Impact of Biochar Amendment on Soil Quality and Crop Yield in a Greenhouse Environment
2019-01-09RossanaMarzaioliElioCoppolaPaolaIovienoAlfonsoPentangeloCatelloPaneFloraAngelaRutigliano
Rossana Marzaioli,Elio Coppola,Paola Iovieno,Alfonso Pentangelo,Catello Pane,Flora Angela Rutigliano
1Dipartimento di Scienze e Tecnologie Ambientali,Biologiche e Farmaceutiche,Università degli Studi della Campania “Luigi Vanvitelli”,Caserta 81100,Italy
2Consiglio per la ricerca in agricoltura e l’analisi dell’economia agraria – Centro di ricerca Orticoltura e Florovivaismo,Pontecagnano 84091,Italy
Keywords Biochar Greenhouse agriculture Crop yield Microbial biomass Microbial activity Rhizoctonia solani Suppressiveness
Abstract Greenhouse agriculture,a widespread practice in the Mediterranean basin,is prone to impoverishment in soil organic carbon because of crop removal and high decompositionrate.Open- field experimentationhas shown that the addition of biochar,a product of thermochemical conversion of biomass,under a limited concentration of oxygen,increases the soil organic C pool,enhances crop productivity and improves C terrestrial sink.The present study investigates the effect of biochar amendment in a greenhouse environment in a Southern Italy organic farm.Two doses(10 or 20 t ha-1)of biochar from conifer pruning wastes were applied immediately before planting 1-week old plants of pepper(Capsicum annuum L.).Plant growthandcrop yieldwere evaluatedsix monthslater,at the endof cultivation,in biochar-treated and in control(without biochar)plots.Soil samples were collected in the same plots immediately after biochar addition and six months later and were analyzed for the following parameters:bulk density,water-holdingcapacity,pH,electrical conductivity,organiccarbon,mineral nitrogen,total microbial biomass and fungal mycelium contents,soil respiration,nitrogen mineralization,potential nitrification,soil suppressiveness to Rhizoctonia solani.A single biochar application caused no apparent damage to the crop;on the other hand,no improvement was observed in crop yield or soil suppressiveness to R.solani.In contrast,the single char application positively affected soil respiration,nitrogen mineralization and potential nitrification.These preliminary results suggest that soil amendment with biochar is a potentially useful practice in greenhouse agriculture,yet furtherexperimentationis necessary to assess optimal amounts for better crop productivity and soil quality.
1 Introduction
Greenhouse cultivation is a growing agricultural sector,with about 2 million hectares employed in the world and over 400,000 ha in the Mediterranean Basin,mainly Spain,Italy,France,Greece,and Turkey(Scarascia-Mugnozza et al.,2011).Greenhouse cultivation in Italy currently covers an area of about 42,000 ha,mainly in Sicily,Campania,Lazio,Veneto and Liguria Regions(ISTAT,2010).Of these,37,000 ha are employed for the production of vegetables and fruit,the rest for cultivation of ornamental plants and flowers(ISTAT,2010).
The expansion of this agricultural sector depends on several advantages offered by greenhouse cultivation.Controlled microclimatic conditions,relatively low building costs and high crop yields throughout the year(also thanks to pest control)afford farmers high net earnings(Lamont,2005).On the other hand,this type of cultivation entails a steady decline in soil quality due to organic matter loss;this depends on high decomposition rate,due to optimal temperature and water conditions,and continuous removal of crop residues(Bonanomi et al.,2011).The reduction in organic matter content negatively impacts on other soil properties,such as waterholding capacity,cation-exchange capacity,nutrient content(Karlen et al.,1997)and,consequently,on soil microbial growth and activity(Bonanomi et al.,2014).An increase in soil salinity is also frequent because of unbalance between upward movement of soil water(transporting salts to the soil surface),caused by high evapotranspiration,and water input by irrigation(Chen et al.,2004).Maintaining and/or improving the pool of soil organic matter is crucial for sustainable agriculture(Vanlauwe et al.,2010),as this parameter affects fundamental soil services such as nutrient cycling and carbon sequestration(Adhikari and Hartemink,2016).
