5个基因在小尾寒羊和苏尼特羊性腺轴相关组织中表达分析
2018-12-27张壮彪狄冉刘秋月胡文萍王翔宇田志龙张效生张金龙储明星
张壮彪,狄冉,刘秋月,胡文萍,王翔宇,田志龙,张效生,张金龙, 储明星
5个基因在小尾寒羊和苏尼特羊性腺轴相关组织中表达分析
张壮彪1,狄冉1,刘秋月1,胡文萍1,王翔宇1,田志龙1,张效生2,张金龙2, 储明星1
(1中国农业科学院北京畜牧兽医研究所/农业部动物遗传育种与繁殖重点实验室,北京 100193;2天津市畜牧兽医研究所,天津 300381)
【背景】随着人们生活水平不断提高,蛋白含量丰富、胆固醇含量较少的羊肉在日常生活中越来越受青睐,羊肉总体消费需求呈逐年增长趋势。但是近年来以羊肉为主的羊产品短缺,导致羊肉的价格一直居高不下,造成羊肉供需之间的矛盾。在早期对绵羊各项生产性能研究中发现,其繁殖性能高低对羊肉生产有重要影响。因此,提高绵羊繁殖力对改变我国肉羊生产周转慢、效益差的局面有重要意义。产羔数是最重要的繁殖性状,但产羔数是一个低遗传力的数量性状,且受微效多基因的控制,故传统的育种方法难以快速改良产羔数性状。近年来随着分子标记技术的出现,研究人员发现了一些影响绵羊繁殖力的主效基因,比如、等,所以后期人们开始利用常规育种结合这些分子标记进行高繁殖力绵羊品种选育。研究表明除了这些已经发现的主效基因外,仍有一些基因对绵羊的繁殖力具有一定的调控作用。【目的】探究影响绵羊繁殖力的候选基因、、、和在小尾寒羊和苏尼特羊性腺轴相关组织(大脑、小脑、下丘脑、垂体、子宫、卵巢、输卵管)的表达差异,为阐明绵羊高繁殖力机理提供参考。【方法】以产多羔的小尾寒羊和产单羔的苏尼特羊为对象,利用实时荧光定量PCR检测上述5个基因在两个绵羊品种与性腺轴相关的7种组织中的表达差异。【结果】在小尾寒羊和苏尼特羊的性腺轴7种组织中均有表达,在小尾寒羊下丘脑中的表达量高于苏尼特羊(<0.05),在小尾寒羊输卵管、卵巢、垂体、小脑的表达量高于苏尼特羊(<0.01),但该基因在2种绵羊的大脑和子宫中表达量差异并不显著(>0.05);在小尾寒羊垂体和卵巢以及输卵管中表达量高于苏尼特羊(<0.01),虽然该基因在小尾寒羊的下丘脑、子宫中的表达量高于苏尼特羊,但其表达差异并不显著(0.05);在小尾寒羊垂体、大脑的表达量高于苏尼特羊<0.05),在小尾寒羊下丘脑、输卵管、卵巢中的表达量高于苏尼特羊(<0.01),但该基因在小尾寒羊和苏尼特羊的小脑和子宫中表达量差异并不显著(>0.05);在小尾寒羊和苏尼特羊的下丘脑、垂体中呈痕量表达,在其它组织中均有较高表达量,其在小尾寒羊输卵管和子宫中的表达量高于苏尼特羊(<0.01),但在小尾寒羊和苏尼特羊大脑中的表达量几乎相同(>0.05);在2种绵羊下丘脑中有较高表达量,在苏尼特羊垂体中的表达量高于小尾寒羊(<0.05),在小尾寒羊大脑中的表达量高于苏尼特羊(0.01),而该基因在2种绵羊其它组织中的表达量差异并不显著(>0.05)。【结论】暗示这5个基因可能对绵羊繁殖力具有一定的调控作用。
绵羊;产羔数;候选基因;性腺轴;组织表达
0 引言
【研究意义】对于绵羊来说,排卵数[1]和产羔数是衡量繁殖力高低的两个重要因素。不同物种之间的后代数和排卵数差别很大,比如人和牛一般被认为是胎产单个后代的物种,而小鼠和猪一般被看作窝产多仔的物种。绵羊排卵数一般仅有1—2个,但其有较大的“可塑性”。有研究表明某些羊的排卵数可达10个左右[2]。绵羊繁殖力受营养、羊群公母比例等多种因素调控,其中遗传因素对绵羊的繁殖力影响较大。如的发现,携带该基因的母羊在排卵数方面存在剂量效应,即每增加一个拷贝数其排卵数可增加1.5个,产羔数可增加1—1.5个左右[3,4],该基因的发现为选育高繁殖力母羊提供了科学依据。但近年来随着研究不断深入,研究人员发现还有一些其他基因比如骨形态发生蛋白2、6、7(bone morphogenetic protein 2、6、7,2、6、7)、钙蛋白酶抑制蛋白(calpastatin,)和可卡因-苯丙胺调节转录肽(cocaine and amphetamine regulated transcript,)[1, 5-7]可以影响绵羊的繁殖力。探究这些基因在单羔、多羔羊性腺轴相关组织中的表达差异对于阐明相关分子机理具有重要意义。【前人研究进展】BMPs家族属于转化生子因子亚家族的成员[8],其发挥作用主要是通过BMP II型受体(BMPR-II)以及BMPR IA或IB型受体形成异低聚物的形式发挥信号转导作用。