Human adult pluripotency: Facts and questions
2019-02-20LuminitaLabuscaKavehMashayekhi
Luminita Labusca, Kaveh Mashayekhi
Abstract Cellular reprogramming and induced pluripotent stem cell (IPSC) technology demonstrated the plasticity of adult cell fate, opening a new era of cellular modelling and introducing a versatile therapeutic tool for regenerative medicine.While IPSCs are already involved in clinical trials for various regenerative purposes, critical questions concerning their medium- and long-term genetic and epigenetic stability still need to be answered. Pluripotent stem cells have been described in the last decades in various mammalian and human tissues (such as bone marrow, blood and adipose tissue). We briefly describe the characteristics of human-derived adult stem cells displaying in vitro and/or in vivo pluripotency while highlighting that the common denominators of their isolation or occurrence within tissue are represented by extreme cellular stress. Spontaneous cellular reprogramming as a survival mechanism favoured by senescence and cellular scarcity could represent an adaptative mechanism. Reprogrammed cells could initiate tissue regeneration or tumour formation dependent on the microenvironment characteristics. Systems biology approaches and lineage tracing within living tissues can be used to clarify the origin of adult pluripotent stem cells and their significance for regeneration and disease.
Key words: Human adult pluripotent stem cells; Induced pluripotent stem cells;Reprogramming; Cellular stress
INTRODUCTION
During the development of multicellular organisms, cells evolve from the initial undifferentiated, totipotent state of the fertilized egg and early embryo to sequentially restricted states while gradually losing their differentiation potential. The capability of generating more committed progenitors is referred to as the degree of “potency” that defines the “stem” status of a cell. Adult organisms are composed of a large panel of differentiated cell types that accomplish various functions within the body. Among them, a variable amount of tissue-resident stem cells have been documented in various human tissues, accounting for tissue turnover and repair after injury. Tissue specific adult stem cells [such as mesenchymal stem cells (MSCs), neural stem cells,and haematopoietic stem cells] exist in dormant states in adult tissues and are thought to be lineage-restricted, meaning they only give rise to progeny of their tissue of origin. Differentiation implies epigenetic silencing of the so-called pluripotency genes and transcriptional activation of protein-coding genes with cell-type specific functions.
CELLULAR REPROGRAMMING - CHALLENGING THE DOGMA OF LOCKED ADULT DIFFERENTIATION
The ultimate differentiated state associated with loss of cell division, which is known as terminal differentiation, was long considered irreversible. Seminal Nobel-winning research has gradually deconstructed this dogma. Envisaged by Nobel Prize-winner Hans Spemann in 1935, “the fantastical experiment”[1]was performed several decades later. Using the previously established nuclear transfer technology[2], somatic cell nuclei transferred to an enucleated egg cytoplasm were shown to generate a viable adult organism. The experiment confirmed that somatic, fully differentiated adult cells not only retain an intact full genome but can also revert to pluripotent stages under permissive conditions[3]. However, the locked differentiation dogma and definitive rolling down of the epigenetic Waddington landscape[4]was challenged even more dramatically several decades later. In 2006, Shinya Yamanaka used forced expression of several “pluripotency” transcription factors (Oct3/4, Klf4, Sox2 and c-Myc, which was later called the OKSM cocktail) to “reprogram” differentiated somatic cells (mouse fibroblasts) to a cellular status equivalent to embryonic counterparts [embryonic stem cells (ESCs)]. The “reprogrammed” elements, termed induced pluripotent stem cells (IPSCs) were similar to ES cells in morphology,proliferation, surface antigens, gene expression, epigenetic status of pluripotent cellspecific genes, and telomerase activity; the IPSCs were capable of generating various cell types in a teratoma assay and contributed to chimeric animals when injected into mouse blastocysts[5]. The IPSC reprogramming process was further refined to generate cells with potential for germ line transmission[6]. One year later, human fibroblast cells were converted to IPSCs, launching a new era of