pH和光对复合型乳液拓扑结构的调控
2019-01-23王喆贾康乐刘同庆胡俊文李学丰董金凤
王喆,贾康乐,刘同庆,胡俊文,李学丰,董金凤
武汉大学化学与分子科学学院,武汉430072
1 Introduction
Complex emulsions, especially multiple emulsions (smaller droplets of another liquid contained in dispersed droplets within a continuous phase) and Janus emulsions (dispersed droplets composed of two immiscible oils), have attracted increasing attention due to their potential applications in pharmaceuticals1,2,chemicals or microparticles synthesis3, and optical or biological sensors4. The unique and tunable morphology of complex emulsions endows them with unique behaviors, extraordinary properties and suitable applications. For example, Nagelberg and coworkers5presented a new generation of fluidic tunable compound micro-lenses, in which the stable bi-phase emulsion droplets composed of hydrocarbon and fluorocarbon liquids in aqueous media were primary. The droplets showed different refraction of the light depending on their configuration,switching between converging and diverging lens geometries.Double emulsions with the optically denser fluid as the dropletcore phase strongly focused light while that as the droplet-shell phase strongly scattered light, and Janus droplets could change focal length of the light by varying the internal interfacial curvature. In Zarzar et al. subsequent work6, they modulated the transmission of light through a sample to monitor enzyme kinetics through changes in the morphology of those complex emulsions, driven by enzyme-responsive surfactants. In addition,complex emulsions are excellent templates for microparticles synthesis, especially for synthesizing microparticles of special structures such as Janus particles7,8. Therefore, it is greatly necessary to exactly control the complex emulsion morphology for their potential and realistic applications.
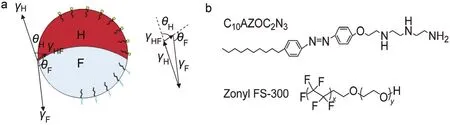
Fig. 1 (a) Illustration of the H/F/W complex emulsion droplet stabilized by a fluorosurfactant and a stimuli-responsive surfactant.(b) Molecular structures of C10AZOC2N3 and Zonyl FS-300.
Significant advance has been achieved in stability mechanism, preparation methods and morphology control of complex emulsions in recent years9–11. The correlation between interfacial tensions and the topology of the droplets has been illustrated, which provides the basis for precise control of the droplet topology12,13. The topology of the complex emulsion droplets can be determined by interfacial tensions at the contact line14. For instance, in a stable bi-phase emulsion droplet composed of hydrocarbon (H) and fluorocarbon (F) liquids in aqueous solution (W), the balance of three concurrent forces at the contact line, the interfacial tensions of the H/W interface, γH,the F/W interface, γF, and the H/F interface, γHF, stabilizes the complex emulsion droplet (Fig. 1a). Following a Neumann triangle, θHbetween the H/W and H/F interfaces and θFbetween the F/W and H/F interfaces are changed with the variation of γHand γF. These two contact angles characterize three thermodynamically permissible internal configurations including the double-emulsion and Janus states or to invert entirely. Numerous fundamental investigations were carried out on the topology parameters including the contact angles, the location of contact plane and the volume ratio of two hemispheres15. Although the topology of complex emulsions can be precisely tuned to the required morphology via microfluidic devices16–18, whereas the limited amount of products greatly restricts its applications in a large-scale. In this case, Hasinovic et al.19developed the one-step vibrational preparation of Janus emulsions by the traditional high intensity emulsification processes firstly. In the subsequent researches,however, most reports focused on that changing the volume ratio of the inner immiscible liquids to adjust the geometries of Janus droplets from “snowman” to “dumbbell”20, varying the fraction of different surfactants to control the topology of the complex emulsion droplets switching between core-shell and Janus configurations21, and controlling the emulsification energy to change the droplet size from a few hundred micrometers to hundreds of nanometers22. Only a few reports focused on tuning the internal interfacial curvature of the complex emulsion droplets, especially driven by the external stimuli such as temperature, pH, light, and so forth.