这种复合体会使II型受体磷酸化I型受体,进而导致Smad蛋白磷酸化,形成异二聚体之后导致靶基因转录改变[9-10],从而影响母羊的繁殖力。该家族对哺乳动物繁殖力影响首先是在1999年由SHIMASAKI等[11]报道的。研究表明在哺乳动物生殖系统中,组织特异性表达的BMPs家族成员能够影响某些结构和器官的形成以及其生物学功能的发挥[10]。LOCHAB 等[12]发现在原始生殖细胞向配子发育的过程中BMPs家族起主要调控作用。在动物颗粒细胞中它主要是通过调控促性腺激素诱导的生成类固醇以及有丝分裂发挥其调控作用[13]。研究表明BMPs家族成员能明显促进牛、羊的卵泡发育[5,14-16],也有研究发现该家族成员与闭锁卵泡[17]有一定的关系。CCS (calpain-calpastatin system)是机体内重要的系统之一,该系统对包括T细胞[18]等多种细胞或系统具有一定的调控作用。该系统是由钙蛋白酶(calpain)(m和μ型钙蛋白)和组成。作为该系统的重要成员,基因编码钙蛋白酶抑制蛋白,它能专一性地识别相应蛋白酶与钙离子结合后构像的变化,从而发挥蛋白酶抑制剂的调控作用。研究发现它对一些产肉性状比如肌肉形成、初生重、断奶重以及后期肉品质的质量起重要作用[7,19-21]。该基因对绵羊繁殖性能[7,21]以及牛卵泡封闭[22]也有一定影响。前人研究已经证明脑垂体中的性腺激素对于卵巢的卵泡发育和排卵具有重要作用[23-24],故之前研究的关注点是与繁殖相关的性腺激素。但是随着科技的进步和人们对繁殖机理研究的不断深入,越来越多的证据证明对排卵数有明显影响的卵巢局部调节因子[25-27]。作为一种卵巢的局部调节因子在2004年由KOBAYASHI等首先发现[28-29],当时研究人员发现它是一种与体增重和某些神经反应有关的神经因子的存在[30]。该基因对牛、羊体内的某些激素如雌激素具有一定的抑制作用[31-32],对于牛卵巢、卵泡的发育以及卵泡的闭锁具有重要影响[33-35]。该基因在动物采食活动、液体平衡、感官刺激传导以及免疫反应[36-38]中也发挥重要作用。【本研究切入点】家族、、对绵羊繁殖具有一定的调控作用,目前关于这些基因在苏尼特羊、小尾寒羊性腺轴相关组织中表达情况还没有文献详细报道。【拟解决的关键问题】故本文选用家族中具有代表性的、、以及、作为研究对象,以期通过表达谱的方式说明其在单羔、多羔绵羊性腺轴各组织表达量的差异情况,为进一步阐明上述5个基因影响绵羊繁殖力的分子机理提供一定的参考,为“分子编写育种”[39]提供一定的遗传学材料。
1 材料与方法
1.1 试验时间、地点
本研究室内试验于2017年12月至2018年3月在中国农业科学院北京畜牧兽医研究所肉羊遗传育种科研团队实验室进行。
1.2 实验样品的采集和保存
随机选取2—3周岁健康、经产的空怀苏尼特母羊和小尾寒羊母羊各3只,于2017年10月饲养于天津市畜牧兽医研究所试验羊场,所有试验羊的饲养环境和饲料均相同。2017年11月进行屠宰,取其大脑、小脑、下丘脑、垂体、子宫、卵巢、输卵管,取样后迅速装入1.8mLRNase-Free冻存管中(最大样品量为冻存管体积的2/3)。所有样品采集要在半小时内完成,样品采完后迅速放入液氮中冷冻保存,用干冰带回实验室,放入-80℃冰箱冷冻保存,备用。
1.3 RNA的提取及质量检测
将采集的小尾寒羊和苏尼特羊性腺轴7种相关组织研磨后,用Trizol(Invitrogen,美国)进行裂解,之后按照动物组织RNA提取试剂盒(天根,北京)的说明进行总RNA的提取,用1.0%琼脂糖凝胶电泳和NANODROP2000检测提取RNA的质量和浓度。经检验合格的组织总RNA置于-80℃保存备用。
1.4 引物设计
参考文献中已报道的[40]、[40]、[41]、[1]基因序列并结合Primer Premier 5.0进行引物的合成(NCBI的登录号分别为XM_ 004014353.3、NM_001308564.1、XM_015102858.1、XM_015101190.1)。根据GenBank提供的绵羊、基因序列(登录号分别为NM_001009788.1、NM_001009784)信息并用Primer Premier5.0软件进行引物设计,其中作为内参基因。引物由北京天一辉远生物科技有限公司合成。各引物浓度均为10 μmol·L-1,其他详细信息见表1。
1.5 cDNA的合成