reprogramming technology with exciting implications in disease modelling and treatment[7]. The initial retroviral-based gene transfer of the OSKM factors was modified to increase reprogramming efficiency and decrease potential tumourigenicity. Non-integrating viruses, stabilized RNAs and proteins, and episomal plasmids are currently used to deliver integration-free reprogramming genes. A large panel of adult somatic or adult stem cells of various species and tissues of origin were shown to be reprogrammable to IPSCs. Some cell types such as neural stem cells required fewer transcription factors for reprogramming[8], while in some cases, small molecules can substitute for the forced expression of one or several OSKM factors[9]. Direct reprogramming of adult differentiated cells to adult cells of different lineages (e.g., fibroblasts to neurons[10])without conversion to intermediary pluripotent stages was further demonstrated.Using reprogramming methods, adult somatic cells could “go back” to pluripotent stages or directly convert to lineages with distinct developmental origins. Extreme somatic cell plasticity was therefore shown possible under defined in vitro conditions.A holistic systems biology approach was applied to existing large “-omic” datasets from pluripotent cell populations to discover genes important for pluripotency and cell reprogramming[11]. Bioinformatics analysis of several data bases on naïve and primed (pluripotent) ESCs revealed a network of functionally interrelated genes in which the OSKM factors are nodes (Table 1 and Figure 1). Contextual ontology enrichment and quantitative gene expression signatures revealed the mouse pluripotency gene interaction network, the hierarchical importance of genes and pathways, and their significance in pluripotency.
REPROGRAMMING AT WORK
IPSC-based or direct cell reprogramming further advanced to investigating the effect of somatic cell reprogramming in vivo.
Short term in vivo activation of OKSM factors in transgenic “reprogrammable” mice carrying a tetracycline-inducible OSKM polycistronic cassette crossed with progeria models reduced signs of premature ageing[12]. The same method improved recovery from metabolic disease and muscle injury in older wild-type mice[13]. Cellular epigenetic reprogramming after short-term cyclic in vivo activation of OKSM factors(termed partial reprogramming) does not cause tumour formation and probably acts by reverting epigenetic dysregulation associated with older age, offering a platform to study the disease of ageing. In other work, long-term induction of OKSM factors in reprogrammable mice lead to teratoma formation and IPSC induction in a large variety of tissues including haematopoietic lineages. Transcriptomic analysis showed that in vivo-produced IPSCs are more similar to ESCs compared to their in vitro counterparts; in vivo-produced IPSCs are also totipotent as they could generate all embryonic layers and trophectoderm, a property that ESCs are lacking[14].Intriguingly, in vivo forced expression of OSKM factors, a process known to have low efficiency in vitro, triggers reprogramming of few cells and induces cellular senescence and apoptosis in many other surrounding cells in vivo. Ageing- and tissue injuryassociated senescent cell-secreted factors (of which proinflammatory cytokine IL-6 plays a major role) improve the in vivo reprogramming process. A similar process might take place under physiological conditions when damage-driven senescent cells promote cell dedifferentiation during tissue repair[15]. In vivo direct reprogramming platforms are currently under intense scrutiny and may be the next generation of regenerative approaches for cardiac, neural, liver or pancreatic islet cells. Anti-aging interventions may be a possible outcome of direct somatic cell manipulation[16]. It is worth mentioning that spontaneous reprogramming mechanisms in mammalian organs do occur after injury. Using lineage tracing, several direct conversions were documented in mice. Adult hepatocytes were shown to spontaneously reprogram in vivo in biliary epithelial cells after toxic liver injury in a NOTCH-dependent mechanism[17]. Glucagon-producing alpha pancreatic cells converted to beta cells in a mouse model of diphtheria-induced acute selective beta cell loss[18]. Due to obvious ethical constraints, such mechanisms have not yet been documented in humans.Controversial reports about adult pluripotent stem cells in various human tissues prompts reconsideration of their origin and/or causative mechanisms.