The application of organic amendments,such as compost,is a reliable and effective practice to increase soil organic carbon and consequently soil fertility and crop yield(Smith et al.,1997).However,accelerated decomposition in greenhouse conditions demands high doses and repeated applications of organic amendments,and,consequently,increased costs and carbon dioxide emissions(Kaur et al.,2008).A particularly stable organic amendment is carbonized material known as“biochar”,obtained by thermochemical conversion of biomass(such as plant wastes)in an oxygen-limited environment(IBI,2013).Biochar addition to soil has been reported to produce increased plant growth and higher yields(Asai et al.,2009;Vaccari et al.,2011,2015).Moreover,biochar was found to improve plant resistance to pathogens by stimulating antagonistic micro flora and/or introducing biochar-associated organic antifungal compounds(De Corato et al.,2015).Biochar improves long-term soil fertility by increasing the pH and enhancing the water-holding capacity,bulk density and micro-aeration,as well as the cation-exchange capacity,nutrient retention,and organic-matter adsorption(Lehmann et al.,2006;Laird et al.,2010).Biochar addition may also positively affect the soil microbial community,which is fundamental for the soil functioning and ecosystem service provisioning(Nannipieri et al.,2012;Schmidt et al.,2014).
Because of its molecular structure dominated by aromatic C,biochar is much more resistant to microbial decomposition(from 1,000 to 10,000 years;Warnock et al.,2007)than uncharred organic matter(Baldock and Smernik,2002),therefore biochar employment for soil amendment is considered a promising way of increasing the terrestrial C sink.Biochar treatment also reduces emission of greenhouse gas N2O(Castaldi et al.,2011;Xu et al.,2014).Moreover,biochar production from organic wastes for use in agriculture is an effective strategy for waste disposal,with positive effects on economy and environment(Raveendran et al.,1995;Nik-Azar et al.,1997).
Biochar application has been reported to have either positive or negative effects on soil microbial community,depending of its characteristics.An increase in total microbial biomass due to biochar treatment probably depends on the release of organic and inorganic nutrients(Lehmann et al.,2011).Moreover,the microporous structure of biochar is thought to provide microbes with a favorable habitat(Thies and Rillig,2009)and a refuge from predators(Warnock et al.,2007).Onthe other hand,the high pHand the abundance of aromatic compounds of biochar could negatively affect the soil microbial community,in particular the fungal populations(Warnock et al.,2010).
The effects of biochar application in open- field crops have been investigated quite extensively(Steiner et al.,2008;Asai et al.,2009;Vaccari et al.,2011;Rutigliano et al.,2014);several studies have been conducted on the use of biochar as a substrate for pot plants(Baronti et al.,2010;2014;Deenik et al.2010;Gartler et al.,2013;Smider and Singh,2014;Xu et al.,2014),but as far as we are aware there is no report on the effects of in-situ biochar addition to greenhouse soil.
The Italian legislation(D.L.vo 75/2010,as modified in 2015)has recently included biochar in the list of amendments approved in agriculture.In contrast to the considerable body of information on the use biochar amendment in open field,as of yet the general effects and dosage application of biochar in greenhouse environment have not been directly tested.
The aim of the present study was i)to verify the absence of negative effects of biochar on crops and soil and ii)to assess benefits from biochar addition on crop yield and soil quality in a greenhouse environment.In combination with chemical and physical soil properties,we investigated microbial biomass and activity because the soil micro flora is a highly dynamic soil component that reacts to change of environmental conditions long before chemical parameters,such as organic C or total N content(Powlson et al.,1987;Jørgensen and Emmerling,2006).
2 Material and methods
2.1 Study area
The study was carried out in 2016 in a large greenhouse belonging to an organic farm located in Eboli(Southern Italy).The soil present in the greenhouse is classified as Vertic Calcisols,with clay texture(Scotti et al.,2016).Soil physical and chemical properties were reported by Scotti et al.(2016).
Before the greenhouse was built,in 2009,the area used to be an orchard.Until 2014,the greenhouse has been employed for cultivation of cabbage and lettuce in winter,melon and watermelon in summer.Before annual cultivation cycle(generally in June),the soil was amended with a compost produced in the same farm from cultural residues(as described by Scotti et al.,2016),worked up to 50 cm depth and solarized.Soil solarization was realized bringing soil to field capacity with 90 m3ha-1of water and covering it with a plastic film of polyethylene from June to all August.Soil temperature reached 60-65◦C in the first 10 cm of depth.Sub-alkaline well water(pH=7.7-8.0)was used for irrigation.In line with organic farming rules,no pesticides were applied to the crops.The greenhouse had not been in use for two years before the present study.
