以4,4′-(1-咪唑基亚甲基)二苯甲酸为配体的锌配合物的合成、晶体结构和荧光性质
2019-01-14刘光祥
喻 敏 宣 芳 刘光祥
(南京晓庄学院环境科学学院,新型功能材料南京市重点实验室,南京 211171)
The design and synthesis of coordination polymers (CPs)have continued unabated over the past decade.These materials possess diverse and enticing applications in gas storage and separation,sensing,ion exchange,heterogeneous catalysis,non-linear optics,explosives residue detection,luminescence and even drug delivery[1-8].However,the applications of CPs are directly related to their structural features.Thus,the fabrication of desired CPs with the targeted structures and properties has become a great challenge for research scientists owing to many key factors including metal ions,organic ligands,metalligand ratio,pH,reaction solvents,temperature,as well as the oxidation state of the metal ion[9-12].Among them,the design and judicious selection of organic ligands are of crucial concern in the CP construction because the structure,symmetry,solubility,size,and coordination mode of these ligands directly correlate with the architectures of CPs[13-16].Generally speaking,multifunctional ligands are the first choice for the construction of coordination polymers because they are sensitive to pH value of the reaction solution and can adopt variety of coordination modes to bridge metal ions[17-20].Here, the 4,4′-((1H-imidazol-1-yl)methylene)dibenzoic acid (H2IMB)ligand was chosen as the linker,and the reasons are as follows:flexible carboxyphenyl and rigid imidazolyl group can provide multidentate metal-binding sites and diverse coordination modes;the varying coordination modes of the ligands have great potential in synthesizing different coordination polymers with intriguing structures and unique properties[21-23].Moreover,it is equally important that the metal ions have a d10configuration to construct coordination polymers with an excellent luminescent property[24-26]. Motivated by these abovementioned aspects,we obtain two new coordination polymers,namely,[Zn(HIMB)2]n(1)and{[Zn(IMB)]·1.5H2O}n(2).Herein,we report their syntheses,crystal structures and luminescent properties.
1 Experimental
1.1 Materials and general methods
All reagents and solvents employed in this work were commercially available and used without further purification.The H2IMB ligand was purchased from WuXi AppTec Group.Elemental analyses(C,H and N)were performed on a Vario ELⅢelemental analyzer.Infrared spectra were recorded on KBr discs using a Nicolet Avatar 360 spectrophotometer in the range of 4 000~400 cm-1.The luminescent spectra for the powdered solid samples were measured at ambient temperature on a Horiba FluoroMax-4P-TCSPC fluorescence spectrophotometer.Powder X-ray diffraction(PXRD)measurements were performed on a Bruker D8 Advance diffractometer at 40 kV,40 mA with Cu Kα radiation (λ=0.154 056 nm)and a graphite monochromator scanning from 5°to 50°at room temperature.
1.2 Synthesis of[Zn(HIMB)2]n(1)
A mixture containing Zn(NO3)2·6H2O (29.6 mg,0.1 mmol)and H2IMB (32.2 mg,0.1 mmol)in 15 mL of DMF/CH3OH/H2O (1∶2∶2,V/V)mixed solution was sealed in a 25 mL Teflon lined stainless steel container and heated at 100℃for 3 days.Colorless block crystals of 1 were collected by filtration and washed with water and ethanol several times with a yield of 44%based on H2IMB ligand.Anal.Calcd.for C36H26N4O8Zn(%):C,61.07;H,3.70;N,7.91.Found(%):C,60.98;H,3.72;N,7.93.IR (KBr,cm-1):3 457 (br),3 139 (w),1 702 (s),1 561 (s),1 521 (s),1 419 (m),1 391 (s),1 273 (w),1 208 (m),1 122 (w),997 (w),892 (m),835 (w),782 (w),651 (w),565 (w),542 (w).