ADULT PLURIPOTENT CELL- TYPES AND CONTROVERSIES
Bone marrow-derived pluripotent cells
Starting in the early 2000s, several reports about spontaneously occurring pluripotent cell types emerged. Derived from mice and human bone marrow by negative depletion of CD45 (+)/glycophorin (+) cells, multipotent adult progenitor cells(MAPCs) were reported to undergo triploblastic differentiation under defined conditions in vitro. MAPCs did not form teratomas, contributed to chimaera formation when injected into mouse blastocysts, and contributed to cardiac regeneration in severe combined immune-deficient (SCID) mice[19,20]. Marrow-isolated adult multilineage-inducible cells derived from the bone marrow of vertebral bodies under low oxygen conditions were reported to be particularly efficient in differentiating into neural lineages without displaying features of pluripotency[21]. Very small embryoniclike cells (VSELs) were isolated from murine bone marrow by positive selection for the chemokine receptor CXCR4 and were shown to display features of embryonic cells (cell and nuclei size, chromatin characteristics, telomerase activity). The authors hypothesized that such cells with embryonic-like surface markers [stage-specific embryonic antigen (SSEA), OCT-4, and NANOG] could be epiblast-derived pluripotent cell remnants of embryonic developmental stages; these cells could be a less controversial source for regenerative approaches[22]. The existence of VSELs waschallenged a couple of year later as other groups failed to replicate their isolation from bone marrow Remarkably, almost all reports of bone marrow-derived cells that claimed to retain embryonic-like stem cell features were isolated in modified culture conditions (such as low oxygen tension or serum deprivation). Arguments that such cells are early MSC progenitors or culture condition-modified MSCs have not been