Stimuli-responsive surfactants have become very popular for their interesting properties change in response to environmental stimuli such as temperature, pH, CO2, light and redox23–27. There are numerous studies on their interfacial behavior under single stimuli, such as photo-manipulation of a droplet28,demulsification with pH variation29, defoaming by redox30,temperature-induced emulsion configuration transformation31,and so on. Recently, studies of using single stimuli with stimuliresponsive surfactant to precisely control the morphology of complex emulsions have been reported14,32,33. However, the stimuli-responsive surfactants in each system only respond to a single external stimulus, which greatly restricts those complex emulsions for a wider controllable range. Thus, it is of great importance to fabricate multi-stimuli responsive complex emulsions using multi-stimuli responsive surfactants for multistimuli response and higher precisely control.
In this work, the influences of pH and UV/Blue light irradiation on the morphology control of the three-phase complex emulsions,consisting of hydrocarbon and fluorocarbon liquids in aqueous solution (H/F/W), were investigated. The three-phase complex emulsion composed of 1:1 volume ratio of heptane (H) and perfluorohexane (F) in aqueous surfactant solution (W) was stabilized by the mixture of surfactants, F(CF2)x(CH2CH2O)yH(Zonyl FS-300) and 1-[2-(4-decylphenylazo-phenoxy)-ethyl]-1-diethylenetriamine (C10AZOC2N3). It is expected that C10AZOC2N3would play a major role in complex emulsion morphology control because the molecular conformation and interfacial activity of C10AZOC2N3 are responsive to both pH and UV/Blue light irradiation.
2 Experimental
2.1 Materials
Heptane (97%), perfluorohexane (99%), Sudan Red 7B (98%),Zonyl FS-300 (40% solids) and sodium chloride (AR) were purchased from Sinopharm Chemical Reagent Co., Ltd. (China)and used as received. The pH and light dual-responsive surfactant,1-[2-(4-decylphenylazo-phenoxy)-ethyl]-1-di-ethylenetriamine(C10AZOC2N3), was synthesized according to Scheme S1 in the Supporting Information. pH of aqueous surfactant solutions were adjusted with 1 mol·L-1HCl and NaOH aqueous solutions using an acidometer (PB-10, Sartorius, Germany). Ultrapure water was made by Milli-Q (18.2 MΩ·cm at 25 °C, Millipore-Q).
2.2 Emulsions preparation
The three-phase complex emulsions were prepared by onestep temperature-induced phase separation method. The inner oil phase was the mixture of heptane dyed with Sudan Red 7B and perfluorohexane in equal volumes. A mixture surfactant aqueous solution of 0.1% (mass fraction) Zonyl FS-300 and various concentration of C10AZOC2N3was the water phase. 0.1 mol·L-1NaCl was added to the aqueous phase to maintain ionic strength when adjusting pH. The volume ratio of the oil phase and aqueous phase was 1:1 for all complex emulsions. Heptane and perfluorohexane were heated above their suspension’s upper critical solution temperature (Tc, 40.9 °C) until miscible, and the preheated aqueous surfactant solution (heated above Tc) was then added. The resulting mixture was emulsified using a micro vortex mixing apparatus (WH-3, 2800 r·min-1, Shanghai Huxi Analysis Instrument Factory Co., Ltd.) for 1 min and then cooled at 25 °C to induce the inner oil phase separation, equilibrating for at least 12 h at 25 °C.
2.3 Emulsions characterization
The stability of these prepared emulsions was conducted by visual observation after the emulsions were store over 1 month at 25 °C. The morphology of the complex emulsion droplets was observed with an optical microscope (Olympus BX51, Japan)and recorded using MiE microscopic image processing software at room temperature (25–28 °C).
2.4 Light irradiation
A 1.2 mL sample, placed in a glass cuvette, was irradiated 3 cm away from UV/Blue light for 30 min and then observed by microscopy. Herein, a flashlight LED UV lamp (OPX-365, λ =365 nm, 15 mW·cm-2, Spectronics, USA) was used as the UV light resource and an OPX-450 (λ = 450 nm, 7 mW·cm-2,Spectronics, USA) was as the Blue light resource.
