In vitro evaluation of transdermal permeation effects of Fu’s cupping therapy via six diffusion kinetics models
2019-01-09WeiJieXieYuMeiWuShuaiShuaiChenJianXuFangFangYangYongPingZhangXiaoBoSun
Wei-Jie Xie , Yu-Mei Wu Shuai-Shuai Chen Jian Xu Fang-Fang Yang Yong-Ping Zhang*, Xiao-Bo Sun*
1School of Pharmacy, Guiyang University of Chinese Medicine, Guiyang, China. 2Institute of Medicinal Plant Development, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, China.
Background
The earliest record of cupping therapy appeared in theWushier Bing Fangpublished in Xihan Dynasty of China(202 B.C. - 8 A.D.). During the Tang Dynasty of China,there was a detailed record of the use of “Corner canister”(made of horns) for disease treatment. Cupping therapy is administration of the drug after cupping, because the pores of the skin open as well as the magnetic field effect is conducive to the transdermal absorption of the drug, so that the drug directly reaches the affected part. The pain and the side effect caused by oral or intravenous or intramuscular administration to the liver and kidney can be avoided by cupping therapy. Besides, it has the ideal effect with a small amount of medicine, and has the effect of acupoint injection, and has no pain in the skin [1].Therefore, the canister is the original form of percutaneous administration of cupping, and transdermal administration after cupping is an important clinical application of traditional Chinese medicine (TCM). The mechanical and physical stimulation effect on the human skin by cupping therapy is mainly the skin epidermal layer fissure, and the percutaneous absorption rate of the drugs and the clinical curative effect can be improved.
In recent years, it has been found that the skin treated by cupping exhibited significant effects on percutaneous penetration of tetramethylpyrazine, verapamil, and tetrahydropalmatine [2 - 5] and can significantly improve the effects of these drugs against myocardial ischemia and local inflammation. With the development of cupping therapy and the discovery of new methods, as well as the in-depth study of the mechanism of action, cupping has a good therapeutic effect for several diseases, which is considered superior to other conventional Chinese and Western medicines. According to reports [3 - 8], cupping has been widely used in the treatment of the following diseases: pertussis, exogenous cough, asthma, cold,pneumothorax, stroke sequelae, mouth oblique, snoring,hypertension, rheumatoid arthritis, diarrhea, hemorrhoids and so on.
Fu’s cupping therapy (FCT) belongs to a new type of cupping method. It is made of soft silicon and combined with a variety of cupping methods. More different effects can be achieved through the regulation of the microenvironment in the cup than that of the traditional cupping therapy. Similarly, the study found that by regulating the temperature and negative pressure of the drug solution in the tank, it has similar effects of warming,drug application, cupping, and transdermal administration[2 - 5]. In our previous studies, we have developed high-,medium-, and low-concentration of chemical permeation enhancers (CPEs) for the penetration-inducing system and physical penetration, which is iontophoresis [9 - 11].Based on this, the present study used CPEs and iontophoresis as control. The effect of FCT on the kinetic parameters of percutaneous absorption and penetration enhancement of indomethacin patch were studied by using the sixin vitrodrug permeation and diffusion kinetic models. This study was aimed to evaluate the effect and characteristics of FCT penetration on the transdermal absorption of the model drugs, and speculate the effect of FCT on the skin keratin barrier (obstructing the percutaneous absorption of drugs), and evaluate the safety of FCT on the skin, and offer the reference basis for the clinical application of FCT in promoting drug absorption, and provide the current scientific basis for the effect of cupping therapy on skin.
Materials and methods
Materials
The reference substance of Indomethacin (purity about 99.9%), was purchased from National Institutes for Food and Drug Control (batch number:100258-200904);Indomethacin hydrophilic gel patch was provided by the preparation laboratory of Guiyang College of Traditional Chinese Medicine; Acetonitrile and chromatographic grade was obtained from Tianjin Kemiou Chemical Reagent Co., Ltd.; Green tea anti-allergic hair removal cream was purchased from Guangzhou Youxi Cosmetics Co., Ltd.; The purified water was obtained from Wahaha Group Co., Ltd..
Animals
Male and female Sprague Dawley rats with body weight from 260 to 300g were provided by the Experimental Animal Center of the Third Military Medical University of the People’s Liberation Army, certificate number:SCXK - (Army) 2012-0011. The animals were housed at 25 ± 1 ℃ with access to food and water ad libitum in a specific pathogen-free environment. All animal experiments were carried out in accordance with protocols evaluated and approved by the ethics committee of Guiyang College of Traditional Chinese Medicine.