利用反转录试剂盒合成cDNA,反转录体系和反应条件见表2,全程在冰上操作。将反转录完成之后的cDNA产物稀释后,用持家基因进行PCR检测,质量合格后-20℃保存以备检测基因mRNA表达。
1.6 实时荧光定量PCR
1.6.1 实时荧光定量PCR体系和程序 反应体系总体积为20 μL,具体各试剂用量见表3。PCR程序:95℃预变性5min ,95℃变性5 s,60℃ 30 s,40个循环;反应结束后进行熔解曲线分析。
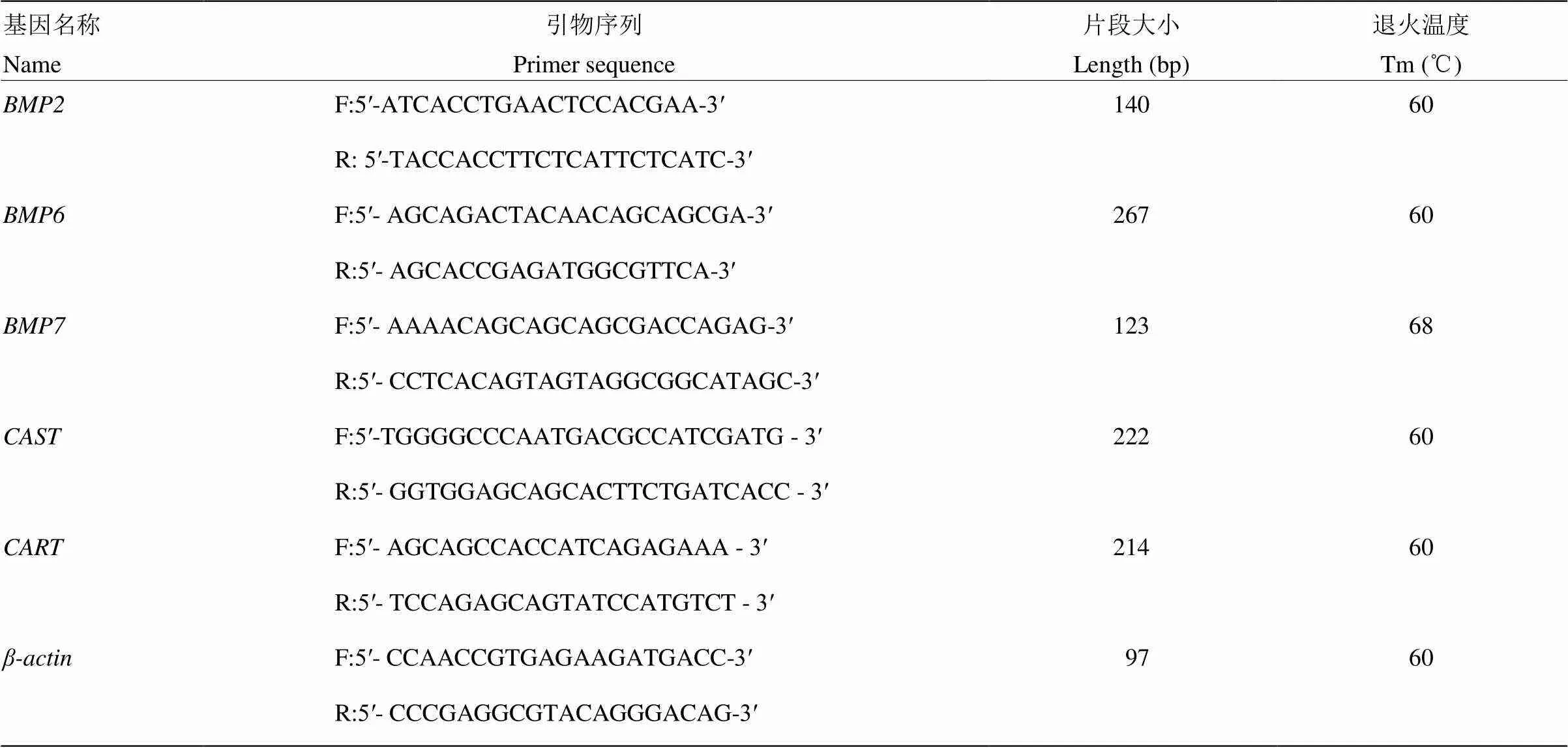
表1 引物的序列、扩增片段大小及退火温度

表2 反转录体系
1.6.2 标准曲线的建立 将cDNA样本5倍稀释后,进行2倍梯度稀释获得5个浓度梯度(1、1/2、1/4、1/8、1/16)的cDNA样品。用这些cDNA作为模板对目的基因和持家基因进行荧光定量PCR,以浓度梯度的对数值(10为底数)为横坐标,以检测所得Ct值为纵坐标,绘制目的基因和持家基因标准曲线。

表3 实时荧光定量PCR体系
1.6.3 实时荧光定量检测与数据分析 使用Roche Light Cycler®480Ⅱ型荧光定量PCR仪进行荧光定量检测,采用Excel进行数据处理,用2-△△Ct的方法计算基因的相对表达量。获得的表达量数据用SPSS19.0进行单因素方差分析(One-way ANOVA),分为差异显著(<0.05);差异极显著(<0.01)。
2 结果
2.1 RNA的提取
提取后的总RNA用1%琼脂糖凝胶电泳进行检测,其完整性如图1。结果发现28S条带明显亮于18S条带,条带完整性较好,表明该RNA质量合格,可用于后续试验。
2.2 BMP2、BMP6、BMP7、CAST、CART组织表达分析
2.2.1组织表达分析 从图2中可以看出该基因在7种组织中均有表达,其中在小尾寒羊的输卵管表达量最高;在小尾寒羊输卵管、卵巢、垂体和小脑该基因的表达量极显著高于苏尼特羊(<0.01);该基因在小尾寒羊下丘脑的表达量显著高于苏尼特羊(<0.05);其他组织差异均不显著(>0.05)。

图1 RNA电泳检测

*(P<0.05); **(P<0.01)。其中上述1-7分别代表大脑、小脑、下丘脑、垂体、子宫、卵巢、输卵管。下同
2.2.2组织表达分析 由图3可见,在两种绵羊的7种组织中,该基因在小尾寒羊的卵巢中表达量最高;该基因在小尾寒羊卵巢、输卵管、垂体的表达量极显著高于苏尼特羊(<0.01);该基因在苏尼特羊垂体和卵巢表达量很少或基本不表达,其他组织差异都不显著(>0.05)。

图3 BMP6在小尾寒羊和苏尼特羊各组织中的表达
2.2.3基因组织表达分析 如图4所示,该基因在小尾寒羊垂体中表达量最高;该基因在小尾寒羊下丘脑、输卵管和卵巢中的表达量极显著高于苏尼特羊(<0.01);在小尾寒羊垂体中该基因的表达量显著高于苏尼特羊(<0.05),在苏尼特羊大脑中该基因的表达量显著高于小尾寒羊(<0.05);其他组织差异均不显著(>0.05)。

图4 BMP7在小尾寒羊和苏尼特羊各组织中的表达
2.2.4组织表达分析 如图5所示在小尾寒羊输卵管和子宫中的表达量极显著高于苏尼特羊(<0.01);在苏尼特羊小脑中该基因的表达量显著高于小尾寒羊(<0.05);而该基因在两种绵羊的下丘脑、垂体中均呈痕量表达。
2.2.5组织表达分析 如图6所示,该基因在两种绵羊下丘脑表达量最高;该基因在小尾寒羊大脑中表达量极显著高于苏尼特羊(<0.01);在苏尼特羊垂体中该基因的表达量显著高于小尾寒羊(<0.05);该基因在小尾寒羊和苏尼特羊卵巢、子宫和输卵管均呈痕量表达。

图5 CAST在小尾寒羊和苏尼特羊各组织中的表达
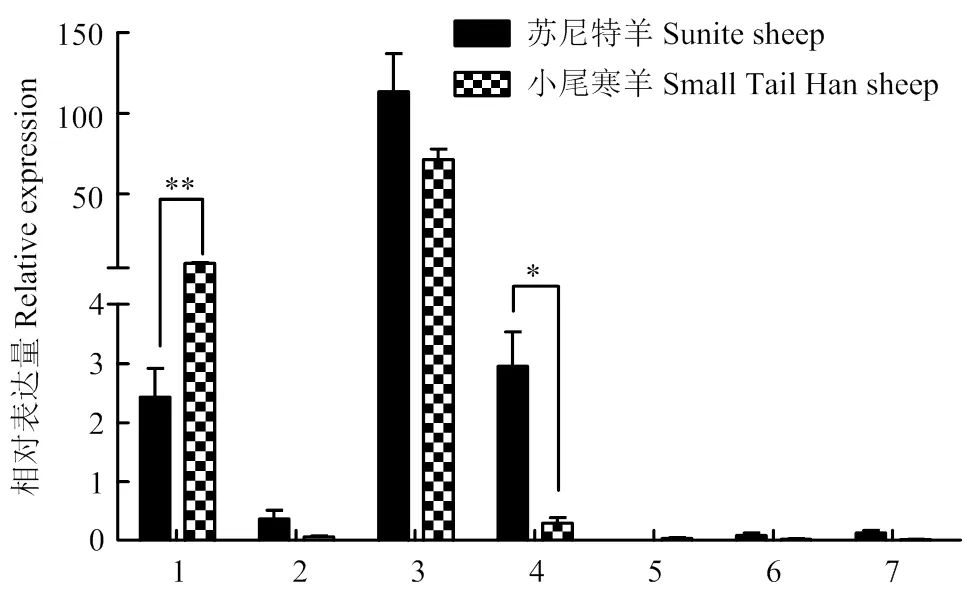
图6 CART在小尾寒羊和苏尼特羊各组织中的表达
3 讨论
3.1 BMPs家族对繁殖的调控
卵巢、子宫等是动物最重要的繁殖器官,探究影响繁殖相关基因在这些组织中的表达的差异对于阐明产羔数差异和繁殖力的高低具有一定的促进作用。研究表明不同的BMPs家族成员在不同的物种体内均有表达[8,42-46],说明该基因家族可能在动物体内发挥着重要调控作用。有研究表明在绵羊卵巢的卵母细胞、颗粒细胞、膜细胞均存在BMPs受体,说明BMPs家族可能会在卵巢发挥其局部调节因子的作用,从而影响绵羊的繁殖活动[4,47]。邝美倩等[48]研究表明在产羔数较高的湖羊的子宫中有较高表达量。在本实验中,不仅在产多羔的小尾寒羊的子宫有一定的表达量,而且在苏尼特羊也有一定的表达量,但二者差异不显著(>0.05)。徐业芬等[40]在湖羊的相关组织表达谱中发现在卵巢和输卵管有表达,但在子宫中不表达;、在卵巢组织中有表达,但是上述3个基因在单、双羔湖羊的卵巢表达差异并不显著(>0.05)。本研究显示不仅在卵巢和输卵管中有表达,而且在小尾寒羊(多羔)和苏尼特羊(单羔)的子宫中均有表达;并且3个基因在小尾寒羊(多羔)卵巢中均有表达且表达量极显著高于苏尼特羊(单羔)(<0.01)。也有研究发现当卵巢发生某些病变如肿性卵巢疾病时,BMPs家族的、等的表达量会发生改变[49],所以说某些卵巢的病变也有可能是导致其基因表达量差异的重要原因。