1.3 Synthesis of{[Zn(IMB)]·1.5H 2O}n(2)
A mixture containing Zn(NO3)2·6H2O (29.6 mg,0.1 mmol)and H2IMB (32.2 mg,0.1 mmol)in 15 mL of DMF/H2O (2∶1,V/V)mixed solution was sealed in a 25 mL Teflon lined stainless steel container and heated at 100℃for 3 days.Colorless pillar crystals of 2 were collected by filtration and washed with water and ethanol several times with a yield of 26%based on H2IMB ligand.Anal.Calcd.for C18H15N2O5.5Zn (%):C,52.38;H,3.66;N,6.79.Found (%):C,52.47;H,3.65;N,6.81.IR (KBr,cm-1):3 473 (br),3 165 (w),1 608 (s),1 519 (m),1 422 (s),1 377 (s),1 273 (m),1 123 (w),1 034 (w),1 010 (s),871 (m),835 (w),778 (w),659 (m),527 (m).
1.4 X-ray crystallography
Two single crystals with dimensions of 0.22 mm×0.16 mm×0.08 mm for 1 and 0.18 mm×0.14 mm×0.10 mm for 2 were mounted on glass fibers for measurement,respectively.X-ray diffraction intensity data were collected on a Bruker APEXⅡCCD diffractometer equipped with a graphite-monochromatic Mo Kα radiation (λ=0.071 073 nm)using the φ-ω scan mode at 293(2)K.Data reduction and empirical absorption correction were performed using the SAINT and SADABSprogram[27],respectively.The structures were solved by the direct method using SHELXS-2016[28]and refined by full-matrix least squares on F2using SHELXL-2016[29].All of the non-hydrogen atoms were refined anisotropically.The solvent molecules in 2 were highly disordered and were removed from the diffraction data by the SQUEEZE routine of PLATON program.The final formula of 2 were determined by single-crystal structures,elemental analysis results and TGA.The details of the crystal parameters,data collection and refinement for 1 and 2 are summarized in Table 1,and selected bond lengths and angles with their estimated standard deviations are listed in Table 2.
CCDC:1851301,1;1851302,2.

Table 1 Crystal data and structure refinement for 1 and 2
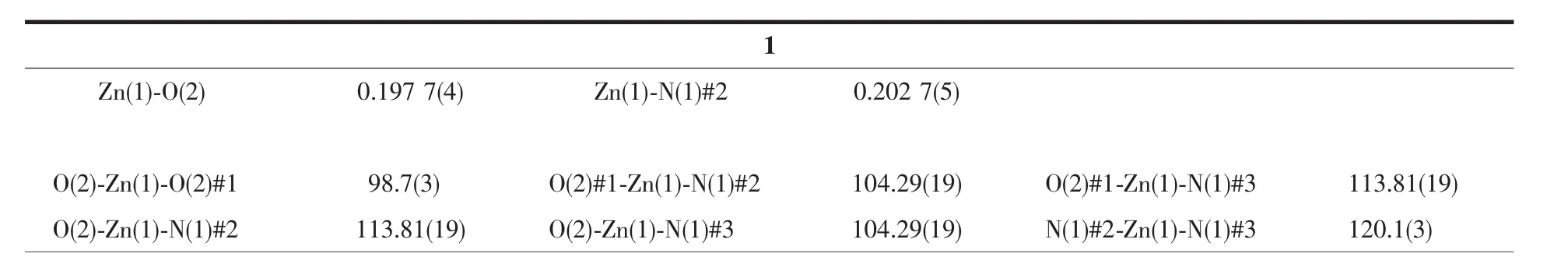
Table 2 Selected bond lengths(nm)and angles(°)for 1 and 2

Continued Table 2
2 Results and discussion
2.1 Crystal structure
Single crystal X-ray diffraction analysis revealed that complex 1 crystallizes in the orthorhombic space group C2221and features a 2D→2D polycatenane of 2-fold interpenetrated sql layer.As illustrated in Fig.1,the asymmetric unit of complex 1 contains a half zinc ion located on a crystallographic 2-fold axis and one crystallography independent HIMB-anion.The central ZnⅡion is four-coordinated by two carboxylate oxygen atoms from two HIMB-anions,and two nitrogen atoms from two other HIMB-anions in a distorted tetrahedral coordination geometry.Bond lengths and angles about the zinc ion are standard for tetrahedral coordination (Table 2).The central carbon of HIMB-anion shows sp3hybridization,exhibiting C(N)-Ncentral-C angles of 111.9(5)°,111.7(6)° and 114.8(5)°.The dihedral angles between the phenyl rings of the HIMB-anion are 88.25°,84.64°and 66.18°,and the Ncentral-C(N)bond lengths are 0.148 0(8),0.153 3(9)and 0.153 2(9)nm,respectively.In 1,adjacent ZnⅡcenters are linked by partially deprotonation HIMB-anions to generate Zn-HIMB chains,which are further extended by HIMB-anions to form a puckered sheet with Zn…Zn distance of 1.171 6 nm(Fig.2).The sheet has only a kind of rhombic window of Zn4(HIMB)4with dimensions of 1.870 4 nm×1.396 4 nm.
