Efficiency of tight monitoring by nurse practitioners in rheumatoid arthritis patients in remission after treatment with rituximab:study protocol for a randomized,open-label,controlled trial
2019-01-07borahLebedieffneBoulardatriceGodonMarineGrandjeanChristianMarcelliOlivierVittecoq
Déborah Lebedieff ,Hélène Boulard,Béatrice GodonMarine GrandjeanChristian Marcelli,Olivier Vittecoq
1 Department of Rheumatology,Rouen University Hospital,Rouen,France
2 Department of Nursing,Rouen University Hospital,Rouen,France
3 Department of Rheumatology,Caen University Hospital,Caen,France
Abstract
Key words:rheumatoid arthritis; tight monitoring; nurse practitioner; rituximab; remission; relapse; telephone follow-up; randomized controlled trial
INTRODUCTION
Research background
Rheumatoid arthritis(RA)is the most common form of chronic in flammatory rheumatism.1RA mainly affects small-and medium-sized joints and causes progressive joint destruction with functional,psychological,social and occupational repercussions.Clinically,it results in pain,swelling and tender joints,morning stiffness,night awakenings and significant fatigue.
Rituximab(RTX)is one of the biologics available for rheumatologists to treat patients with RA.The schema of administration for RTX is not clearly established unlike other biologic agent.RTX differs from other biologics by a long interval between treatment cures because the selective depletion of CD20+B cells by RTX results in a longer therapeutic response time.2This singularity makes this drug one of the most complex to manage in routine.Indeed,although the Eu-ropean League against Rheumatism(EULAR)recommends evaluation of the therapeutic response from the 16thweek,and a minimum interval of 6 months between two treatment cycles,it recommends carrying out new research to establish the optimal treatment strategy.3RTX is currently re-administered when clinical relapse occurs(6 to 18 months after initiation of treatment,beyond 24 months for some patients).However,this reprocessing method is not satisfactory because the resumption of symptomatology is generally detected too late.For patients,this delay can lead to increased doses of background and symptomatic treatments(corticosteroids,analgesics and non-steroidal anti-in flammatory drugs(NSAIDs)),with proven potentially harmful side effects(cardiovascular,osteoporosis,etc.),an altered quality of life due to increased pain intensity,joint swelling,nocturnal awakening and morning stiffness,aggravation of joint destruction.The persistence of RA activity,even moderate,has been shown to be harmful in terms of joint destruction and functional capacity.4,5Therefore,it is essential to identify resumption of RA activity before clinical relapse.
In particular,our team explored clinical and biological monitoring every 2 months to predict the resumption of disease activity by detecting the reemergence of CD19+B lymphocytes.6However,different approaches are possible to detect a new flare of RA leading to early retreatment.In this respect,the role of nurse practitioners might be relevant.Indeed,EULAR recommendations have highlighted the role of nurse practitioners in the assessment of chronic in flammatory rheumatism.7The EULAR working group advocated that patients should have access to:education sessions,given by a nurse practitioner to improve their knowledge of their pathology and how to evaluate it; a nursing consultation and nursing telephone follow-up to improve continuity of care.Similarly,it specifies that nurse practitioner may:participate in disease activity monitoring to reduce symptoms and improve patient outcomes; promote patient self-assessment of the disease.Unlike other chronic conditions,RA cannot be assessed only on the basis of clinical or biological criteria.8The latest EULAR recommendations are in favor of using a validated composite index,including joint count(number of painful and swollen joints),to assess RA activity in daily practice and guide treatment decisions.9The American College of Rheumatology has published specific recommendations on the indexes to be used in current practice10:disease activity score based on 28 joints(DAS28),validated by EULAR,11is currently the most widely used criterion for evaluating RA activity in current practice and clinical trials.It is based on a complex mathematical formula,but easily usable at the bedside with pre-programmed calculators or digital applications.It takes into account:the number of painful joints out of a total of 28 joints(shoulders,elbows,wrists,metacarpophalangeal,proximal interphalangeal,knees); the number of swollen joints out of a total of 28 joints; the erythrocyte sedimentation rate(ESR)at thefirst hour or the C-reactive protein(CRP); self-assessment of overall disease activity by the patient rated between 0 and 100.12,13Given that the values of the ESR can rise out of in flammatory context,the DAS28 was also developed using the CRP instead of the ESR.14Several studies have shown a good correlation between DAS28-ESR and DAS28-CRP results.15-18
Strengths and limitations
Strengths
-A long follow-up period of 24 months per patient for a clinical relapse appearing between 6 to 18 months.