Instruments
Agilent 1260 High Performance Liquid Chromatograph(Agilent, USA); TK-12B Transdermal Diffusion Tester(Shanghai Yukai Technology Trade Co., Ltd.); AE240 Electronic Balance (METTLER); JA2003 Analytical Balance (Shanghai Liangping Instrument Co., Ltd.);TGL-16C centrifuge (Shanghai Anting Scientific Instrument Factory); HYS-339 digital meridian therapeutic instrument (ion importer, Shenzhen Haoyisheng Electronic Technology Co., Ltd.). Scanning Electron Microscope (EM-30Plus, Korea COXEM Company)
HPLC determination
The concentration of indomethacin (IM) was determined by the High Performance Liquid Chromatography System(HPLC) with the VWD detector (Agilent, USA) and a Diamonsil C18 column (250 × 4.6 mm, 5 μm) maintained at 35 ℃. The mobile phase consisted of acetonitrile and 0.1% phosphoric acid water (60 : 40), was pumped at a flow rate of 1.0 mL/min and monitored at a wavelength of 228 nm.
Reference solution preparation.The appropriate amount of IM reference substance was precisely weighed,and dissolved in pH7.3 PBS buffered saline solution, and then the concentration of IM 236.80 μg/mL was obtained.The solution was diluted to different concentrations as needed with the PBS solution.
Preparation of test samples.From each group of IM hydrophilic gel patch, 2mLin vitrotransdermal receptor solution was taken, and then filtered with 0.45 μm membrane, and 10 μL of the sample was injected for HPLC analysis.
Specificity.The blank gel patch transdermal receiving solution was filtered through 0.45 μm membrane after centrifugation and used as a negative sample. The above test sample, control substance, and negative sample respectively were analyzed by the above chromatographic conditions. As shown in Figure 1, there was no other interfering peak at the retention time of IM, this showed that the method is specific..

Figure 1 Quantitative HPLC chromatogram specificity investigation
Standard curve.The IM standard PBS solution was diluted to 0.47 μg/mL, 3.32 μg/mL, 14.21 μg/mL, 33.15 μg/mL, 47.36 μg/mL, 71.04 μg/mL and analyzed by the HPLC. The peak area was taken as the abscissa (X) and the concentration (μg/mL) was as the ordinate (Y) to plot the standard curve. The linear regression equation was Y= 0.031X - 0.114 (r = 1). It showed that the method was linear in concentration range of 0.47 to 71.04 μg/mL.
Precision.The IM standard solution was analyzed 6 times and the relative standard deviation (RSD) was calculated. The result was found 0.64%, suggesting a good precision under the chromatographic conditions.
Stability.The same sample was analyzed at 0 h, 6 h, 10 h,16 h, 20 h, and 24 h under the chromatographic conditions and the RSD was found to be 0.21%, which indicating the acceptable stability of the sample for 24 h.
Repeatability.Six samples of the same concentration from the same batch of transdermal receptor solution were prepared and analyzed, and the RSD was found to be 0.13%, indicating a good repeatability.
Recovery.Six samples of known concentration of transdermal receptor were taken and IM reference solution was added and analyzed, and then the recovery rate was calculated. As shown in Table 1, the recovery rate was 101.2 % ± 1.56.

Table 1 Sample recovery test
In vitro transdermal diffusion
In vitr o skin and patch preparation.The rats were selected according to the requirements and the skin was isolated after abdominal injection of anesthesia [4 - 7],and then stored in the refrigerator and used up within one month. According to the prescription and preparation process of the matrix [5], the IM gel patch without penetration enhancer and with different penetration enhancers were prepared for which the drug loading was 12.5 mg.