2.5 UV-Vis spectra measurements
UV-Vis spectra measurements were carried out on a UV-Vis Tu-1901 spectrophotometer (Pgeneral, China) using ultrapure water (18.2 MΩ·cm, Millipore) as a blank at 25 °C.
2.6 Interfacial tension measurement
Equilibrated and dynamic interfacial tensions of the interface between heptane and aqueous surfactant solutions were measured by the spinning drop tensiometer (SVT 20N,Dataphysics, Germany) under 25 °C. The tensiometer consists of a horizontal glass capillary rotating about its long axis with a maximum speed of 10000 r·min-1and a camera with a microscopic zoom lens to capture the image of the spinning droplet. The capillary was filled with aqueous surfactant solution at working pH, and then heptane was carefully injected with a microsyringe, avoiding any air bubble. The heptane droplet stopped elongating when the centrifugal force was balanced by the interfacial tension force. The interfacial tension was determined from Vonnegut’s equation34by measuring the cylinder radius. Every measurement was measured for at least 1 h until equilibrium. The dynamic interfacial tension of heptane and aqueous surfactant solution under UV/Blue light irradiation was measured in a dark room. UV/Blue light was irradiating vertically from 3 cm right above the spinning heptane droplet in aqueous surfactant solution, with an opaque curtain covering the whole equipment.

Fig. 2 Images of the complex emulsion droplets in aqueous solutions of 0.1% Zonyl FS-300 and different C10AZOC2N3 concentration.(a) 0, (b) 0.01%, (c) 0.03%, (d) 0.05%, and (e) 0.1%. Scale bar, 100 µm.

Fig. 3 Oil/water interfacial tension between heptane and aqueous solution of 0.1% Zonyl FS-300at different concentration of C10AZOC2N3.
3 Results and discussion
3.1 Inner phase inversion controlled by C10AZOC2N3 concentration
All the prepared complex emulsions were stable without apparent phase separation over 1 month, which were evaluated as “stable emulsion” with the averaged size about tens of micrometers (Fig. S1, Supporting Information). Fig. 2 shows the microscopy images of the complex emulsion droplets composed of 1 : 1 volume ratio of heptane (red) and perfluorohexane in aqueous solution stabilized by 0.1% Zonyl FS-300 and different concentration of C10AZOC2N3 ([C10AZOC2N3]) (Fig. S2,Supporting Information). The complex emulsion was changed from a single-core H/F/W double emulsion to entirely inverted F/H/W double emulsion passing through a spherical Janus emulsion morphology upon increasing [C10AZOC2N3]gradually. This change suggests that the morphology of the H/F/W complex emulsions can be controlled by [C10AZOC2N3],in which the inner phase inversion is achieved by increasing the concentration of C10AZOC2N3.