fully investigated to date[23]. MSCs derived from bone marrow as well as other sources(excluding adipose tissue) were shown to foster a population of SSEA-positive cells with enhanced expansive and clonogenic potential. Arguments that SSEA-positive cells are a culture artefact have not been addressed yet[24]. The existence of adult pluripotent cell populations proved hard to replicate, leading to doubt concerning the accuracy of the reported findings and concept of naturally occurring pluripotency.However, the diversity of reports on enigmatic cells with morphology similar to embryonic counterparts that were isolated under harsh conditions may signal that this is an unelucidated phenomena.
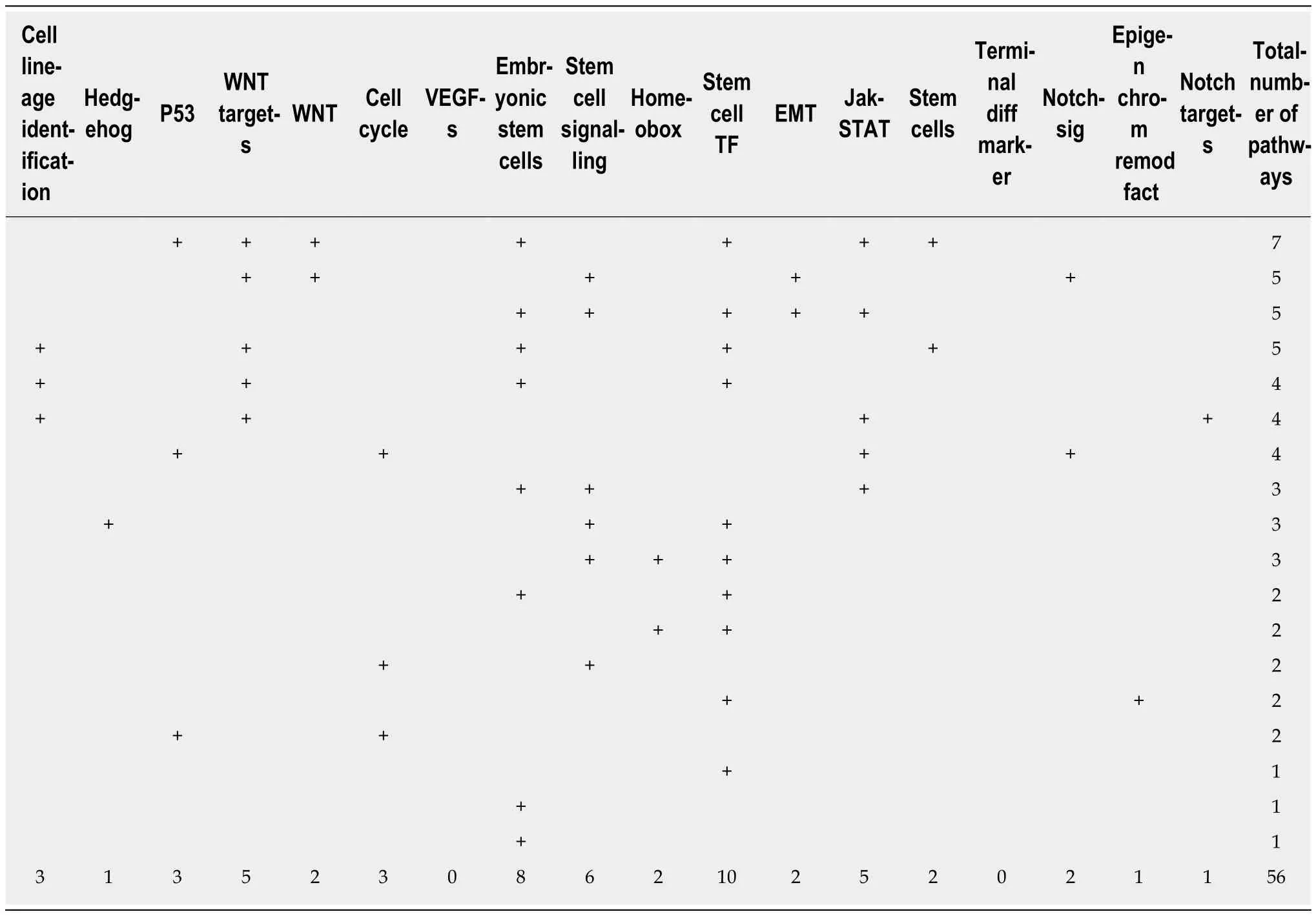
Table 1 Pathway population
Adipose-derived pluripotent cells
Isolated from adipose-derived stromal vascular fraction, adipose-derived MSCs(ADSCs) were shown to differentiate to non-mesodermal lineages under special culture conditions in vitro[25]. Notably, the majority of non-mesenchymal lineage differentiation protocols involve an intermediary step including suspension culture,spheroid formation of intermediary progenitors and sometimes serum deprivation.Undifferentiated or in vitro pre-differentiated ADSCs were shown in several reports to contribute to liver, Schwann cell and glial cell regeneration[26]. The advent of IPSCs and the enthusiasm for their potential in generating patient-specific pluripotent cells for research and therapy seemed to throw the controversy of adult pluripotency into oblivion. However, two special cell types continue to capture research interest:multilineage differentiating stress-enduring cells (MUSE) and dedifferentiated fat cells.
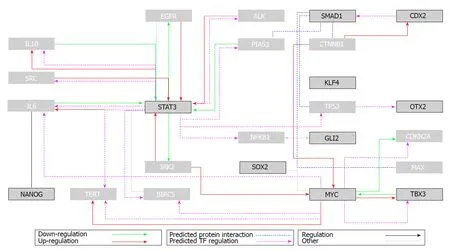
Figure 1 Flux diagram of the top 10 ranked genes related to pluripotency (interaction data obtained from GNCPro, SABiosciences).
