D cardiomyocyte studies have displayed similar results[49,50]. These studies suggest a role for 3D culture in maturing hiPSC-CMs into adult-like states and improving disease models. 3D culture of hiPSC-CMs may be furthering maturation through provision of an environment closer to in vivo heart development. Culturing these cells in organoid formations could improve cell-cell contact and increase expression of various genes expressed in mature cardiomyocytes although the exact mechanism of maturation is yet to be elucidated. While promising,3D hiPSC-CM models display some key disadvantages in disease modelling. First,many disease models require the use of single-cells to characterize disease phenotypes. Efficient dissociation and re-plating of 3D hiPSC-CMs is a known problem as many cells do not survive post-dissociation. This also poses a problem for potential clinical applications as typically, protocols involve the use of single cardiomyocytes for injection into a recipient animal myocardium[14,15]. Second, unless organoid cell numbers and aggregate size are not carefully optimized, drug testing may be inaccurate as organoids may not be exposed to the same dose of drugs.Further, routine cell sorting may be required to ensure the cellular homogeneity of cultured organoids. The recent development of tissue-culture plates such as AggreWell (STEMCELL Technologies), and cardiomyocyte recovery/dissociation medium (STEMCELL Technologies) may prove useful in regulating cardiac organoid cell size and optimizing cell recovery; however, further research in this area must be done to definitively address these concerns.
CONCLUSION
Current protocols for the derivation of cardiomyocytes from iPSCs are highly efficient; however, hiPSC-CM culture conditions have not been adequately understood. Addition of various cytokines, environmental cues, and mechanical/electrical stimulation have yet to be optimized. As a result, current protocols result in cardiomyocytes that are most consistent in their properties with fetal cells which potentially limits their use for disease modelling and clinical translation of adult diseases. This review outlined several strategies for the maturation of hiPSC-CMs. The combination of several of these approaches may lead to the optimal maturation conditions.

Figure 3 Induced pluripotent stem cell-derived cardiomyocytes in a 3-dimensional structure.