In vitr o transdermal experiment.The transdermal experiment was conducted by the existing methods with modification [5 - 12]. Briefly, the isolated skin of the animals was divided into 8 groups (n = 6): blank penetration group, 3 groups of CPEs (3% Azone, 5%Azone, 3% Azone-5% Mint oil), iontophoresis physical penetration reference group (the mode parameter:acupuncture mode, low frequency, strength 5, 15 min),and 3 groups of FCT (high, medium and low). Franz transdermal diffusion device was used to conduct the experiment. The receptor compartment volume (V) was 7.0 mL, contact area (A) was 2.92 cm2, human simulated buffer pH = 7.3 PBS solution was used as the receiving solution. The stratum corneum of the skin was closely adhered to the patch sheet with the area of 3.14 cm2and fixed on the diffusion cell to ensure the absence of air bubbles, and the top was sealed with a plastic film. The water bath thermostat was maintained to 37 °C ± 1 °C,electromagnetic constant speed stirring was set as 320 r/min, all the solution was taken out at 3 h, 6 h, 9 h, 12 h,16 h, 24 h, 30 h, 36 h, and then the same amount of the blank receiving solution was replaced. The samples were analyzed by HPLC to determine the concentration of IM and then the cumulative penetration amount and the per unit area cumulative amount Q (μg/cm2) at each time point were calculated.
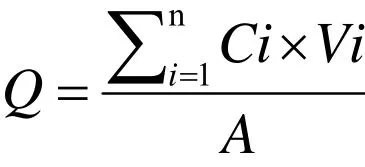
Where, Q is the per unit area cumulative amount, A is the contact area of the skin, Ci is the concentration of the IM and “Vi” is the volume of the receiving solution withdrawn.
Iontophoresis.The conditions used in iontophoresis was:electrode orientation (+, −), processing mode, current intensity (3, 5, 10, 15), frequency (low, medium, high),processing time (5 min, 10 min, 15 min), combined with clinical use conditions andin vivotests [3], the conditions determined: acupuncture mode, low frequency, intensity 5,time was 15 min. The IM patch was connected to the negative electrode, and the intermediate frequency was used in the human bodies, and the rest remain unchanged,this was a low strength treatment condition commonly used for iontophoresis. Since the transdermal rate of the drug attached to the negative electrode was relatively high and adhered to the patch, and then the patch was adhered to the surface of the skin.
FCT’s percutaneous penetration.The main influencing factors of cupping [2 - 9] are cupping method, pressure and time. In this study, different sizes of cups were used to control the pressure of cups [9]. The cups of No. 2, 3, 4(Figure 2A) were used to regulate the cupping pressure and adjust the strength of the human body stimulation, a certain volume (V) of internal air was drawn out via the cupping reserved mouth (Figure 2B) to form a quantitative negative pressure environment. Each group of cupping was operated according to clinical requirements [2 - 9]. The method of “retaining cup” is to leave the cup in the extracted part or to follow the meridian to retain the cup for 5 to 15 min, preferably 7 min. It has warming effect, so it is also called for the“warm cupping method”. The “shake cupping” method is also known as the spin cupping method. Firstly, the cup was sucked on the skin and then the canister was shaken rhythmically. During shaking, the attention should be paid to softness of the force, the speed should not be too fast, the angle of shaking should be appropriate, and the operation should be used once per 2 seconds. The“movable cupping”, also known as the sliding cup method, the “scraping cup” is mostly applied to the parts with large lesions and thick muscles. Firstly, a layer of massage oil and petrolatum (or glycerin) was applied to the area, and the cup body was tightened on the skin with left hand to make it tight. The cup body was pulled by the right hand to slide in a certain direction and walked along the meridian with the degree of skin flushing. Different types of cups and cupping methods were used to establish a high-, medium-, and low- intensity of permeability system [9]. The cups of No. 2 was used and shaken cupping for 6 minutes in FCT low-intensity group. The cups of No. 3 was used and retained cupping for 11 minutes in FCT medium-intensity group. The cups of No.4 was used and moved cupping for 15 minutes in FCT high-intensity group.