2.2 Experimental design
The biochar experiment was carried out following an experimental design with randomized blocks,defined by three tunnels,each 40 m in length and 2.4 m in width.Each tunnel was divided into three 10-m-long plots that were treated with biochar in amounts equivalent to 10 t ha-1(B10),20 t ha-1(B20),0 t ha-1(control).A strip of about 2.5 m was left between neighboring plots to avoid interference between treatments.Before biochar treatment(15 April 2016),the soil underwent milling;sprinkler irrigation(5 minutes)was applied immediately after biochar addition,followed by new mild vertical milling to incorporate the biochar in the soil.The following day(16 April 2016),one-week-old pepper seedlings(Capsicum annuum L.,Kaptur F1 variety from Monsanto vegetable seeds)from a nursery were planted in the experimental plots.
During pepper cultivation,12 treatments with the organic liquid fertilizer BIO ENERGY(Biolchim)were applied by fertigation every ten days at rate of 30 L ha-1.As reported in a previous study carried out in the same farm(Scotti et al.,2016),the routinely employment of compost fertilization and fertigation by farmers resulted in a mean soil N content of about 1.3 g kg-1dry soil.
Thebiochar applied(obtained fromAgrindustria s.n.c.;Cuneo,Italy)wasproduced bygasification(1000◦C),in low oxygen concentration,from spruce wood(2-8 cm)of Northwestern Italy(Bedussi et al.,2015).Characteristics of the resulting biochar(ø≤4.4 mm)and measurement methods are reported in Table 1.
2.3 Sampling and analyses
Plant growth was determined by measuring the mean height(cm)of 20 randomly-collected plants from each plot(60 plants for treatment).Harvesting of berries was performed six times in the period July-October,and data were cumulated.Crop yield was evaluated by determining the average number of berries for plant,average weight(g)of a single berry,and total weight(kg)of berries for square meter,assayed on the whole plot(=50 plants;150 plants for treatment).
Two soil samplings were carried out:the first immediately after biochar addition and before transplanting the pepper seedlings(time t1h;15/04/2016);the second soil sampling was performed immediately after crop collection(14/10/2016;time t6M).Each time,two soil replicates were collected(15 cm depth)from each plot,thus obtaining 6 replicates for treatment,for a total of 36 samples(2 replicates x 3 plots with the same treatment×3 treatments×2 sampling times).Immediately after sampling,the samples were transferred to the laboratory and sieved(sieve mesh:2 mm)to remove coarser fragments(roots,stones,etc.).Each sample was then separated into two aliquots;the first was air dried to constant weight for determination of organic carbon content,pH and electrical conductivity,the second was stored at 4◦C for determination of water content,NH+4-N and NO-3-N content,microbial biomass,fungal mycelium,potential respiration,nitrogen mineralization and potential nitrification,as well as suppressiveness against Rhizoctonia,a plant pathogen(see below).In addition,an undisturbed soil core(15 cm depth)was collected each time from each plot to assess water-holding capacity and bulk density.