Since the topology of the complex emulsion droplets is primarily determined by three interfacial tensions including the H/W interface (γH), the F/W interface (γF), and the H/F interface(γHF) at the contact line. The interfacial tensions between heptane and aqueous surfactant solution at different concentration of C10AZOC2N3were measured (Fig. 3) to illustrate the dependence of the complex emulsion droplet morphology on the concentration of C10AZOC2N3. In order to make a better description about the change of the H/F interfacial curvature, “+” and “-” were employed to characterize the orientation of the heptaneperfluorohexane interface inside the complex emulsion droplet,where H/F/W double emulsion is “+”, F/H/W double emulsion is “-”, and a perfect Janus droplet is “0” representing an upright interface. As shown in Fig. 3, the H/W interfacial tension, γH,was gradually decreasing with increasing the concentration of C10AZOC2N3. Initially, 0.1% Zonyl FS-300 was added into aqueous solution to reduce the interfacial tension of the perfluorohexane-water interface, γF, to control the morphology of complex emulsions. At this point, γH≥ γF+ γHF, θF≈ 0, where θF is the contact angle between the F/W interface and the H/F interface, heptane was encapsulated within perfluorohexane,resulting in the H/F/W double emulsion (Fig. 2a). And then,C10AZOC2N3was added to lower the heptane-water interfacial tension, γH, to decrease the θHand increase the θFsimultaneously, and the Janus emulsion was formed. The H/F interfacial curvature inside the Janus droplet could be gradually decreased upon increasing the concentration of C10AZOC2N3,tuned from “+” passing through “0” to “-” (Fig. 2b–d). When the H/W interfacial tension was lowered further by C10AZOC2N3and resulted in γF≥ γH+ γHF, θH≈ 0, where θHis the contact angle between the H/W interface and the H/F interface,perfluorohexane was encapsulated by heptane completely that caused the formation of F/H/W double emulsion (Fig. 2e). These results illustrate that C10AZOC2N3can adjust the interfacial tension between heptane and aqueous solution, and the morphology of complex emulsions is exactly controlled between the double emulsion and Janus emulsion or to invert entirely by varying the concentration of C10AZOC2N3. It is worthwhile to note that aqueous phase is always the continuous phase in the studied surfactant concentration range.

Fig. 4 (a) Species distribution of C10AZOC2N3 in aqueous solution at different pH. (b) pH-dependent interfacial tensions between heptane and aqueous solution containing 0.1% Zonyl FS-300 and 0.07% C10AZOC2N3.

Fig. 5 Micrographs of the complex emulsion droplets in aqueous solutions of 0.1% Zonyl FS-300 and 0.07% C10AZOC2N3 at different pH.(a) 3.59, (b) 6.19, (c) 7.92, (d) 9.62, and (e) 11.30. Scale bar, 100 µm.
3.2 Inner phase curvature tuned by pH
The presence of N-amido groups endows C10AZOC2N3with pH-sensitivity at the interfacial activity. C10AZOC2N3could be triple-protonated or less due to the presence of three amido or imino groups in the head group, corresponding to three acid dissociation constants, pKa13.43, pKa26.20 and pKa39.68 (Fig.S3, Supporting Information). The species distribution fraction,δ, of C10AZOC2N3was then calculated according to pKa(Fig.4a). The result shows clearly that C10AZOC2N3,C10AZOC2N3H+, C10AZOC2N3and C10AZOC2N3can be presented at respective pH value. Since the surfactant is insoluble in water when pH was below 3, the effect of C10AZOC2N3is not considered in this work. Obviously,C10AZOC2N3at different protonated state endows this surfactant with different surface activity. The interfacial tension between heptane and the aqueous phase at different pH is shown in Fig.4b. Apparently, the H/W interfacial tension was reduced gradually upon increasing pH to 10, but it was increased sharply at pH 11. The pH-dependent H/W interfacial tension might be explained as the following. The protonation degree of C10AZOC2N3was decreased with a pH increase, which resulted in weakening the electrostatic shielding effect of head group,reducing the occupied area of surfactant per molecule at the H/W interface and decreasing the Gibbs free energy at the interface,and so the interfacial tension was decreased gradually. However,C10AZOC2N3as a nonionic surfactant may be less surface active than its ionic partner, thus, the H/W interfacial tension was increased at pH 11 instead. This interfacial tension change caused by the variation of C10AZOC2N3protonation status results in the reconfiguration of the complex emulsion morphology by pH variation.