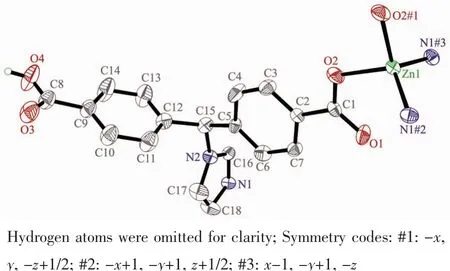
Fig.1 Coordination environment of the ZnⅡions in 1 with the ellipsoids drawn at the 30%probabilitylevel
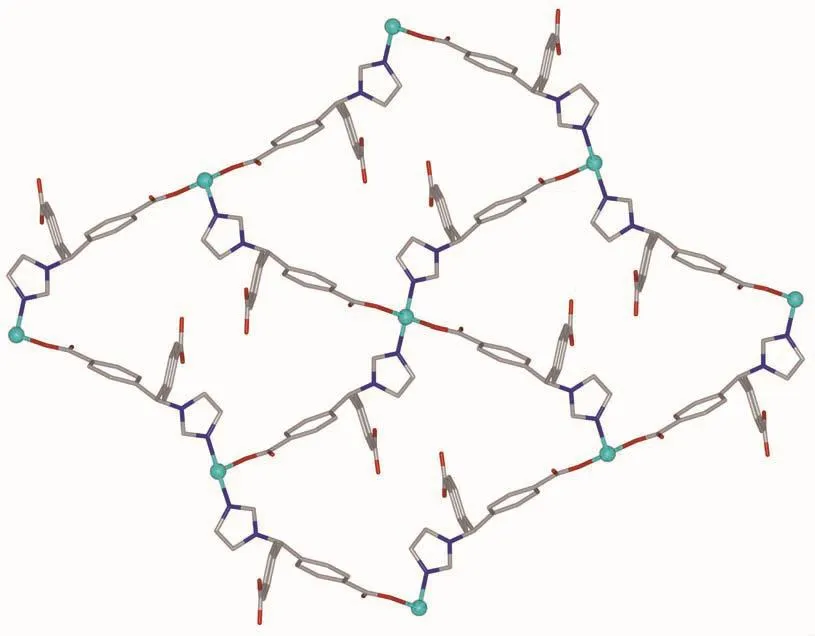
Fig.2 Perspective view of the sql layer in 1
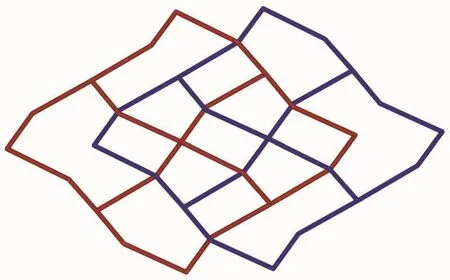
Fig.3 Schematic view of the 2D→2D polycatenane of 2-fold interpenetrated layers
Based on the concept of topology,the layer can be simplified into a (4,4)-connected sql net.The potential voids are filled via mutual interpenetration of two independent equivalent networks in a normal mode,giving rise to a 2D→2Dpolycatenane framework of 2-fold interpenetration (Fig.3).Their mean planes are parallel and coincident.In addition,intramolecular O4-H4…O1 (O4…O1 0.264 1(5)nm)hydrogen-bonding interactions between the layers further stabilize the 2D structure of 1.