Figure 2 Treatment of percutaneous permeation by cups
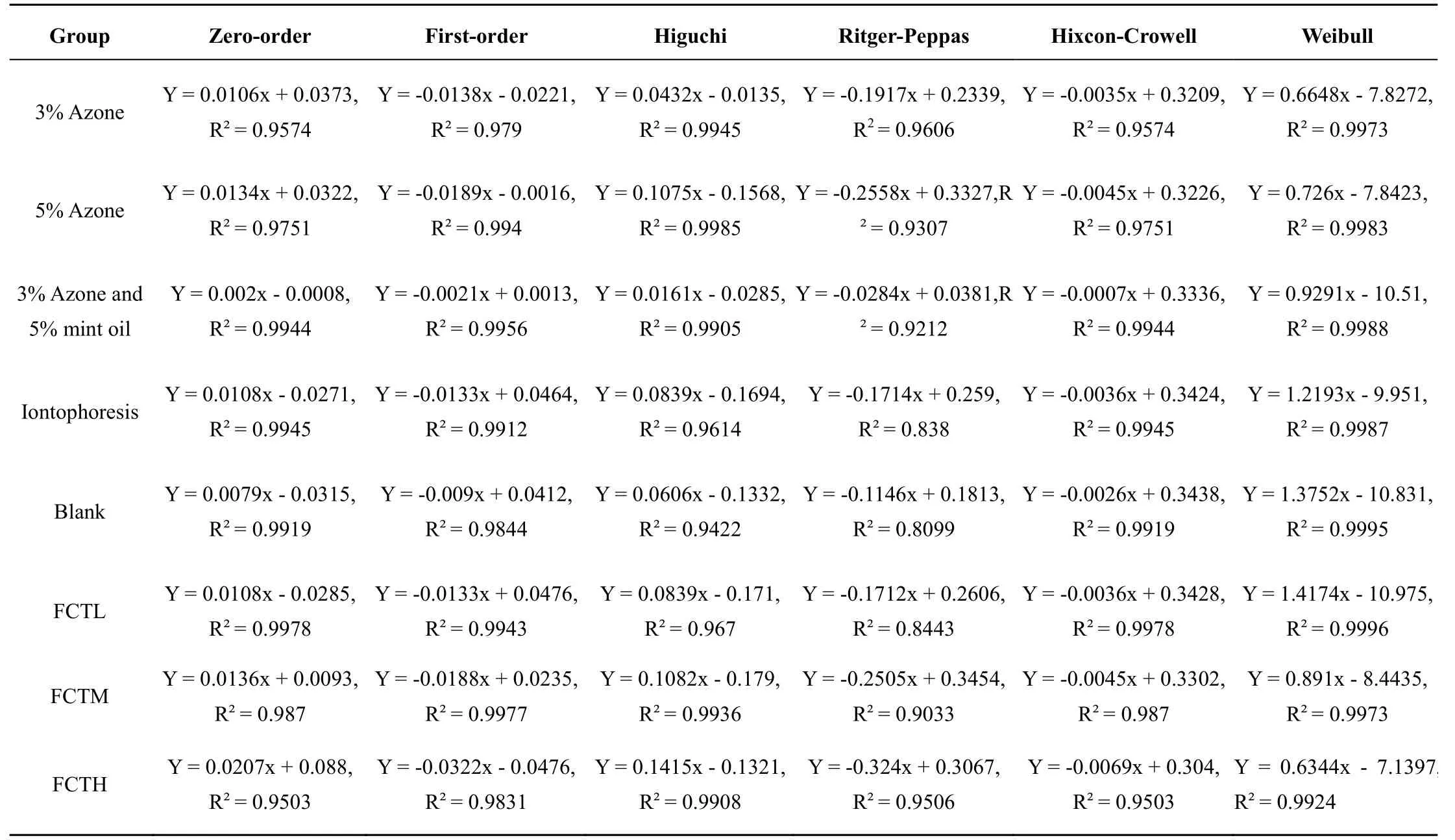
Table 2 Equations of diffusion kinetics of each group
Kinetic model establishment
In vitr o transdermal kinetic model.After the IM cumulative permeation amount “Q” (μg) at each time point was obtained, the cumulative permeation percentage “Q%” was calculated from the ratio of the cumulative permeate to the total amount, and “1-Q%”was the percentage of drug content in the patch matrix at different time point. The “Q% - t” curve of patch in vitro transdermal diffusion was obtained [9].
Sixin vitrotransdermal diffusion kinetic models [10 -17] were selected as the evaluation methods. In the equation, “Q” and t represents the cumulative permeation per unit area (μg/cm2), and different time points (h),respectively. Details as follows:

When Q was adjusted to Q% = Mi/M∞, it means the cumulative permeation percentage Q%, calculated according to the formula, and the kinetic model is analyzed. Among them, 1/2, 1/3, 2/3 and n are all indices in the equation. As follows: zero-order dynamic equation is Q% = Kt + b. First-order dynamic equation is ln (1-Q %) = -Kt + b. Higuchi plane-diffusion equation: Q% =Kt1/2+ b. Retger-peppas power Equation is ln (Q%) = b +Klnt. Hixcon-Crowell dynamic equation is (1 - Q%) 1/3 =-Kt + b. Weibull dynamic equation is lnP = Kln (t - τ) + b,ln [ 1/(1 - Q%)] = P.