As expected, in the chosen pH values corresponding to different ionic species of C10AZOC2N3in aqueous solutions, the curvature of the inner phase of the heptane-perfluorohexanewater complex emulsions (Fig. S4, Supporting Information) was pH-responsive. The morphology of the complex emulsion droplets at different pH is shown in Fig. 5. The droplet was a perfect Janus droplet at pH 3.59, where the H/F interfacial curvature was “0”. At pH 6.19 and 7.92, the H/F interfacial curvature decreased to “-”further, and the droplet was tuned to an imperfect Janus droplet. At pH 9.62, they became F/H/W double emulsion droplets. But the H/F interfacial curvature increased close to “0” at pH 11.30, the droplet was tuned back to Janus droplet once again. The results illustrate that the H/F interfacial curvature was gradually decreased from “0” to “-”with pH increasing when pH was blow 11. This change of the complex emulsion morphology was consistent with the interfacial tension decrease of the H/W interface well (Fig. 4b).γHwas gradually reduced along with pH increasing, leading to a θHdecrease and a θFincrease, which characterized that the H/W interface inside the Janus droplet was bent gradually as the trend from Janus state to F/H/W double-emulsion. However, γH was increased abruptly when pH was above 11, causing the F/H/W double emulsion droplets switching back to Janus droplets. In addition, for the Janus droplets fabricated at the low concentrations of C10AZOC2N3such as 0.01% and 0.03%, the H/F interfacial curvature was changed little and the droplet topology was changed slightly. As a result, only near perfect Janus droplet was obtained because insufficient surfactant was participated at the interface in this condition (Fig. S5, Supporting Information).

Fig. 6 (a) Molecular structures of trans-isomer and cis-isomer of C10AZOC2N3 and UV-Vis absorbance spectra of C10AZOC2N3 via UV/Blue light irradiation. (b) Dynamic interfacial tension variation of the H/W interface under UV/Blue light irradiation. (c) Micrographs of the complex emulsion droplets in aqueous solution of 0.1% FS-300 and 0.05% C10AZOC2N3 after UV/Blue light irradiation, scale bar, 100 µm.
3.3 Inner phase curvature switched by light
The photo-response of C10AZOC2N3is attributed to the existence of the azobenzene group in its molecule. Azobenzene has a reversible conformational transition between trans-isomer and cis-isomer by UV/Blue light irradiation, causing the properties variation of molecules and emulsions35. C10AZOC2N3was photo-responsive as it switched to cis-C10AZOC2N3after UV irradiation and reversibly switched back to trans-C10AZOC2N3 after Blue light irradiation, but not all of the cisisomer switched back to trans-isomer (Fig. 6a). The dynamic interfacial tension of the H/W interface under UV/Blue light irradiation showed that the H/W interfacial tension was instantaneously increased slightly in the initial UV irradiation stage and then sharply decreased upon increasing the UV irradiation time, while it was increased abruptly after Blue light irradiation but was not able to reach its initial level (Fig. 6b). The instantaneously increase slightly of the H/W interfacial tension could be explained by the occupied area of C10AZOC2N3 per molecule at the H/W interface that was reduced suddenly when trans-isomer was switched to cis-isomer under UV irradiation,causing a temporary and partial direct contact of heptane and aqueous solution at the interface and inducing a slight temporary increase in the interfacial tension35. However, the interfacial tension activity of cis-C10AZOC2N3 was higher than that of trans-C10AZOC2N336, and thereby resulting in the lower equilibrium interfacial tension. In the reverse process, major cis-C10AZOC2N3was switched back to trans-C10AZOC2N3after Blue light irradiation and the H/W interfacial tension was increased again but not equilibrating at the initial value because of partial transition of cis-C10AZOC2N3. Therefore, the decrease of γHafter UV irradiation could induce the H/F interfacial curvature decreasing from “+” to “-”, and then Blue light irradiation could induce the droplets tuned back reversibly with the increase of γH.
The H/F/W complex emulsions prepared by C10AZOC2N3containing aqueous solutions were irradiated with UV/Blue light(Fig. S6, Supporting Information), and the effect of light irradiation on the morphological change of emulsion droplets fabricated with different concentration of C10AZOC2N3at different pH was studied in detail. The morphological change of complex emulsions observed by light microscopy was shown in Fig. 6c. It is clear that the H/F/W complex emulsion was switched from Janus emulsion to F/H/W double emulsion after UV irradiation and was reversibly switched back after Blue light irradiation, whereas it cannot turn back to the original state completely after a UV/Blue/UV light irradiation cycle. The Janus droplet as the H/F interfacial curvature was “-”, which was fabricated by the high concentration of C10AZOC2N3, could be tuned to F/H/W double emulsion after UV irradiation.However, when the initial droplet morphology was Janus droplet fabricated at the low concentration of C10AZOC2N3 or at low pH,the reduction in the H/F interfacial curvature was very limited,only tuned near “0” after UV irradiation. Hence, the change of the H/F interfacial tension at these two conditions after UV/Blue light irradiation was too small to induce significant change of the droplet topology.