-A large sample size of patients.
-All patients with RA in remission receiving RTX treatment may participate in the study regardless of the number of RTX treatments received.
-The telephone follow-up prevents patients being lost to follow-up.
-Patients perform self-assessment of their painful and swollen joints after a training session with a nurse practitioner and therefore are active in their own management of RA.
Limitation
-This is a bicentric clinical trial conducted in two centers located in the same region of France.
A classification of RA activity according to CRP levels has been proposed as follows:high activity if DAS28-CRP ≥ 4.1;moderate activity if DAS28-CRP > 2.7 and < 4.1; low activity if DAS28-CRP ≤ 2.7; remission if DAS28-CRP ≤ 2.3.
Main objective
To demonstrate the efficiency of tight monitoring by a nurse practitioner of RA patients in remission receiving RTX treatment to detect very early relapse of the disease.
Secondary objectives
To demonstrate that tight monitoring by a nurse practitioner of RA patients,with good response to RTX,allows reduction in the number of prescriptions for corticosteroids and/or NSAIDs as a result of new flares of the disease,and an improvement in the quality of life of patients.
Study design
This is a prospective,controlled,randomized,open-label,bicentric clinical trial.The inclusion period is 24 months.The maximum duration of participation per patient is 24 months.Number of visits per patient:the minimum number of visits expected per arm is 2.Since patients are followed up to clinical relapse,the number of follow-up visits varies among patients.The total research time is 48 months.The mainfindings are reported in Table 1.
Study setting
Department of Rheumatology,Rouen University Hospital and Department of Rheumatology,Caen University Hospital,France.
Eligibility criteria
Inclusion criteria
-Male or female patients(aged 18 years or older)
METHODS/DESIGN

Table 1:The schedule of enrollment,intervention,and assessments
-Patients with RA fulfilling the American College of Rhumatology/European League Against Rheumatoid Arthritis criteria(ACR/EULAR 2010)19
-Patients prescribed treatment with RTX or who received treatment with RTX in the last 6 months
-Patients with active RA prior to treatment with RTX(disease activity score based on 28 joints-C-reactive protein[DAS28-CRP] > 2.7)
-Patients with a DAS28-CRP of less than 2.7 at 6 months from the last administration of RTX
Exclusion criteria
-Patients not responding to the last treatment with RTX(DAS28-CRP > 2.7 at 6 months)
-Patients under the age of 18 years
-Patients with chronic pain due to another pathology rather than RA,which may interfere with the assessment
-Patients with a contraindication to treatment with RTX
-Women of childbearing age not taking effective contraception
-Pregnant or lactating women
Recruitment
All patients who require treatment with RTX or who have been treated with RTX for less than 6 months receive an information note from the rheumatology departments of Rouen or Caen University Hospitals.
Baseline evaluation
The patient signs a consent form beforehand.The following investigations are undertaken prior to review by the investigator:
-A clinical examination of the number of painful and swollen joints(out of 28)
-The latest biology analyses including complete bold count,ESR,CRP,aspartate aminotransferase and alanine aminotransferase
-An interview to collect the patient's self-assessment of pain intensity,fatigue and disease activity(rated between 0 and 100),as well as the morning stiffness time and the number of night awakenings
-Calculation of the DAS28-CRP
-An interview to collect the names and dosages of background and symptomatic treatments(glucocorticosteroids,NSAIDs and analgesics)taken by the patient
-An interview to collect any side effect(s)attributable to RTX treatment
All these data allow the physician to evaluate the response to RTX.
In case RTX efficacy is not demonstrated by a decrease in RA activity(DAS28-CRP > 2.7),the rheumatologist may decide to refer the patient to day hospital for a new administration of RTX or to switch the biologic agent.In this case,the patient is not eligible for the study.In case RTX efficacy is demonstrated by a decrease in RA activity(DAS28-CRP <2.7),follow-up visits are scheduled with the attending rheumatologist,every 3 to 4 months,until relapse of the disease;in this case,the patient is eligible for this study.
Randomization
The randomization list by block has been established by the Biostatistics Unit of Rouen University Hospital using SAS 9.3 software(SAS Institute,Cary,NC,USA).This list is then centrally saved and readable by Ennov Clinical software(version 6.2,Ennov,Paris,France).Upon request,the investigator may obtain information on the arm to which the patient has been assigned.This information is sent by the systemviae-mail to both the investigator of the center and the study sponsor.A document describing the randomization procedure is kept confidentially within the Biostatistics Unit.Randomization is planned the day the patient is included in the study.