MUSE cells
MUSE cells were initially identified by applying stressful culture conditions to several cell populations such as MSCs[27,28]; they have been further obtained from adipose tissue by positive immune-separation for the mesenchymal marker CD105 and SSEA-3[29]. MUSE cells are capable of triploblastic differentiation without tumour formation after in vivo injection into SCID mice; these were considered safer sources for pluripotent cells than ESCs or IPSCs[30]. With several distinctive properties in vitro and in vivo, MUSE cells display low telomerase activity and a normal karyotype. MUSE cells form distinctive clusters in vivo (the so-called M clusters) that resemble ES or IPSCs behaviours in similar conditions. These cells express pluripotent markers, such as NANOG, Oct3/4, Par-4, and Sox2, and are capable of spontaneous or induced expression of mesodermal, endodermal or ectodermal markers[31]. The low levels of cell proliferation and oncogenesis gene expression might account for their low proliferation and absence of tumourigenic activity, while the expression of gene clusters related to death and survival that are shared with non-mammalian species might represent a highly-conserved mechanism of cell survival during extreme conditions[32]. Several preclinical studies have reported their migratory potential due to expression of chemokines involved in cell homing and their capability to participate in liver, kidney, and neural regeneration in relevant animal models (for review see 30). Muse cells also have immunomodulatory properties in lipopolysaccharide-stimulated macrophages and antigen-challenged T-cell assays through downregulating the secretion of pro-inflammatory cytokines (interferon-γ and tumour necrosis factor-α); this effect is probably acquired by transforming growth factor-β1 expression that decreases the immune-regulatory activity through T-box transcription factors in T cells[33]. Interestingly, MUSE cells have been identified in very low numbers in the blood stream of early stage patients with acute stroke where they probably mobilized from bone marrow; MUSE cells have also been detected in situ and in post-mortem bone marrow samples harvested from subjects with severe conditions such as stroke and myocardial infarction[34]. Research to harness the therapeutic potential of such cells for regenerative applications is ongoing; however,their anatomical location in niches has not yet been identified. It is unclear whether induced or naturally occurring stressful conditions are sorting or generating MUSE cells through adaptative and potentially “reprogramming” mechanisms attempting regeneration after major insults.
Adipose tissue was one of the first sources reported for generating MUSE cells and another reportedly pluripotent adult human cell source is dedifferentiated adiposederived cells (DFATs). Mature adipocytes isolated from adult human adipose tissue that are subjected to an in vitro dedifferentiation strategy (ceiling culture) revert to a more primitive phenotype and gain proliferative and differentiative abilities[35].Indeed, DFATs were found to have triploblastic differentiation potential in vitro and do not generate teratomas when injected in immuno-deficient mice[36]. As opposed to ADSCs that are obtained by enzymatic digestion of adipose tissue and selection of plastic-adherent fibroblastoid elements, DFATs are homogenous populations. DFATS display surface markers for CD13, CD29, CD44, CD90, CD105, CD9, CD166 and CD54,and do not express CD14, CD31, CD34, CD45, CD66b, CD106, CD117, CD133, CD146,CD271, CD309, HLA-DR and alpha-smooth muscle cell actin; a fraction of DFATs also express SSEA-3[37]. Inter-donor and interspecies variability in the makeup of surface antigens has been reported. Combined with a Poly-D, L-lactic-co-glycolic acid scaffold, rat DFAT cells were able to regenerate periodontal tissue[38], opening exciting avenues for oral and maxillofacial tissue regeneration[39,40].
Dedifferentiation as a source of adult pluripotent cells
Mature adipocytes are not the only cells capable of dedifferentiation. Mature chondrocytes isolated from the well organized and highly structured cartilage ECM dedifferentiate while in monolayer culture. When expanded in MSC growth medium with or without fibroblast growth factor (FGF), costal chondrocytes express features of MSCs but retain their chondrogenic potential when injected in vivo for cartilage defects[41]. Cartilage progenitor cells with clonogenic and migratory potential reside in osteoarthritic cartilage but not in normal mature cartilage[42]. However, further reports identified surprisingly high levels of the stem cells markers Notch-1, Stro-1 and VCAM-1 in normal cartilage and in a stage- and zone-dependent manner in osteoarthritic (OA) cartilage[43]. Despite their controversial nature, these studies revealed the previously ignored dynamic activity of adult cellular cartilage elements that could be metabolic- and/or mechano-stimulation-dependent[44]. Hypothetically,cells with surface markers of pluripotency in adult cartilage could originate from dedifferentiated chondrocytes induced by metabolic and/or mechanical stress.Disturbances in these parameters might lead to abnormal cell clustering and ECM disorganization that synergizes to produce the progressive cartilage breakdown of OA. The fibrous remodelling of joint surfaces seen in advanced OA stages might represent an abnormal differentiation of such dedifferentiated adult chondrocytes.