3.4 Possible mechanism of pH-sensitivity and photo-response of the inner phase curvature
On the basis of these interfacial tensions and emulsion morphologies, a possible mechanism for the morphology change of the heptane-perfluorohexane-water complex emulsions controlled by pH variation and UV/Blue light irradiation is proposed as shown in Fig. 7. Since the concentration of Zonyl FS-300 in aqueous solution was kept constant in this work,which meant that Zonyl FS-300 showed little effect on the F/W interfacial tensions. All the changes in this system came from the H/W interfacial tensions controlled by C10AZOC2N3.C10AZOC2N3is pH-sensitivity and photo-response that causes the H/W interfacial tensions highly depending on pH variation and UV/Blue light irradiation, and there by altering the morphology of complex emulsions accordingly.
On one hand, the pH increase of aqueous solution decreased the protonation degree of C10AZOC2N3that weakened the electrostatic shielding effect of head group and reduced the occupied area of C10AZOC2N3 per molecule at the H/W interface, and thereby lowering the H/W interfacial tension. The decrease in the H/W interfacial tension resulted in a θHdecrease and a θFincrease, and tuned the H/W interfacial curvature from“+” to “-”, which appeared as the topology of the complex emulsion droplet was changed from Janus droplet to F/H/W double emulsion. However, the H/W interfacial tension was suddenly increased above pH 11 when C10AZOC2N3was nonionic form, leading to the complex emulsion droplet changed back to Janus droplet. On the other hand, UV irradiation induced trans-C10AZOC2N3to cis-C10AZOC2N3isomerization reduced the occupied area of C10AZOC2N3per molecule at the H/W interface, which also caused the decrease of H/W interfacial tension. Similarly, the H/W interfacial tension decrease caused the H/W interfacial curvature from “+” to “-” and the complex emulsion droplet changed from Janus droplet to F/H/W double emulsion. After Blue light irradiation, major cis-C10AZOC2N3was switched back to trans-C10AZOC2N3and the H/W interfacial tension was increased again but could not reach its initial value because of the incomplete isomerization of cis-C10AZOC2N3. The F/H/W double emulsion was tuned back to Janus emulsion reversibly under Blue light irradiation.

Fig. 7 Possible mechanism of the complex emulsion droplet morphology change with pH variation and UV/Blue light irradiation.
4 Conclusions
In this work, the morphological control of a heptaneperfluorohexane-water (H/F/W) three-phase complex emulsion was realized solely with a pH and light dual-responsive surfactant, C10AZOC2N3, by pH variation and UV/Blue light irradiation. With increasing the concentration of C10AZOC2N3,the phase inversion from the H/F/W double emulsion to the F/H/W double emulsion passing through the Janus droplets intermediate was achieved. This is because C10AZOC2N3 can continuously decrease the interfacial tension between heptane and aqueous solution upon increasing its concentration, and thereby tuning the complex emulsion droplet topology.Moreover, the H/W interfacial tension could also be altered by pH variation and UV/Blue light irradiation in the presence of C10AZOC2N3, which was creatively applied to control the morphology of complex emulsions further. This work provides a novel idea of controlling the morphology of complex emulsions with external multi-stimulus solely by simply introducing a multi-responsive surfactant. The multi-responsive complex emulsions would be applied in drug delivery taking advantage of the stimuli-responsive morphology change or as templates to generate Janus particles for further applications.
Supporting Information:available free of charge via the internet at http://www.whxb.pku.edu.cn.