在动物的繁殖周期中,激素是一类非常重要的调控发情或繁殖的因素。BMPs家族基因对繁殖相关的激素具有重要调控作用。LIU等[50]研究发现在鸡胚胎的下丘脑大量表达。TAKEDA等[51]研究表明BMPs家族成员在人垂体中有表达。也有研究表明家族成员在湖羊的子宫[39,48]和卵巢[40]中有表达。VINODKUMAR等[52]研究发现在哺乳动物的输卵管上皮细胞中存在BMPs家族成员的相关受体。上述文献结果表明BMPs家族成员在生物体性腺轴相关组织有一定的表达量。在本试验中与激素分泌相关的下丘脑、垂体、卵巢等组织中均有表达。OTSUKA等[53]在小鼠体内研究发现,能够抑制孕酮含量的上升。绵羊的体外实验研究表明直接卵巢灌注会导致抑制素A、孕酮和雌激素含量的快速变化以及排卵前LH峰的提前[5]。VINOD等[54]在体外直接培养绵羊颗粒细胞并用不同剂量处理颗粒细胞,发现不同的剂量均能增加雌激素含量。上述文献表明BMPs家族的成员对动物各种激素或激素受体都有较明显的调控作用。综上,由于BMPs家族基因在分泌性激素的垂体、下丘脑等组织中有表达,且该基因家族对激素具有调控作用,故推测对绵羊繁殖力具有一定的调控作用。
3.2 CART和CAST对动物繁殖的调控
研究发现在蛋鸡[55]、猪[56]以及小鼠[57]的下丘脑中有表达;在体外培养的小鼠垂体细胞中也发现了的表达,同时研究人员也发现该基因的不同剂量可以明显影响PRL/ACTH/TSH和GH的含量[58]。在本试验中在小尾寒羊和苏尼特羊的下丘脑和垂体中均有表达,并且该基因在苏尼特羊(单羔)两种组织的表达量均高于小尾寒羊(多羔)。李鹏飞等[58-59]研究发现该基因对FSH诱导的卵泡细胞中的雌激素含量具有一定的抑制作用,由于下丘脑和垂体是性腺轴两个重要的促性腺激素分泌组织,结合本试验结果,进一步为抑制雌激素的分泌提供了证据。若在蛋鸡中导入干扰表达的dsRNA,发现其对卵泡细胞分泌雌激素具有促进作用[60]。综上推测该基因与雌激素的分泌呈一定的负相关。李鹏飞等[60]研究发现该基因在蛋鸡卵巢中有表达,在本试验中,在两种绵羊的卵巢组织中该基因均呈痕量表达,但JONES等[57]研究发现该基因在小鼠的卵巢内不表达。其可能原因是物种之间的差异,或者所处的生理条件不同。
张菊等[61]利用半定量RT-PCR方法研究发现的2型转录本在陶赛特羊卵巢和大脑中有表达。在本实验中在两种绵羊的大脑中均有表达,且该基因在小尾寒羊卵巢中的表达量显著高于苏尼特羊(<0.01),与上述文献结果基本一致。KAPPES等[62,63]研究发现位于牛科动物的7号染色体上,并且在该区域存在潜在的与产双羔相关的基因座。BYUN等[64]通过对大群体统计分析,发现与奶牛繁殖活动以及寿命长短有关;但是GARCIA等[65]研究发现,该基因与罗姆尼羊、美利奴羊、考力代羊的繁殖力以及寿命不存在明显的相关性。在本研究中在小尾寒羊和苏尼特羊子宫、卵巢、输卵管中表达量较高,并且在小尾寒羊子宫和输卵管中的表达量极显著高于苏尼特羊(<0.01),故推测该基因对绵羊的繁殖力具有一定的影响。目前,关于该基因影响动物繁殖力相关的研究几乎处于空白,所以对该基因影响绵羊繁殖力的机制需要进一步探究。
4 结论
本试验利用实时荧光定量PCR探究了、、、和在产多羔的小尾寒羊和产单羔的苏尼特羊与性腺轴相关的7种组织中的表达量差异,研究结果表明上述5个基因在单、多羔性腺轴相关组织中的表达有较大差异,暗示这5个基因可能对绵羊繁殖力具有一定的调控作用,为进一步阐明绵羊高繁殖力的分子机理提供了新的思路。
[1] JUENGEL J L, FRENCH M C, QUIRKE L D, KAUFF A, SMITH G W, JOHNSTONE P D. Differential expression of CART in ewes with differing ovulation rates., 2017, 9(23):471-479.