Regression modeling.According to diffusion dynamics model and regression principle, modeling of each group was performed using Excel software: zero-order equation uses Q to t for linear regression, the first-order equation uses ln (1-Q%) to t for linear regression, the Higuchi plane diffusion equation uses Q% to t1/2for linearly regress, retger-peppas dynamic equation uses ln (Q%) to lnt for linear regression, hixcon-Crowell’s dynamic equation uses (1 - Q%)1/3to t for linear regression, the Weibull dynamic equation uses ln[1/(1 - Q%)] to ln(t - τ)for linear regression. Diffusion dynamics model equations and determination coefficients R (Table 2). The results showed that the Higuchi and Weibull dynamic equation regression models are better, and coefficient R ≥0.99, the kinetic model parameters obtained are available for analysis between groups.
Transdermal parameter.According to the model, the Higuchi and Weibull dynamic equations were used to analyze the diffusion kinetic parameters of each group.The slope of the Higuchi equation is the transdermal rate Js (μg·cm-2/h), the intercept is time lag (TL, h).According to the formula Js = P × C0, the percutaneous permeability coefficient P of each group (cm/h) was calculated. C0represents the drug concentration in the skin contact medium (patch). At the same time, the parameters of Weibull were calculated: the shape parameter m represents the slope of the fitted curve, lnt β represents the intercept of the linear fit, β represents the scale parameter and Td represents the time that 63.2% of the drug dissolution cumulatively through the medium,position parameter τ indicates the lag time of the drug.The Weibull dynamic equation parameters of each group have theoretical reference values. The results are shown in Table 3 and Table 4.
Results
Comparative analysis
According to the analysis of modeling results, we have analyzed the Higuchi dynamic model parameters (Table 3). According to previous studies [8, 9], in vitro results indicate the effect of FCTL and FCTL M group promoting penetration is remarkable, and is higher than that of the CPEs and the iontophoresis group, so FCT has a percutaneous penetration effect for IM. At the same time, the ER was markedly improved at 24 h and 36 h,respectively, as compared with the blank group, which was slightly higher than CPEs and iontophoresis.However, the decrease in ER after a period of time might be related to the recovery of skin barrier function with time. Later studies need to evaluate the recoverability of skin barrier function after FCT penetration.
In order to further explore the characteristics of percutaneous penetration in vitro, the transdermal parameter of the Higuchi and Weibull dynamic equations.As shown in Table 3, the transdermal rate Js, the permeability coefficient P and the time-delay TL are negatively correlated, and the transdermal rate Js is positively correlated with the permeability coefficient P.The trend of change between the three is basically consistent and can be used for comparative analysis between groups. In Table 3, Higuchi kinetic parameters:compared with the blank group, the single chemical penetration enhancer group, the iontophoresis group, and the FCT group all reduced the drug transdermal retention time T to varying degrees, and improved the transdermal rate of the drug and the permeability coefficient of the skin. It shows that FCT and CPEs can weaken the barrier function of the skin. At the same time, the transdermal rate and skin permeability coefficient after FCT treatment were significantly higher than other groups, indicating that FCT has a greater effect on skin barrier and stimulation. However, the 3% Azone - 5% Mint oil system reduced the IM transdermal rate by only 14.1%and the 9h ER was 0.41 times as compared with the blank group, which was shown to inhibit IM transdermal action.This could be due to the presence of drug crystals which is consistent with the literature reports [10 - 11].
In the Weibull dynamic equation, the results showed that FCT can significantly reduce the fitted intercept “τ,Td”, scale parameter β (equivalent to TL), increase the slope of the curve m, indicating that FCT can weaken the skin barrier and improve the transdermal absorption of drugs. The transdermal sampling point of the FCT high-intensity group (FCTH) group was only carried out for 24 h, so it was impossible to analyze.

Table 3 Transdermal kinetic parameters of Higuchi equation analysis (n = 6)

Table 4 Transdermal kinetic parameters of Weibull equation analysis (n = 6)
Statistical analysis
SPSS 21.0 was used for single-factor and multi-group LSD analysis.P≤ 0.05 indicated that the difference was statistically significant. The blank group was used as a reference, and the ER-t curve changes for each group was plotted. After the permeation treatment, the skin barrier function is changed, and the transdermal permeability of the drug is improved. As the skin barrier function is restored, the transdermal performance of the drug is lowered. Based on this point, compared with the blank group, the ER-t curve was analyzed, and the ER of each group at each time point was statistically analyzed, and the time point t with no statistical difference was found as the critical point for evaluating the initial recovery of the skin barrier (Tables 5 - 8).