Changing the solution from DMF/CH3OH/H2O to DMF/H2O for the same reaction mixture of 1 affords complex 2,which crystallizes in the tetragonal I41/acd space group and possesses a 3D framework with a dinuclear structure.The asymmetric unit of 2 contains one crystallographically independent ZnⅡion,one individual IMB2-anion as well as one and a half free water molecule.As depicted in Fig.4,each ZnⅡion is four-coordinated by three oxygen atoms from three different IMB2-anions and one nitrogen atom from the imidazole group of IMB2-anions,exhibiting a slightly distorted tetrahedral geometry.All chemical bonds fall in the normal ranges[30].

Fig.4 Coordination environment of the ZnⅡions in 2 with the ellipsoids drawn at the 30%probability level
The central carbon of HIMB-anion of 2 shows sp3hybridization,exhibiting C(N)-Ncentral-C angles of 111.9(5)°,111.7(6)°and 114.8(5)°.The dihedral angles between the phenyl rings of the HIMB-anion are 88.25°,84.64°and 66.18°,and the Ncentral-C (N)bond lengths are 0.148 0(8),0.153 3(9)and 0.153 2(9)nm,respectively.In 2,the H2IMB ligands are fully deprotonated and the carboxyl groups of IMB2-adopt two coordination modes: μ2-η2∶η1(bis-bridging mode)and μ1-η1∶η0(monodentate mode),and connect two ZnⅡ ions to generate the dinuclear SBUs[Zn2(COO)2]with Zn…Zn distance of 0.375 5 nm (Fig.5).Each IMB2-links three SUBs to give a lattice-shaped 2D layer structure along the c-axis (Fig.6),which is further expanded by IMB2-to form a 3D framework.

Fig.5 Two dimensional network of 2 viewing along the c-axis
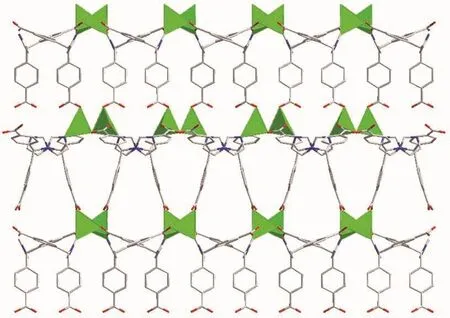
Fig.6 Three dimensional framework of 2 viewing along b direction
By topological analysis,when the dinuclear SBUs are regarded as 6-connected node and the IMB2-as 3-connected node,respectively,the structure of 2 can be simplified as a 2-nodal (3,6)-connected network with the point symbol of (611.84)(63)2.Interestingly,two independent equivalent frameworks interlace each other,resulting in a 2-fold interpenetrated 3D framework (Fig.7).
Two different complexes 1 and 2 were synthesized on the basis of the selection of reaction solvent systems,while the other synthetic parameters were intentionally held constant.The dimensionality and topology of the networks produced in this work are determined by the coordination environments of ZnⅡion and linking modes of H2IMB ligand,which are clearly dictated by the solvent molecules.The solvent not only can affect the extent of deprotonation of organic multicarboxylate ligand but also can induce different conformation of the flexible organic mutilcarboxylate ligand[31-33].During the self-assemble process,the H2IMB ligand deprotonated one proton into HIMB-for 1,two protons into IMB2-for 2,respectively.In complex 1,HIMB-ligand links two different ZnⅡions inμ2-N,O coordination mode.While in complex 2,IMB2-ligand links four different ZnⅡ ions in μ2-N,(η2-O′,O″),(η1-O′)coordination mode.The different conformations and coordination modes for HIMB-and IMB2-resulted in structural variation from 2D layer to 3D framework.Thus,the solvent of reaction mixture plays a key role in controlling the structures of the coordination compounds.