[2] JUENGEL J L, DAVIS G H, MCNATTY K P. Using sheep lines with mutations in single genes to better understand ovarian function., 2013, 146(4):111-123.
[3] MULSANT P, LECERF F, FABRE S,FRÉDÉRIC L, STÉPHANE F,LAURENT S, PHILIPPE M, ISABELLE L, CLAUDINE P, JULIETTE R, DANIELLE M, ISABELLE C, EDMOND C, JACQUES T, JACQUES T, LOYS B, YVES C, NOUR C, JEAN-MICHEL E. Mutation in bone morphogenetic protein receptor-IB is associated with increased ovulation rate in Booroola Merino ewes., 2001, 98(9):5104-5109.
[4] WILSON T, WU X Y, JUENGEL J L, ROSS I K, LUMSDEN J M, LORD E A, DODDS K G, WALLING G A, MCEWAN J C, O'CONNELL A R, MCNATTY K P, MONTGOMERY G W. Highly prolific Booroola sheep have a mutation in the intracellular kinase domain of bone morphogenetic protein IB receptor (ALK-6) that is expressed in both oocytes and granulosa cells., 2001, 64(4):1225-1235.
[5] CAMPBELLB K, KENDALLN R, BAIRD D T. Effect of direct ovarian infusion of bone morphogenetic protein 6 (BMP6) on ovarian function in sheep., 2009, 81(5):1016-1023.
[6] JUENGEL J L, FRENCH M C, QUIRKE L D, KAUFF A, SMITH G W, JOHNSTONE P D. The role of bone morphogenetic proteins 2, 4, 6 and 7 during ovarian follicular development in sheep: contrast to rat., 2006,131(3): 501-513..
[7] DEHNAVI E, AZARI M A, HASANI S, NASSIRY M R, MOHAJER M, AHMADI A. Genetic variability of calpastatin and calpain genes in Iranian Zel sheep using PCR-RFLP and PCR-SSCP methods., 2012, 10(2):136-139.
[8] SHIMASAKI S, ZACHOW R J, LI D, KIM H, IEMURA S, UENO N, SAMPATH K, CHANG R J, ERICKSON G F. A functional bone morphogenetic protein system in the ovary., 1999, 96(6):7282-7287.
[9] MASSAGUE J. TGFβ signaling: receptors, transducers, and Mad proteins.,1996, 85(7):947-950.
[10] MIYAZONO K, MAEDA S, IMAMURA T. BMP receptor signaling: transcriptional targets, regulation of signals, and signaling cross-talk ., 2005, 16(3):251-263.