Other cell types were shown to successfully dedifferentiate in vitro into multipotent or pluripotent progenitors. Adult human thyrocytes regained multipotency,proliferated and differentiated to neurogenic and adipogenic lineages in vitro[45].Terminally differentiated keratinocytes were converted to their progenitor cells under FGF induction[46], while pancreatic islet cells morphed into duct-like progenitor cells under epidermal growth factor exposure[47]. It is noteworthy to mention that these reports involve in vitro cell populations. Isolation protocols require breakdown of ECM structures, a process commonly achieved by maintaining cells in monolayer cultures. Intriguingly, reports about in vivo formation of DFAT cells after induced local mechanical stress in mice might suggest that this process occurs as a natural adaptative mechanism to local stressful conditions[48]. Dedifferentiation, a common mechanism in plants and a limited number of vertebrates that is used for regeneration, involves switching off genes responsible for cell-specific functions, reentering the cell cycle and proliferating, and switching on “pluripotency”-related genes. This might be a conserved phenomenon in mammalian organisms including humans. Several factors such as hypoxia, prolonged stress and injury are known to induce dedifferentiated cells after in vitro manipulation or in vivo. Factors that naturally induce such phenomena in vivo and the fate of the regenerative processes they launch need further investigation. Reports about dedifferentiation processes occurring in human malignant tumours, such as liposarcomas dedifferentiating to osteosarcomatous components[49]or soft tissue sarcomas to liposarcomas[50], reflect several rare situations of pathological dedifferentiation processes. Physiological lung myofibroblast dedifferentiation after tissue injury and inflammation accounts for adaptative apoptosis and bronchiolar re-epithelialization. During ageing, impaired dedifferentiation accounts for continued myofibroblast accumulation, excessive matrix deposition and subsequent interstitial lung fibrosis[51].
STAP CONTROVERSY
In early 2014, a paper described a “unique cellular reprogramming phenomenon” of exposing adult differentiated cells to low pH. CD45-positive spleen lymphocytes from 1-week-old C57BL/6 mice carrying an Oct4-gfp transgene and adult cells derived from the brain, skin, muscle, fat, bone marrow, lung and liver that were transiently exposed to low pH were reported to acquire pluripotency in vitro. A portion of such cells, which the authors termed stimulus-triggered acquisition of pluripotency (STAP)stem cells, were shown to express pluripotency markers, differentiate to triploblastic lineages under specified conditions, and contribute to chimaeras and germline transmission when injected into mouse blastocysts. Compared to mouse ESCs, STAP cells displayed limited self-renewal capability in ES-specific media and did not form colonies in dissociated culture[52]. The authors hypostatised that “unknown cellular mechanisms” triggered by sublethal stress unlocked the cells from their differentiated state and allowed re-expression of pluripotency-related genes, reflecting early embryonic stages. Such phenomena do not likely occur in vivo-at least not in mammalian organisms-as presumed mechanisms block progression from the initial OCT-4 activation to further reprogramming. Several months later, the paper was retracted due to “errors classified as misconduct” by the institutional investigation committee[53]. The negative impact of the retraction was further combined with news about possible “honour suicide” of one of the senior authors. However, while the“multiple errors” could indeed impact the study reproducibility and the credibility of the reported data, they could not rule out the existence of the STAP phenomenon.Interestingly, a recent paper reported a method of preconditioning adult human umbilical cord blood-derived stem cells to increase survival after transplantation.Exposure to oxidative stress and serum deprivation increased cell resistance in vitro,possible pointing to an adaptative mechanism for cell survival[54].
MSCS AND THE “STEM CELL STATE”
The “classical model of hierarchical MSC differentiation depicts a MSC at the top of the potency ladder and subsequent progenies with reduced differentiation potential;this model was challenged by a report showing that murine bone marrow-derived MSCs clonally lost and regained differentiation ability. Fluctuating differentiation potential was even demonstrated at the single-cell level and was closely dependent on culture conditions. Oxygen tension and sparse culture density imposed by clonal expansion altered gene expression and the epigenetic profile that accounts for cell potency. Wnt activation in sparse cultured cells was directly visualized using a green fluorescent protein-tcf/lef reporter, while DNA microarray analysis revealed enrichment of histone methylation in EMT/MET-, MSC-, and Wnt-related genes; this process was found to be oxygen- and tension-dependent[55]. Isolation of cells away from the ECM and exposure to stressful culture conditions might influence mesenchymal cell potency. The authors discussed the potential stress-induced reversibility of cell fate in mammalian mesenchymal cells in vivo as a sort of adaptative mechanisms[56].