Since there is no intra-group error in the blank group,the reference point of 5% Azone and iontophoresis were used to evaluate the critical point of the iontophoresis group, FCTL, and FCTM. As shown in Figure 3, there was no significant difference between the FCTL and the reference group after 16h. Compared with the blank group, the critical time point of the 5% Azone group exceeded 30 h, and there was no statistical difference,indicating that there was no difference in the skin barrier function between the 5% Azone group and the blank skin.Based on comprehensive inference, it was initially considered that the skin barrier function of the FCTL group was initially restored after 46 hours, and there was no statistical difference compared with normal skin.Among them, within the 36 h experimental time, the FCTM and FCTH group did not show any statistical difference. If further investigation is needed, the time ofin vitrostudy should be prolonged.

Table 5 Multiple comparison of enhancing rate between groups at 12 h

Table 6 Multiple comparisons of enhancing rate between groups at 16 h

Table 7 Multiple comparisons of enhancing rate between groups of at 24 h

Table 8 Multiple comparisons of enhancing rate between groups at 30 h

Figure 3 ER-t curve and time of critical point (TCP) of each groups
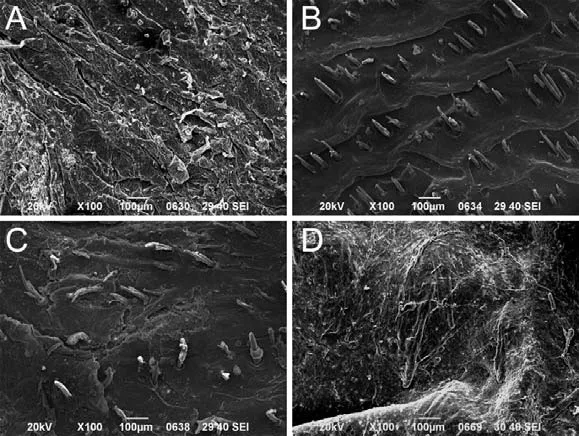
Figure 4 SEM image of the effect of FCT treatment on animal skin microstructure
The vascular structure below the epidermis
The microscopic observation revealed that FCTH group exhibited multiple skin ruptures within 24 h, and therefore, transdermal release study could not be performed. As indicated by the yellow arrow, the stratum corneum of FCTH group was almost completely destroyed after Fu’s cupping process compared with other groups (Figure 4). It suggests that FCT has a strong destructive effect on the stratum corneum of the skin in the animal skin modell, and greatly reducing or even causing the barrier function to be lost, resulting in a dramatic decrease in the mechanical support, protection and resistance to external substances. However, in fact, in the clinical practice of TCM, the middle and high FCT are commonly used for the treatment of human skin meridians, which may be related to the better tolerance of human skin.
Discussions
In this study, thein vitrotransdermal method was used to preliminarily evaluate the penetration-promoting effect of different cupping methods and the penetration-promoting system of FCT. The effects of FCT on rat skin and the kinetic characteristics of drug transdermal permeation were analyzed. Firstly, based on six diffusion kinetic models, 3% Azone, 5% Azone CPE and iontophoresis were used as reference [15 - 27] to evaluate and compare the effects and characteristics of FCT on percutaneous penetration. The results demonstrated that FCT exhibited a significant percutaneous permeation promoting effect on IM. The permeation enhancing effect of FCTL were comparable to that of iontophoresis and CPEs, while the effect of FCTM and FCTH was significantly higher than that of iontophoresis and CPEs. Weibull and Hguchi dynamics model parameters showed that compared with the blank group, the single CPE group, the iontophoresis group and the FCT group all reduced the drug transdermal retention time TL, intercept lnt β and Td to some extent, improved the percutaneous penetration rate,skin permeability coefficient and shape parameter m,which indicated that FCT and CPE could weaken the barrier effect of the skin and improve the transdermal absorption of the drug by skin. Meanwhile, the transdermal rate and skin permeability coefficient after FCT treatment were significantly higher than those of other groups, indicating that FCT had a greater effect on skin barrier.