[11] SHIMASAKI S, MOORE K, OTSUKA F, ERICKSON G F. The bone morphogenetic protein system in mammalian reproduction., 2004, 25(1):72-101.
[12] LOCHAB A K, EXTAVOUR C G. Bone morphogenetic protein (BMP) signaling in animal reproductive system development and function., 2017, 427(2):258-269.
[13] LIU Z, SHEN J, PU K, KATUS H A, PLÖGER F, TIEFENBACHER C P, CHEN X, BRAUN T. GDF5 and BMP2 inhibit apoptosis via activation of BMPR2 and subsequent stabilization of XIAP., 2009, 1793(12):1819-1827.
[14] OTSUKA F, YAO Z, LEE T, YAMAMOTO S, ERICKSON G F, SHIMASAKI S. Bone morphogenetic protein-15-Identification of target cells and biological functions., 2000, 275(50):39523-39528.
[15] ROSSIRO D A, CUNHA E V, PORTELA A M, PASSOS J R, COSTA J J, SILVA A W, SARAIVA M V, PEIXOTO C A, DONATO M A, VAN D R, SILVA J R. Influence of BMP-2 on early follicular development and mRNA expression of oocyte specific genes in bovine preantral follicles cultured., 2016, 31(3):339-348.
[16] DA C E, LRF M, SOUSA G B, ARAúJO V R, VASCONCELOS G L, AWB S, SILVA J. Effect of bone morphogenetic proteins 2 and 4 on survival and development of bovine secondary follicles cultured., 2018, 110(1):44-51.
[17] FOROUGHINIA G, FAZILEH A, EGHBALSAIED S. Expression of genes involved in BMP and estrogen signaling and AMPK production can be important factors affecting total number of antral follicles in ewes., 2017, 91(5):36-43.
[18] MIKOSIK A, JASIULEWICZ A, DACA A, HENC I, FRĄCKOWIAK J E. Roles of calpain-calpastatin system (CCS) in human T cell activation., 2016, 7(47):76479-76495.
[19] MOHAMMADI M, NASIRI M B, ALAMI-SAEID K, FAYAZI J, MAMOEE M, SADR A. Polymorphism of calpastatin gene in Arabic sheep using PCR-RFLP., 2008, 7(15):2682-2684.
[20] CHUNG H, DAVIS M. PCR-RFLP of the ovine calpastatin and its association with growth., 2012, 7(8):641-652.
[21] RANJBARI M, HASHEMI A, MARDANI K, DARVISHZADEH R. Allelic polymorphism of Makoei sheep calpastatin gene identified by polymerase chain reaction and single strand conformation polymorphism., 2012, 14(3):533-538.
[22] TAIT R G, CUSHMAN R A, MCNEEL A K, CASAS E, SMITH T P, FREETLY H C, BENNETT G L.μ-Calpain (CAPN1), calpastatin (CAST), and growth hormone receptor (GHR) genetic effects on Angus beef heifer performance traits and reproduction., 2018, 113(3):1-7.
[23] MIHM M, EVANS A C. Mechanisms for dominant follicle selection in monovulatory species: a comparison of morphological, endocrine and intraovarian events in cows, mares and women., 2008, 43(Suppl.2):48-56.
[24] WEBB R, CAMPBELL B K. Development of the dominant follicle: mechanisms of selection and maintenance of oocyte quality., 2007, 64(1):141-163.
[25] GILCHRIST R B, RITTER L J, ARMSTRONG D T. Oocyte-somatic cell interactions during follicle development in mammals., 2004, 82-83:431-446.
[26] JUENGEL J L, MCNATTY K P. The role of proteins of the transforming growth factor-beta superfamily in the intraovarian regulation of follicular development., 2005, 11(2):143-160.
[27] SILVA J R, FIGUEIREDO J R, VAN D H. Involvement of growth hormone (GH) and insulin-like growth factor (IGF) system in ovarian folliculogenesis., 2009, 71(8):1193-1208.
[28] KOBAYASHI Y, JIMENEZ-KRASSEL F, LI Q, YAO J, HUANG R, IRELAND J J, COUSSENS P M, SMITH G W. Evidence that cocaine- and amphetamine-regulated transcript is a novel intraovarian regulator of follicular atresia., 2004, 145(11): 5373-5383.
[29] YAO J, REN X, IRELAND J J, COUSSENS P M, SMITH T P, SMITH G W. Generation of a bovine oocyte cDNA library and microarray: resources for identification of genes important for follicular development and early embryogenesis., 2005, 19(1):84-92.
[30] ROGGE G, JONES D, HUBERT G W, LIN Y, KUHAR M J. CART peptides: regulators of body weight, reward and other functions ., 2010,11(3):218.
[31] KOBAYASHI Y, JIMENEZ-KRASSEL F, IRELAND J J, SMITH G W.Evidence of a local negative role for cocaine and amphetamine regulated transcript (CART), inhibins and low molecular weight insulin like growth factor binding proteins in regulation of granulosa cell estradiol production during follicular waves in cattle., 2006, 4(1):22-31.
[32] HUANG Y, YAO X L, MENG J Z, LIU Y, JIANG X L, CHEN J W, LI P F, REN Y S, LIU W Z, YAO J B, FOLGER J K, SMITH G W, LV L H.Intrafollicular expression and potential regulatory role of cocaine- and amphetamine-regulated transcript in the ovine ovary., 2016, 54(1):30-36.
[33] SEN A, BETTEGOWDA A, JIMENEZKRASSEL F, IRELAND J J, SMITH G W. Cocaine- and amphetamine-regulated transcript regulation of follicle-stimulating hormone signal transduction in bovine granulosa cells., 2007, 148(9):4400-4410.
[34] LV L, JIMENEZ-KRASSEL F, SEN A, BETTEGOWDA A, MONDAL M, FOLGER J K, LEE K B, IRELAND J J, SMITH G W. Evidence supporting a role for cocaine- and amphetamine-regulated transcript (CARTPT) in control of granulosa cell estradiol production associated with dominant follicle selection in cattle., 2009, 81(3):580-586.