ADULT PLURIPOTENCY AND AGING
A decline in the reprogramming efficiency of cells derived from older donors in both mice and humans has been reported. However, IPSs with complete set of pluripotency markers as well as differentiation capabilities could be derived from older donors[57,58]. Several epigenetic barriers as well as the increased number of senescent cells, could quantitatively limit the reprograming process in older organisms, process that once initiated remains possible and qualitative. “Aging pathways” (IGF-1 pathway, mTOR,) or “longevity: related ones (AMPK or sirtuins)have been found to influence reprogramming and IPS generation (for review see 57).It is not clear, to date, how these functional and metabolic pathways are involved in potential spontaneous adult cell reprogramming. It is noteworthy to mention that all the above mentioned pathways are highly conserved modalities to sense multidirectional stress (such as energy status, DNA damage, protein damage, and hypoxia) and to orchestrate adaptative cellular responses[59]. Their connection and interference with potential spontaneous induced pluripotency needs to be further studied. Activation of Integrated stress response has been proposed as a mechanism for increased longevity in yeasts[60]and recently was found to facilitate human hematopoetic stem cells survival ans well as to mark leukemia stem cells[61]Cellular stress increasingly appears as a turning point between stemness and malignization in adult as well as aging organisms.
SUMMARY AND SOME QUESTIONS
Reprogramming and the advent of Nobel-awarded IPSC technology has shifted our understanding of cell plasticity. Although previously thought to be “terminally”differentiated, adult cells have been shown to “climb back up” the potency hill to regain multiple differentiation potentials, similar to cells from early developmental stages. The revolutionary technology opened a promising era of cell manipulation for modelling and therapeutic use. IPSCs have gained momentum and are quickly moving towards clinical use for regeneration[57]. Several methods have been proposed to tackle genetic instability forced-reprogrammed cells; however, it their application and utility in designing IPSC-based therapies is not clear[58]. The medium- and longterm fates of therapeutic IPSCs and their progeny after implantation are still unclear,as are the influences on the safety and efficacy of regenerative therapies. The potentially safer alternatives of adult pluripotent stem cells (MUSE, DFAT) have not achieved similar impacts in research interest or clinical translation. Perhaps this is due to their variability, limited reproducibility of production conditions, and poorly explained mechanisms that account for their presence or appearance within adult tissues. It is not clear if adult pluripotent cells already exist in various tissues (such as bone marrow or adipose), or if they are a mere isolation or culture artefact. It remains unknown whether adult pluripotent cells are rare remnants of developmental stages in dormant states within tissues or spontaneously reprogrammed elements. The possibility that severe stress, cell loss or even ageing can induce human adult pluripotent cells both in vitro and in vivo cannot be ruled out and should warrant further investigation (Figure 2). Can adult “terminally differentiated” cells switch their fate and return to earlier developmental stages in human tissues and during isolation procedures under extreme conditions? If so, what is the threshold of“cellular stability?” Can protocols for steering cellular “destabilization” towards regeneration rather than malignancy be designed with computer modelling? What are the nature and gradient of “stressors” that potentially induce spontaneous reprogramming? What is the role of organism and cellular senescence in promoting or quenching such phenomena? What is the role of immune-mediated inflammation and the senescence-associated inflammatory background in promoting, controlling and halting in vivo reprogramming? What potential roles do spontaneously reprogrammed cells have in tissue regeneration and tumour formation? A closer and fundamental investigation of in vivo spontaneous-reprogramming phenomena in mammalian cells and humanized animal models could help answer these questions and impact both regenerative medicine and cancer research. Systems biology approaches could be used to discover key switches in biological pathways involved in adult pluripotency and may potentially derive targets for their identification within human tissues.
Figure 2 The possibility that severe stress, cell loss or even ageing can induce human adult pluripotent cells both in vitro and in vivo cannot be ruled out and should warrant further investigation.
ACKNOWLEDGEMENTS
The authors wish to thank Dr. Florin Zugun-Eloae for his helpful counselling during the manuscript design.