However, during the course of the experiment, we found that the FCTH group exhibited multiple skin ruptures within 24 h. In addition, the stratum corneum of FCTH group was almost completely destroyed after Fu’s cupping process. However, in the clinical practice of TCM, the middle and high FCT are commonly used for the treatment of human skin meridians. The treatment of intensive cupping may also have the therapeutic purpose that requires more stimulating cupping to treat the body.Therefore, this study used three different levels of cupping therapy in high, medium and low level to comprehensively evaluate the percutaneous penetration activity.
At the same time, for the processing method of Fu’s cupping, the previous study used different sizes of cups form negative pressure by exhausting the internal air.First, the value of the individual cupping treatment pressures was not precisely quantified, and used different cupping regulation is more in line with the characteristics of clinical use of TCM. The experimental results have more reference value for TCM clinical practice. Second,it can improve the controllability and repeatability of cupping pressure. Third, the influencing factors for percutaneous penetration of FCT were investigated in our preliminary study. Combining the theory of TCM with cupping therapy [23-26], in the use of negative pressure to attract, iron the skin, pull the shallow muscles,stimulate the meridians, acupoints, pass the sense of transmission, thereby adjusting the blood and Yin and Yang, dredge the meridians, to achieve the role of rickets fitness using modern pharmacy theory to give controlled transdermal drugs, The drug can be controlled, sustained release and targeted, and the therapeutic effect of the drug can be exerted in a timely and effective manner.
Conclusion
The combination of cupping therapy and transdermal drug delivery can improve the transdermal rate of the drug, which is the modernization of traditional cupping,and provides a new idea and method for percutaneous penetration. As a new physical percutaneous penetration technique, FCT has obvious effects and has the characteristics of TCM [2, 9].
However, there are certain problems at the same time[28 - 31], such as different treatment methods for cupping have different effects on skin damage which leads to different effects of percutaneous penetration. Therefore,the investigation of the percutaneous dynamic parameters of cupping therapy will improve the clinical accuracy of the combination of cupping and transdermal administration, and provide scientific data and reference for clinical application and further study.
1. Lu X, Chen ZL, Guo Y,et al.Medicinal cupping:clinical application. Chin Acupunct Moxibustion 2011, 31: 79-81.
2. Xie WJ, Zhang YP, Xu J,et al.Progress of Fu’s cupping therapy as new physical penetration technologies for transdermal administration. Mod Tradit Chin Med Mater Med World Sci Technol 2015, 17: 1530-1536.
3. Umar NK, Tursunbadalov S, Surgun S,et al.The effects of wet cupping therapy on the blood levels of some heavy metals: a pilot study. J Acupunct Meridian Stud 2018, Epub ahead of print.
4. Wang JX, Yang Y, Song Y,et al.Ma, Positive effect of acupuncture and cupping in infertility treatment.Med Acupunct 2018. 30: 96-99.
5. Wang YL, An CM, Song S,et al.Cupping therapy for knee osteoarthritis: a synthesis of evidence.Complement Med Res 2018, 25: 249-255.
6. Zhao P, Wang YF, Gu C,et al.Comparison of the effects of the intervention with electric thermal bian stone and air suction cup on blood perfusion at meridian points. Zhong guo Zhen Jiu 2018, 38:159-64.
7. Sabir SA, Cerier J, Ziegler A. Methods of manufacturing devices for generating skin grafts.2015.http://www.patentsencyclopedia.com/app/2013 0145596
8. Xu J, Zhang YP, Xie WJ. Effect of Fu’s cupping therapy on transdermal absorption of tetrahydropalmatine patches. Chin J Exp Tradit Med Formulae 2013, 19: 43-46.
9. Xie WJ, Zhang YP, Xu J,et al.The effect and mechanism of transdermal penetration enhancement of Fu’s cupping therapy: new physical penetration technology for transdermal administration with traditional Chinese medicine (TCM) Characteristics.Molecules 2017, 22: 525.
10. Xie WJ, Zhang YP, Xu J,et al.Matrix formulation screening of indomethacin hydrophilic gel patch by uniform design. Chin Pharm 2017, 28: 1382-1385.
11. Zhang YP, Lin YP, Qiu DW,et al.Research of promoting skin permeability of verapamil hydrochloride with cupping-glass treatment. Chin Pharm J 2003, 38: 930-931.
12. Inayat BP, Mallikarjuna C. Chemical penetration enhancers for transdermal drug delivery systems.Trop J Pharm Res 2009, 8: 173-179.