The 88 patients who are expected for inclusion are randomly divided into two arms comprising 44 patients in each arm.Randomization is carried out to determine the monitoring mode attributed to the patients:either usual follow-up by the attending rheumatologist(arm 1:no intervention),or usual follow-up by the rheumatologist + tight nursing follow-up(arm 2:intervention group).
Follow-up protocol
Patients randomized in arm 1 are examined by the attending rheumatologist every 3 to 4 months according to common practices.If the rheumatologist detects more active disease,the patient is referred within 15 days to a hospital practitioner independent from the study to confirm clinical relapse.If the relapse is confirmed(DAS28-CRP > 2.7),the patient receives a new cycle of rituximab.
In addition to the usual follow-up by their attending rheumatologist,patients in the intervention arm(arm 2)have:
1.A training session with a nurse practitioner on selfassessing their RA:self-assessment of the number of painful and swollen joints,of pain and disease activity(global assessment by the patient)(self-DAS).During this session,ESR and CRP dosage are planned.
2.A monthly telephone called by a nurse practitioner to collect the results of the patient's self-assessment.If a relapse of RA is suspected,the nurse practitioner schedules a consultation to confirm the relapse or not.For this purpose,the nurse practitioner calculates the DAS28 taking into account the results of the biological tests,the articular count and the disease activity.Clinical relapse is defined as a DAS28-CRP > 2.7.
3.A new cure of RTX prescribed by a hospital practitioner independent from the study.
The study is completed as soon as a hospital practitioner prescribes a new cycle of RTX(Figures 1 and 2).
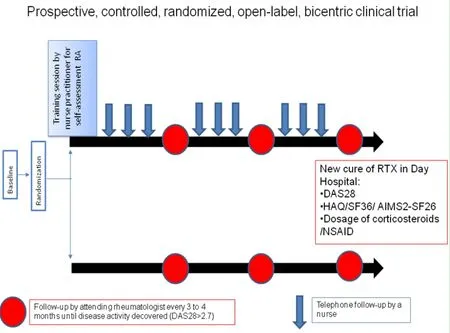
Figure 1:Study flow chart.

Figure 2:Study follow-up.
Outcome measures
Primary outcome measure
Value of DAS28-CRP at the time of a new cycle of RTX prescribed by a physician independent from the study.
Secondary outcome measures
-The cumulative dose of glucocorticosteroids and/or NSAIDs between two cycles of RTX
-The quality of life questionnaires:Health assessment questionnaire,The short form(36)Health Survery,and Short-form of the arthritis Impact Measurement Scales
Statistical analysis and sample size
Statistical analysis will be performed using SAS 9.3 software(SAS Institute).To assess the effectiveness of monthly telephone follow-up of patients,DAS 28 scores will be compared between intervention and non-intervention arms using twosided non parametric Mann-WhitneyUtest.AP-value less than 0.05 will be considered statistically significant.In order to achieve 80% power with respect to a 30% difference between two arms,44 patients will have to be included in each arm,hence 88 patients overall(nQuery 7.0,Statistical Solutions,Cork,Ireland).
Ethical considerations and informed consent
This trial follows the relevant laws and regulations of theDeclaration of Helsinkiand Quality Guidelines of the International Conference on Harmonisation(ICH).
This study has been approved by the Ethics Committee:the Northwestern France ethics committee(approval number:CPP-SC 02/2016)(Additionalfile 1).The trial has been submitted to the Advisory Committee on Information Processing in Health Research and was declared to the National Commission on Information and Freedoms.Written informed consent will be obtained from each patient(Additionalfile 2).
The results will be disseminated through national and international journals and conferences.The protocol adheres to the recommendations provided by the SPIRIT 201320(Additionalfile 3).
Data management
All information required by the protocol must be recorded in the(electronic)case report form and an explanation provided for all missing data.Data are collected as they are obtained.Erroneous data in the case report form are corrected by the investigator or the person authorized to make the correction.The traceability of modifications is automatic,viaan audittrail system.All the data are collected in an electronic case report form(quality of life questionnaires,number of painful and swollen joints,value of ESR and CRP,self-assessment of pain intensity,fatigue and disease activity,name and dosage of treatments taken by the patient).
Quality control of the clinical trial
The Clinical Research Associate,mandated by the sponsor,will visit each study center on a regular basis after the start of the clinical trial,during the trial according to the rhythm of inclusions and at the end of the trial.The Clinical Research Associate ensures that the rights and safety of the subjects are respected,that the data and information transmitted are reliable,of high quality and traceable,and that the study is conducted in accordance with the protocol,Guideline for Good Clinical Practice of ICH and the regulatory and legislative provisions in force.