Soil water content and water-holding capacity were determined gravimetrically(Allen et al.,1989);bulk density was assayed on the basis of dry weight and volume of soil cores(USDA-NRCS,2004);total organic C(Corg)was determined by wet digestion in 0.1667 M of K2Cr2O7(Walkey and Black,1934;Sleutel et al.,2007);pH and electrical conductivity(EC)were measured(USDA-NRCS,2004)in 1:2.5 and 1:2 soil/water suspensions,respectively.Microbial biomass was evaluated with the chloroform fumigation-extraction method(Vance etal.,1987):organic carbon extracted with0.5MK2SO4fromfumigated and non-fumigated soilsamples was determined by chemical digestion with 0.4 N K2Cr2O7at 160◦C,followed by titration with 0.04 N iron(II)sulphate.Microbial biomass,expressed as microbial carbon(Cmic),was calculated from the difference between organic carbon of fumigated and non-fumigated soil samples according to the equation by Vance et al.(1987).Fungal mycelium was assayed with the membrane filter technique(Sundman and Sivelä,1978),evaluating the length of hyphae by the intersection method(Olson,1950).Potential soil respiration was determined by gas chromatography(Kieft et al.,1998,modified).Soil was pre-incubated in 50-ml vials for 3 days in standard conditions(25◦C and 55%water-holding capacity)in order to eliminate abiotic CO2.After pre-incubation,the vials wereflushed with CO2-free air for 3 minutes,sealed and incubated in standard conditions for 1 h(Anderson and Domsch,1978).CO2evolution was quantified by gas chromatography.N mineralization and potential nitrification were measured as described by Castaldi et al.(2009)on soil samples incubated aerobically for 21 days(60%water-holding capacity,25◦C,in the dark),with no substrate or with addition of ammonium sulphate(100 mg N g-1dry soil)to determine net N mineralization and potential nitrification,respectively.After incubation,inorganic nitrogen extracted with 0.5 M K2SO4(1:5 soil:extractant ratio)was measured using selective electrodes for NO-3and NH+4(Castaldi et al.,2009).
Soil natural suppressiveness against Rhizoctonia solani damping-off on the host plant Raphanus sativus was assessed in microcosm experiments on soil samples from treated plots.Five tray cells per treatment were filled with infected 5-mm sieved soil,with control cells fully filled with healthy uninfected soil.Pathogen inoculum preparation,disease incidence calculation and data elaboration were carried-out as described by Pane et al.(2013).The design included three replications(each with five cells)and the experiment was repeated twice.
2.4 Statistical analysis