Fig.7 Three dimensional 2-fold interpenetrated(3,6)-connected framework of 2
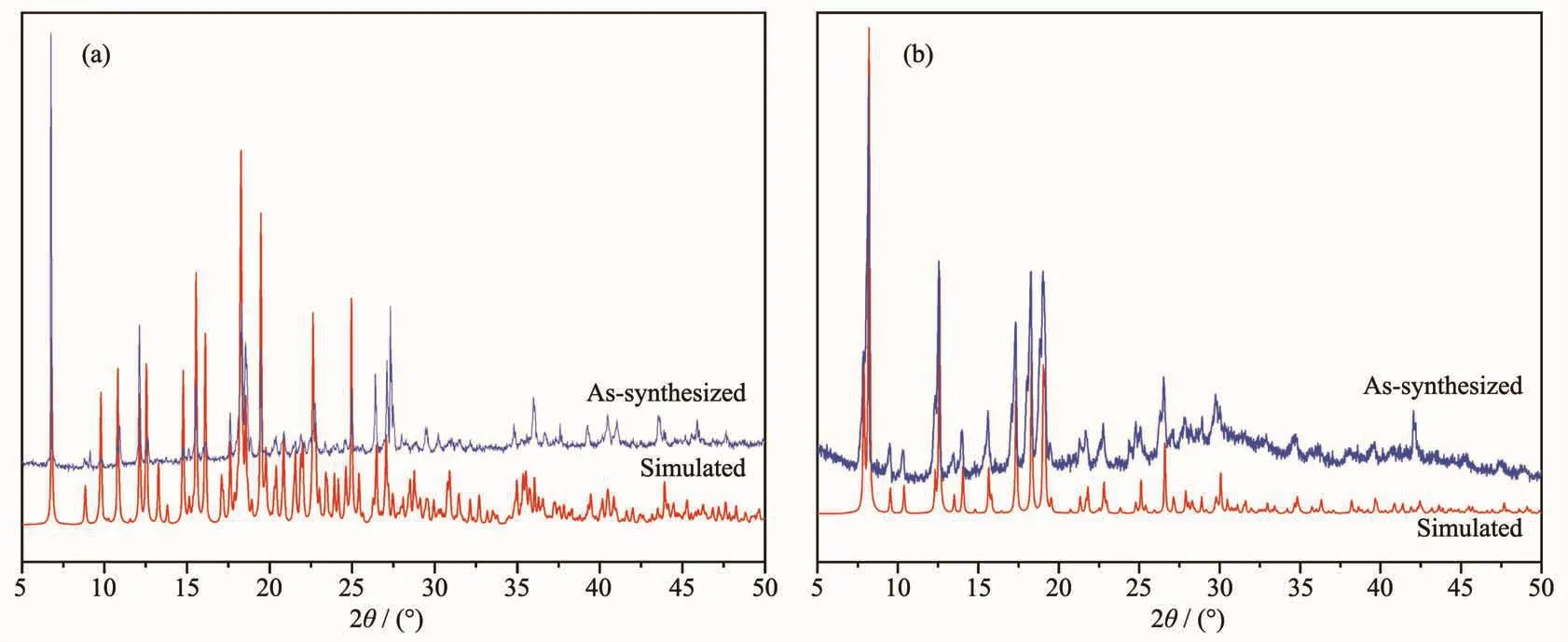
Fig.8 PXRD patterns of complexes 1 (a)and 2 (b)
2.2 FTIR spectra
The IR spectra of 1 showing the presence of the characteristic bands at 1 702 cm-1indicate the partially deprotonation of H2IMB,while the IR spectra of 2 showing the absence of the characteristic bands at around 1 700 cm-1attributed to the protonated carboxylate group indicate that the complete deprotonation upon reaction with metal ion.The characteristic bands of carboxyl groups were shown in the range of 1 560~1 610 cm-1for antisymmetric stretching and 1 370~1 420 cm-1for symmetric stretching.The separations(Δν)between νasym(CO2)and νsym(CO2)bands indicate the presence of different coordination modes.The bands in the region 660~1 300 cm-1are attributed to the-CH-in-plane or out-of-plane bend,ring breathing,and ring deformation absorptions of benzene ring,respectively.Weak absorptions observed at 3 139~3 165 cm-1can be attributed toνC-Hof benzene ring.The IR spectra exhibited the characteristic peaks of imidazole groups at ca.1 520 cm-1[34].