The purpose of the visits will therefore be to verify and validate patient eligibility:compliance with inclusion and non-inclusion criteria; the adherence to patient information procedures ,trial schedule,patient follow-up; quality of data collected in the case report form:accurate,complete and consistent.
At the end of each visit,a standardized monitoring report will be written by the Clinical Research Associate which will be reviewed by the promoter.
DISCUSSION
This study corresponds to the combined management of rheumatoid arthritis by a nurse practitioner and an attending rheumatologist.A monthly telephone interview conducted by the nurse practitioner enables discussion on all aspects of the disease experience and regular follow-up allowing frequent readjustments.Thus,patients with good response to RTX treatment at 6 months might benefit from nursing follow-up alone to detect relapse.Such a practice is easily reproducible for RA patients in clinical remission under other treatments.
TRIAL STATUS
This study was designed in May 2016 and patient recruitment began in May 2017.We are currently recruiting participants at the time of submission.
Additionalfiles
Additionalfile 1:Ethical Approval Documentation.
Additionalfile 2:Model consent form.
Additionalfile 3:SPIRIT checklist.
Acknowledgments
We would like to thank Prof.Thierry Lequerré(Department of Rheumatology,Rouen University Hospital,France),and Dr Nathalie Léon(Department of Rheumatology,Caen University Hospital,France)for clinical assessment,Prof.Jacques Bénichou(Unit of Biostatistics,Rouen University Hospital,France)for statistical analysis,Nikki Sabourin-Gibbs(Unit of Biostatistics,Rouen University Hospital,France)for editing the manuscript,Isabelle Gorvel(Department of Rheumatology,Rouen University Hospital,France)and Yolande Bataille(Department of Rheumatology,Caen University Hospital,France)for management of nurses,Blandine Laurence,Sophie Baude and Virginie Boucher(Department of Rheumatology,Caen University Hospital,France)for patient follow-up,Vincent Ferranti(Department of Clinical Research,Rouen University Hospital,France)for monitoring the study.
Author contributions
Study concept:DL,HB,BG,and OV.Study design and writing:DL,HB,BG,and OV; literature retrieval:HB,DL,BG,and OV; data collection:DL,MG,BG,CM,and OV.All authors approved thefinal version of this manuscript.
Conflicts of interest
The authors declare that they have no con flicts of interest.
Financial support
This study was supported by French Interregional Group of Clinical Research and Innovation(GIRCI).The funder did not participate in data collection and analysis,manuscript writing or submission.
Institutional review board statement
This study protocol was approved by Northwestern France Ethics Committee(approval number:CPP-SC 02/2016),and will be performed in accordance with theDeclaration of Helsinki.This study was registered with ClinicalTrials.gov identifier:NCT03027999 on January 23,2017.
Declaration of patient consent
The authors certify that they will obtain all appropriate patient consent forms.In the form the patients will give their consent for their images and other clinical information to be reported in the journal.The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity,but anonymity cannot be guaranteed.
Reporting statement
This study follows the Standard Protocol Items:Recommendations for Interventional Trials(SPIRIT)guidance for protocol reporting.
Biostatistics statement
The statistical methods of this study were reviewed by the biostatistician of Rouen,University/Hospital in France.
Copyright license agreement
The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement
We confirm that the data are anonymized.The protocol complies with the MR003 standard,the declaration was made on clinical.Trials.gov respecting these principles.
Plagiarism check
Checked twice by iThenticate.
Peer review
Externally peer reviewed.
Open access statement
This is an open access journal,and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
杂志排行
Clinical Trials in Orthopedic Disorder的其它文章
- Ultrasound-guided supine lumbar plexus block versus iliac fascia block for analgesia in older adult patients undergoing hip replacement:a randomized controlled trial
- Efficacy of arthroscopic surgery for discoid lateral meniscus injury in knee joint:a self-control study
- Local anesthetic infiltration before open reduction and internalfixation for ankle fracture:a single-blind randomized controlled study
- Clinicopathological characterization of long bone non-union:a prospective cross-sectional study
- Hemostasis following local versus intravenous tranexamic acid in patients undergoing posterior open reduction and internalfixation of thoracolumbar fractures:study protocol for a parallel-group,randomized controlled trial
- Efficacy of silver needle acupuncture combined with muscle relaxation in the treatment of capulohumeral periarthritis:study protocol for a prospective,single-center,randomized,parallel-controlled trial