Before applying parametric tests,a normality test(Kolmogorov-Smirnov)was performed(SigmaPlot12).The parameters were normalized by log10transformation when not normally distributed.One-Way ANOVA wasused and integrated,when required,by Student-Newman-Keuls test,to evaluate the significance(P<0.05)of differences among treatments(SigmaPlot12).Correlations among parameters were analysed by Pearson’s coefficient test(SigmaPlot12).
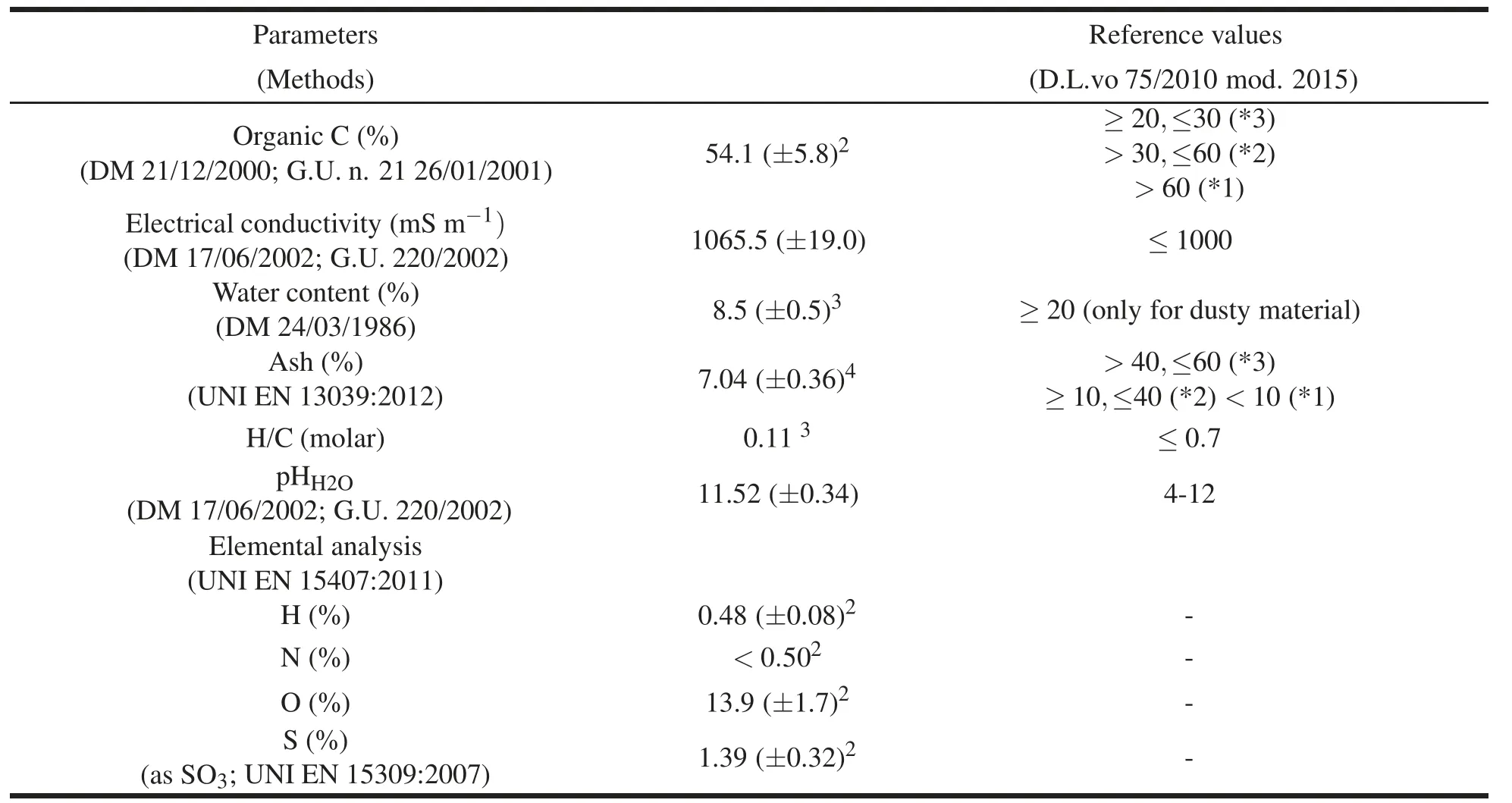
Table 1 Meanvalues(±uncertainties)1ofphysicalandchemicalcharacteristicsofnon-dustybiocharutilizedin the present study.
3 Results
The biochar used in this study was generally compliant with the thresholds imposed by Italian legislation(Legislative Decree 75/2010,as modified in 2015;Official Gazette n.186/2015),except for the electrical conductivity,which was slightly above the maximum level allowed(Table 1).Based on organic C content,the biochar used in this study belonged to quality class 2(≥30%and<60%),whereas,based on ash content it belonged to quality class 1(<10%).
Biochar addition to soil in greenhouse plots caused no apparent negative effects on pepper crop(Table 2)or soil(Table 3;Figs.1,2)with either amounts employed(B10,B20).The alkaline grade of biochar(Table 1)produced only a temporary(t1h)increase of pH in plots treated with 20 t ha-1biochar(B20)compared to both the control and soil treated with a lower biochar dose(B10),this effect being no longer observable 6 months after treatment(t6M;Table 3).The parameters considered showed either no response or a positive response to biochar.In particular,pepper plant growth and crop yield were not affected by biochar treatment(Table 2).Similarly,no effect of biochar was observed on bulk density(BD)and N-mineral content(Table 3).A significant increase was observed in soil water-holding capacity(WHC)and organic carbon(Corg)content(the latter limited to 6 months after treatment,t6M),as well as a slight(non-significant)increase in soil water content(Table 3).Biochar addition did not change microbial biomass(Fig.1A)or fungal mycelium(Fig.1B),yet it stimulated microbial activity as indicated by a significant increase in soil respiration(Fig.2A)and potential nitrification(Fig.2C)at both sampling times,and in N mineralization at the first sampling time only(t1h;Fig.2B).The increase in potential nitrification and,limited to t6M,soil respiration was only observed with the highest biochar dose(B20).Soil respiration and N mineralization were positively correlated with soil water content(respectively,r=0.56 and r=0.67,P<0.001)and negatively correlated with bulk density(r=-0.36 and r=-0.47,P<0.05);potential nitrification was positively correlated with soil pH(r=0.43,P<0.01).

Fig.1 Mean values(+standard deviations)of microbial biomass(Cmic,A)and fungal mycelium(B)in plots treated with 10 t ha-1(B10)and 20 t ha-1(B20)biochar and in control(C),determined1 hour(t1h)and 6 months(t6M)after treatment.N.S.:non-significant differences between treatments for each sampling date.