13. Patel MN, Bharadia PD, Patel MM. Skin penetration enhancement techniques physical approaches. Int J Appl Biol Pharm 2010, 1: 63-72.
14. Suryakanta S, Sarwar B, Astha S,et al.Skin Penetration enhancement techniques physical approaches. Drug Delivery 2011, 8: 456-473.
15. MariellaI A, Sara N, Paolo C,et al.Effect of chemical enhancers and iontophoresis on thiocolchicoside permeation across rabbit and human skin in vitro. J Pharm Sci 2004, 93:2431-2438.
16. Jin Y, Ji X, Liu YQ,et al.Dissolution property of vitamin e-β-cyclodextrin inclusion compound determined by uv and analyzed by Weibull’s model.J Math Med 2010, 23: 344-347.
17. Jiang J, Liang YY, Cui LL,et al.Regulation effect of electrostatic field on stratum corneum of rat skin. J Hebei Univer (Natural Science Edition) 2007, 27:606-609.
18. Pliquett U, Gallo S, Hui S W,et al.Local and transient structural changes in stratum corneum at high electric fields: contribution of Joule heating.Bioelectrochem 2005, 67: 37-46.
19. Li ZH, Li YY, Hou M,et al.Topically applied hypericin exhibits skin penetrability on nude mice.Lasers Med Sci 2018, 33: 1279-1286.
20. Li L, Cai LJ, Yang JR,et al.Study on transdermal absorption of indometacin cataplasm in vitro. Tianjin Med J 2011, 39:165-167.
21. Ruan JH, Gong T, Zhang ZR,et al.Study on the preparation and in vitro percutaneous absorption of indomethacin eutectic-nanoemulsion. West Chin J Pharm Sci 2013, 28: 19-22.
22. Wang F, Ding XY, Gao J,et al.Effect of transdermal penetration enhancers on permeation ability of indomethacin and salbutamol. Dier Junyi Daxue Xuebao 2007, 30: 421-424.
23. Mohammad R, Mohammad A, Sushama T,et al.Enhanced transdermal drug delivery techniques:An extensive review of patents. Recent Pat Drug Deliv Formul 2009, 3: 105-124.
24. Ye Y, Haripriya K, Ajay K,et al.Effects of chemical and physical enhancement techniques on transdermal delivery of cyanocobalamin (Vitamin B12) in vitro. Pharm 2011, 3: 474-484.
25. Jessy S, Dhanila V. Recent advances in physical approaches for transdermal penetration enhancement.Curr Drug Ther 2012, 7: 184-197.
26. Lee WR, Shen CH, Fang CL,et al.Topical delivery of methotrexate via skin pretreated with physical enhancement techniques: Low-Fluence Erbium:YAG Laser and Electroporation. Lasers Surg Med 2008, 40: 468-476.
27. Aboushanab T, AlSanad S. Cupping therapy and animal research: the progress. J Acupunct Meridian Stud 2018, 11: 81-82.
28. Tabakha MM, Sameer FT, Saeed MH,et al.Evaluation of Bloodletting Cupping Therapy in the Management of Hypertension. J Pharm Bioallied Sci 2018, 10: 1-6.
29. Ma SY, Wang Y, Xu JQ,et al.Cupping therapy for treating ankylosing spondylitis: The evidence from systematic review and meta-analysis. Complement Ther Clin Pract 2018, 32: 187-194.
30. Shawaf T, Deeb W, Hussen J,et al.Evaluation of wet cupping therapy on the arterial and venous blood parameters in healthy Arabian horses. Vet World 2018. 11: 620-626.
31. Liu Z, Chen C, Li X,et al.Is cupping blister harmful-A proteomical analysis of blister fluid induced by cupping therapy and scald. Complement Ther Med 2018, 36: 25-29.
杂志排行
Traditional Medicine Research的其它文章
- A multi-center randomized controlled clinical trial of three-step acupuncture and cupping therapy for cervicogenic headache
- The effect of hot intermittent cupping on pain, stiffness and disability of patients with knee osteoarthritis
- Dry cupping therapy and the wellness management of health travelers
- A clinical study on medical cupping for metabolic syndrome with abdominal obesity
- Three-step acupuncture and cupping therapy:an effective approach to treat cervicogenic headache
- Stir up a fire and burn oneself:the secret of fire therapy