[35] KOBAYASHI Y, JIMENEZ K F Q, YAO J, HUANG R, IRELAND J J, COUSSENS P M, SMITH G W. Evidence that cocaine- and amphetamine-regulated transcript is a novel intraovarian regulator of follicular atresia., 2004, 145(11):5373-5383.
[36] HIM L, KRISTENSEN P, LARSEN P J, WULFF B S. CART, a new anorectic peptide., 1998, 30(12):1281-1284.
[37] MAKOWSKA K, GONKOWSKI S. Cocaine-and amphetamine- regulated transcript (cart) peptide in mammals gastrointestinal system–a review., 2017, 17(1):3-21.
[38] VICENTIC A, JONES D C. The CART (cocaine-and amphetamine- regulated transcript) system in appetite and drug addition., 2007, 320(2): 499-506.
[39] 刘志国,王冰源,牟玉莲,魏泓,陈俊海,李奎.分子编写育种——动物育种的发展方向.中国农业科学,2018(12):2398-2409.Liu Z G, Wang B Y, Yan Y l, Wei W, Chen J H, Li K. Breeding by Molecular Writing (BMW): the Future Development of Animal Breeding., 2018(12): 2398-2409.
[40] 徐业芬,李齐发,李二林. 湖羊BMP2、BMP4、BMP6 和BMP7 基因mRNA 表达水平与排卵数关系的研究. 中国农业科学, 2009, 42(10):3655-3661.XU Y F, LI Q F, LI E L. Relationship between mRNA Expression of BMP2, BMP4, BMP6 and BMP7 genes and number of ovulation in Hu sheep., 2009, 42(10):3655-3661. (in Chinese)
[41] 杨燕燕,杜文,刘永斌. BMP6基因在单、双羔蒙古羊不同生理时期卵巢组织中的差异表达及分析. 畜牧与饲料科学,2017, 38(3):25-27.YANG Y Y, DU W, LIU Y B. Difference expression and analysis of BMP6 gene in ovary tissues of single and double lamb Mongolian sheep at different physiological stages. Animal Husbandry And feed Science, 2017, 38(3):25-27. (in Chinese)
[42] DUBE J L, WANG P, ELVIN J, LYONS K M, CELESTE A J, MATZUK M M. The bone morphogenetic protein 15 gene is X-linked and expressed in oocytes., 1998, 12(12):1809-1817.
[43] HINO J, TAKAO M, TAKESHITA N, KONNO Y, NISHIZAWA T, MATSUO H, KANGAWA K. cDNA cloning and genomic structure of human bone morphogenetic protein-3B (BMP-3b)., 1996, 223(2):304-310.
[44] JAATINEN R, ROSEN V, TUURI T, RITVOS O. Identification of ovarian granulosa cells as a novel site of expression for bone morphogenetic protein-3 (BMP-3/osteogenin) and regulation of BMP-3 messenger ribonucleic acids by chorionic gonadotropin in cultured human granulosa-luteal cells., 1996, 81(11):3877-3882.
[45] LYONS K M, PELTON R W, HOGAN B L. Patterns of expression of murine Vgr-1 and BMP-2a RNA suggest that transforming growth factor-beta-like genes coordinately regulate aspects of embryonic development., 1989, 3(11):1657-1668.
[46] TAKAO M, HINO J, TAKESHITA N, KONNO Y, NISHIZAWA T, MATSUO H, KANGAWA K. Identification of rat bone morphogenetic protein-3b (BMP-3b), a new member of BMP-3.ns, 1996, 219(2): 656-662.
[47] SOUZA C J, CAMPBELL B K, MCNEILLY A S, BAIRD D T. Effect of bone morphogenetic protein2 (BMP2) on oestradiol and inhibin A production by sheep granulosa cells, and localization of BMP receptors in the ovary by immunohistochemistry., 2002, 123(3): 363-370.
[48] 邝美倩, 金鹏锦, 王若丞, 孙玲伟,王锋,李鹏,成志军,茆达干. 限饲对湖羊子宫RGMb基因及BMP系统成员表达的影响. 畜牧兽医学报, 2017, 48(5):863-870.RUAN M Q, JIN P J, WANG R J, SUN L W, WANG F, LI P, CHENG Z J, MAO D G. Effect of feed restriction on expression of RGMb gene and BMP system members in Huyang uterus., 2017, 48(5):863-870. (in Chinese)
[49] DÍAZ P U, HEIN G J, BELOTTI E M, RODRÍGUEZ F M, REY F, AMWEG A N, MATILLER V, BARAVALLE M E, ORTEGAH H, SALVETTI N R. BMP2, 4 and 6 and BMPR1B are altered from early stages of bovine cystic ovarian disease development., 2016, 152(4):333-350.
[50] LIU F, PLACZEK M. Axon guidance effects of classical morphogens Shh and BMP7 in the hypothalamo-pituitary system., 2013, 553(8):104-109.
[51] TAKEDA M, OTSUKA F, SUZUKI J, KISHIDA M, OGURA T, TAMIYA T, MAKINO H. Involvement of activin/BMP system in development of human pituitary gonadotropinomas and nonfunctioning adenomas., 2003, 306(4):812-818.
[52] VALDECANTOS P A, MIANA R D C B, GARCÍA E V, GARCÍA D C, ROLDÁN-OLARTE M, MICELI D C. Expression of bone morphogenetic protein receptors in bovine oviductal epithelial cells: Evidence of autocrine BMP signaling., 2017, 185(10):89-96.
[53] OTSUKA F, MOORE R K, SHIMASAKI S. Biological function and cellular mechanism of bone morphogenetic protein-6 in the ovary., 2001, 276(35):32889-32895.