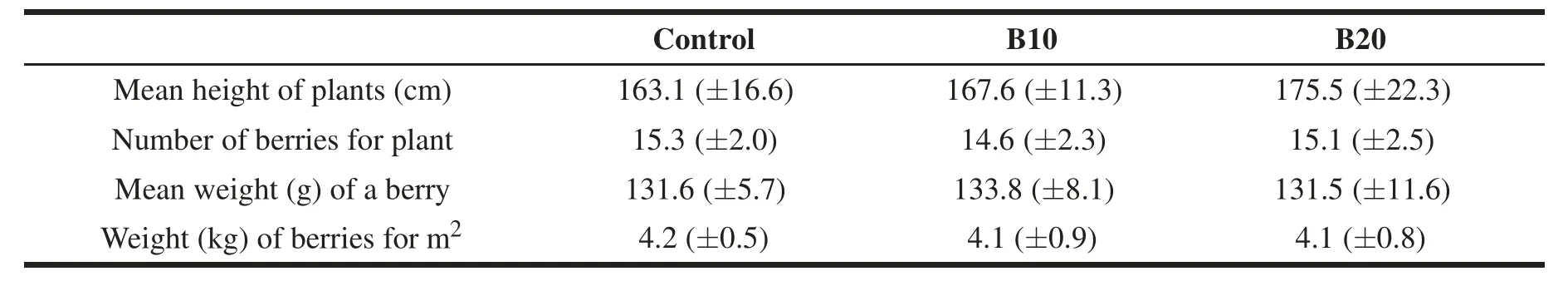
Table 2 Crop yield in plots treated with 10 t ha-1(B10)and 20 t ha-1(B20)biochar and in control.Values are means(±standard deviations).
Biochar application did not enhance soil suppressiveness to R.solani.Our microcosm assays on soil samples from B10,B20 and control plots showed an average disease incidence of 93%,77%and 79%,respectively,with no significant differences between treatments.
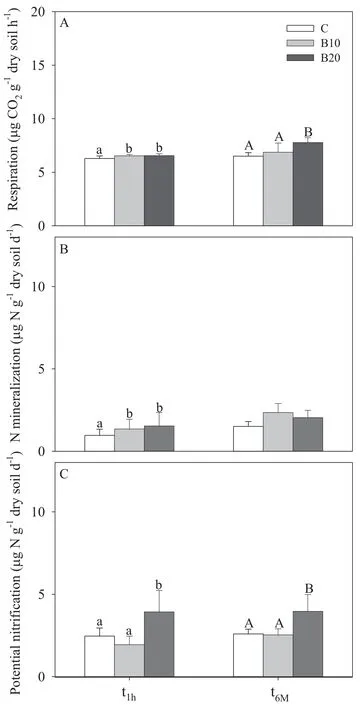
Fig.2 Mean values(+standard deviations)of(A)soil respiration,(B)N mineralization and(C)potential nitrification in plots treated with 10 t ha-1(B10)and 20 t ha-1(B20)biochar and in control(C),determined 1 hour(t1h)and 6 months(t6M)after treatment.Significant(P<0.05)differences among treatments in the first(lower case)and second(upper case)sampling are indicated with different letters on the bars.
4 Discussion
Whilst producing no evidence for major positive effects,our results show that application of 10 and 20 t ha-1biochar does not adversely affect crops and soil properties in a greenhouse environment.Biochar phytotoxicity has been reported by some authors(Deenik et al.,2010;Dutta et al.,2017)and ascribed to polycyclic aromatic hydrocarbons(PAHs),volatile organic compounds(VOCs)and/or trace elements.However,other authors did not observe phytotoxicity(Beesley et al.,2010).Moreover,the absence of negative effects on crop in the present study was confirmed by results obtained six months later(one year after biochar addition),when no negativeeffect on growth and yield of Lactuca sativa L.was recorded(Pentangelo et al.,unpublished data).