2.3 Powder X-ray diffraction(PXRD)and thermal analyses
Powder X-ray diffraction analysis (PXRD)experiments were carried out for 1 and 2 at room temperature to characterize their purity.As shown in Fig.8,the measured peak positions closely match the simulated peak positions,indicative of pure products.The thermal behaviors of complexes 1 and 2 were measured under a dry N2atmosphere at a heating rate of 10℃·min-1from 25 to 700℃ and the TG curves are presented in Fig.9.Complex 1 began to collapse at 210℃.Complex 2 lost water molecules before 158℃,and the framework began to decompose at 320℃.

Fig.9 TGA curves of complexes 1 and 2
2.4 Luminescent properties
Based on the references about the fluorescent properties of Zn complexes[35-38],the solid state luminescent properties of the title complexes and H2IMB ligand were measured at room temperature under the excitation of 285 nm (Fig.10).The H2IMB displayed a maximum peak at 462 nm,which is attributed to the π→π*and n→π*transition of intra-ligands.The emission peaks of two complexes occurred at 386 nm(λex=315 nm)for 1 and 395 nm (λex=323 nm)for 2,respectively.Theemission of 1 and 2 can be essentially ascribed to the intraligand fluorescent emission.The red-shift of the emission can be attributed to the ligand coordination to the metal center,which effectively increases the rigidity of the ligand and reduces the loss of energy by radiationless decay[39-42].The emission strength of 2 was stronger than complex 1,whichmaybecaused bydifferentcoordinationmodes.
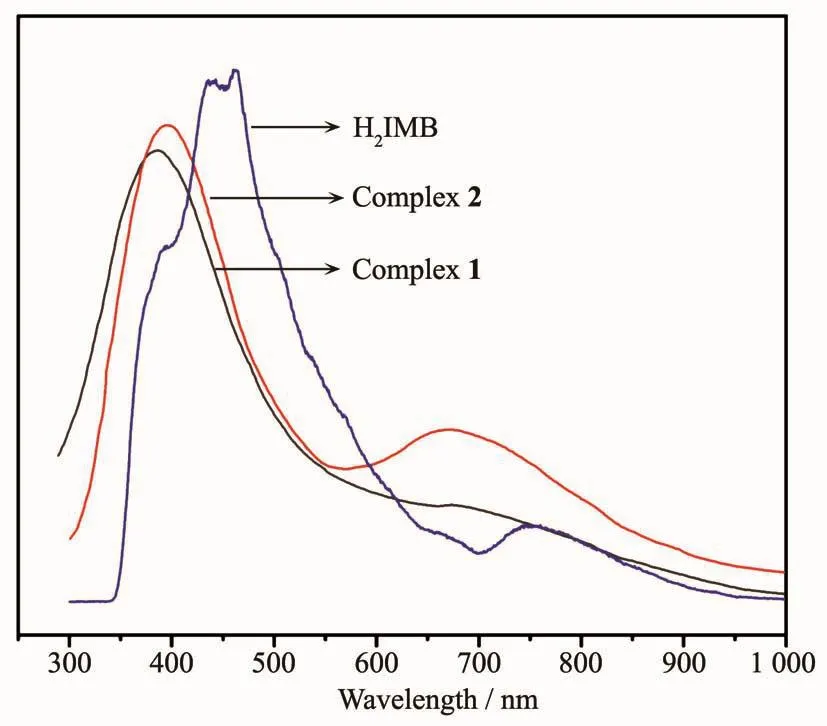
Fig.10 Emission spectra of free H2IMB ligand,1 and 2 at room temperature