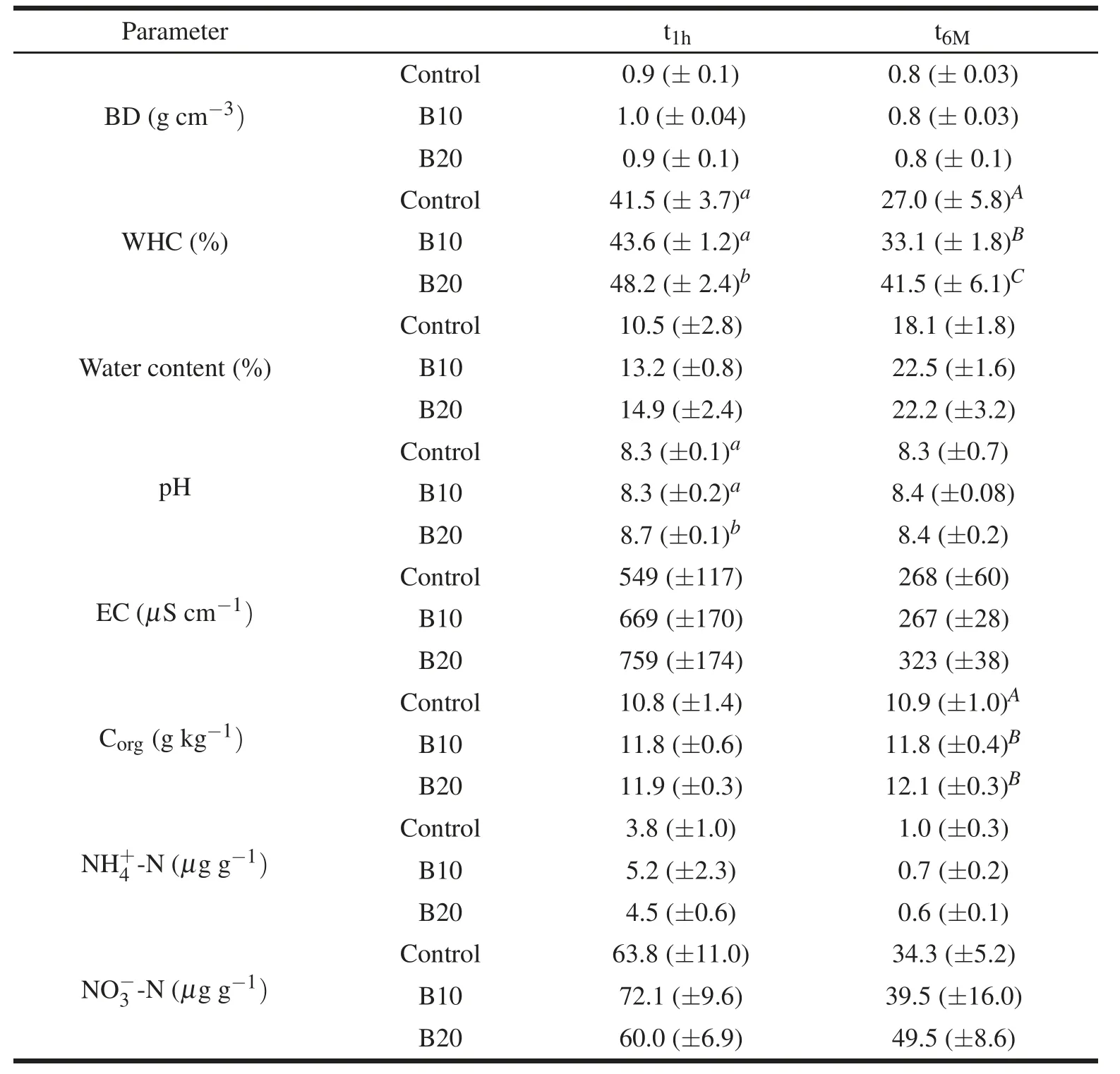
Table 3 Mean values(±standard deviations)of soil bulk density(BD),water-holding capacity(WHC),water content,pH,electrical conductivity(EC),organic carbon content(Corg),and mineral N(NH+4-N,NO-3-N)in plots treated with 10 t ha-1(B10)and 20 t ha-1(B20)biochar and in control,determined 1 hour(t1h)and 6 months(t6M)after biochar addition.Different letters in apex indicated significant(P<0.05)differences among treatments in the first(lower case)and second(upper case)sampling.
Despite a general lack of reactivity to char amendment in the doses employed,several positive effects were observed,notably an increase in soil organic C and water-holding capacity and an enhancement of microbial activity reflected by higher soil respiration,potential nitrification and N mineralization,the last only at the first sampling time.Similar effects have been observed in open- field wheat crop three months after application of biochar derived from coppiced woodlands(Castaldi et al.,2011);in the same study area,a significant increase was also observed in soil respiratory response to certain substrates including succinic,citric,L-ascorbic,gluconic,α-ketoglutaric and fumaric acids and L-asparagine(Rutigliano et al.,2014).Mahmood et al.(2003)reported a significant increase in bacterial activity in soils treated with biochar from hardwood.Likewise,an increase in soil respiration was observed by Smith et al.(2010)after addition of biochar from Panicum virgatum and by Deenik et al.(2010)in soil treated with biochar derived from Macadamia integrifolia nut-shell.Contrasting results were reported by Spokas and Reicosky(2009),who observed either a reduction or stimulation of respiration rate depending on the nature of biochar and the type of soil tested.Similarly,Steinbeiss et al.(2009)observed different effects on soil respiration depending on the type of biochar employed,with yeast-derived biochar stimulating soil respiration much more than glucose-derived biochar.The increase in microbial activ-ity following biochar addition was ascribed to a general improvement in physical and chemical soil properties(Lehmann et al.,2006;Laird et al.,2010).In this study,soil respiration and N mineralization were found to be positively correlated with soil water content and negatively correlated with bulk density,whereas potential nitrification was positively correlated with pH.Other factors besides those considered in this study may account for char-stimulated microbial activity,notably nutrient retention increasing with time as a consequence of char interaction with soil.Cheng et al.(2006)reported a progressive super ficial oxidation of biochar particles,leading to an increase of cation-exchanging capacity due to formation of new carboxylic groups.
In spite of positive effects on microbial activity,no increase was observed in crop yield after biochar addition in the present study,suggesting requirement of higher doses.Vaccari et al.(2011)observed a crop yield increase of over 30%in open- field wheat crop treated with 30 or 60 t ha-1biochar.Our study employed lower biochar amounts to avoid an excessive increase in soil pH,which was already moderately alkaline.Remarkably,we observed a significant increase in pH only 1 hour after addition,but no effect on pH was no detectable at the second sampling(t6M).
The greenhouse soil investigated showed a relatively low suppressiveness to R.solani,an aggressive plant pathogen with low-specificity.Soil ability to reduce Rhizoctonia pathogenicity is possibly due to antagonistic factors(Pane et al.,2011).Soil suppressivenes of R.solani on cucumber plants after biochar treatment was found to vary with biochar type and concentration(Jaiswal et al.,2014).Putative suppression factors were not present and/or not active in untreated soil and biochar treatment had no significant effect under the experimental conditions of the present study.
5 Conclusion
This is the first study focused on effects of biochar treatment on crop and soil physical,chemical and microbial properties in a greenhouse cultivation.Our results show that biochar addition at 10 or 20 t ha-1has no negative effects on plant growth and soil microbial community,yet it does not improve crop yield or soil suppressiveness to R.solani at least during the first six months after treatment.Positive effects on soil microbial activity,probably due to enhanced water retention and organic C content,were mainly observed at the higher dose tested.Further experimentation with biochar doses above 20 t ha-1is needed to test possible positive effects on crop yield.Moreover,longer time intervals should be analyzed for a better assessment of optimal conditions.
Acknowledgements
We thank Idea Natura Soc.Coop.Agr.to allow the experimentation and Prof.Roberto Ligrone for helpful comments during the preparation of the manuscript.
杂志排行
Journal of Environmental Accounting and Management的其它文章
- The Scientific Research On Natural Capital:A Bibliometric Network Analysis
- Spatial Analyses of An Integrated Landscape-seascape Territorial System:The Case of The Overcrowded Gulf of Naples,Southern Italy
- Uptake of Micro and Macronutrients in Relation to Increasing Mn Concentrations in Cistus salvifolius L.Grown in Hydroponic Cultures
- Ecotoxicological Assessment of Virgin Plastic Pellet Leachates in Freshwater Matrices
- Phytotoxic Extracts as Possible Additive in Subsurface Irrigation Drip for Organic Agriculture
- Regional Redistribution Effects of Renewable Energy Subsidies