[54] VINOD K D, GULZAR R, SELVARAJU S, NAZAR S, PARTHIPAN S, PRASAD R V, JAMUNA K V, RAVINDRA J P. Effect of bone morphogenetic protein-2 (bmp-2) on sheep granulosa cell steriodogenic function.2014, 14(2):4233-4236.
[55] 于雪静, 庞钰莹, 贺俊平, 姜晓龙, 吕丽华. 蛋鸡下丘脑CART mRNA序列测定及分析. 畜牧兽医科技信息, 2013, (4):21-23. YU X J, PANG Y Y, HE J P, JIANG X L, LV L H. Determination and analysis of CART mRNA sequence in hypothalamus of laying hens., 2013, (4):21-23. (in Chinese)
[56] 李鹏飞, 李富禄, 于秀菊,吕丽华. 猪CART mRNA全CDS区序列的克隆与表达载体的构建. 福建农林大学学报(自然科学版), 2011, 40(6):52-55.LI P F, LI F L, YU X J, Lü L H. Cloning of the full CDS region of pig CART mRNA and construction of its expression vector., 2011, 40(6):52-55. (in Chinese)
[57] JONES D C, KUHAR M J. Cocaine-amphetamine-regulated transcript expression in the rat nucleus accumbens is regulated by adenylyl cyclase and the cyclic adenosine 5′-monophosphate/protein kinase a second messenger system., 2006, 317(1):454-461.
[58] CHMIELOWSKA M, BARANOWSKA B, WOLINSKA-WITORT E, MARTYNSKA L, KALISZ M, LITWINIUK A, BIK W. The effect of Cart on pituitary hormones release from cultured pituitary cells: harvested from fasted and fed ad libitum male rats., 2017, 9(1): 20-25.
[59] 李鹏飞, 岳文斌, 黄洋, 孙晋艳, 李晓明, 庞钰莹, 于学静, 贺俊平, 孟金柱, 任有蛇, 吕丽华. 可卡因-苯丙胺调控转录肽对绵羊卵巢卵泡颗粒细胞雌激素产生的影响. 畜牧兽医学报, 2013, 44(6): 853-857.LI P F, YUE W B, HUANG Y,SUN J Y, LI X M, PANG Y Y,YU X J, HE J P, MENG J Z, REN Y S, LV L H. Effect of cocaine- amphetamine-controlled transcription peptide on estrogen production of ovarian follicle granulosa cells in sheep., 2013, 44(6):853-857. (in Chinese)
[60] 李鹏飞, 孟金柱, 于雪静, 庞钰莹, 杜海燕, 李晓明, 姚晓磊, 赵妙妙, 吕丽华. 外源性dsRNA对蛋鸡卵泡CART基因表达及雌激素和孕酮分泌的影响. 畜牧兽医学报, 2016, 47(3):515-520.LI P F, MENG J Z, YU X J, PANG Y Y, DU H Y, LI X M, YAO X L, ZHAO M M, LV L H. Effects of exogenous dsRNA on CART gene expression and estrogen and progesterone secretion in follicles of laying hens., 2016, 47(3):515-520. (in Chinese)
[61] 张菊. 绵羊CAST、MC4R和BTGl基因的克隆、组织表达和遗传多态性分析[D]. 中国农业科学院, 2009.ZHANG J. Cloning, tissue expression and genetic polymorphism analysis of CAST, MC4R and BTG1 genes in sheep [D]., 2009.
[62] KAPPES S M, BENNETT G L, KEELE J W, ECHTERNKAMP S E, GREGORY K E, THALLMAN R M. Initial results of genomic scans for ovulation rate in a cattle population selected for increased twinning rate., 2000, 78(12):3053-3059.
[63] LIEN S, KARLSEN A, KLEMETSDAL G, VÅGE D I, OLSAKER I, KLUNGLAND H, AASLAND M, HERINGSTAD B, RUANE J, GOMEZ-RAYA L. A primary screen of the bovine genome for quantitative trait loci affecting twinning rate., 2000, 11(10):877-882.
[64] GARCIA M D, MICHAL J J, GASKINS C T, REEVES J J, OTT T L, LIU Y, JIANG Z. Significant association of the calpastatin gene with fertility and longevity in dairy cattle., 2006, 37(3):确良304-305.
[65] BYUN S O, ZHOU H, FRAMPTON C M, HICKFORD J G. No association between variation in the ovine calpastatin gene and either longevity or fertility in sheep., 2009, 41(2): 222-224.
Expression Analysis of Five Genes in the Gonadal Axis of Small Tail Han Sheep and Sunite Sheep
ZHANG ZhuangBiao1, DI Ran1, LIU QiuYue1, HU WenPing1, WANG XiangYu1, TIAN ZhiLong1,ZHANG XiaoSheng2, ZHANG JinLong2, CHU MingXing1
(1Key Laboratory of Animal Genetics, Breeding and Reproduction of Ministry of Agriculture/ Institute of Animal Science, Chinese Academy of Agricultural Sciences, Beijing 100193;2Tianjin Institute of Animal Sciences, Tianjin 300381)
【Background】With the continuous improvement of living standard, mutton with rich protein and low cholesterol content is increasingly favored in daily life, and the overall consumption demand of mutton is increasing year by year. However, in recent years, the shortage of mutton-based sheep products has caused the price of mutton to remain high, resulting in a contradiction between supply and demand of mutton. In the early researches on the performance of sheep, it was found that the reproductive performance had an important impact on the mutton production. Therefore, improving the fecundity of sheep is of great significance for changing the situation of slow turnaround and poor efficiency of meat sheep production in China. The litter size is the most important reproductive trait, but the litter size is a quantitative trait of low heritability and is controlled by micro-multiple genes. Therefore, traditional breeding methods are difficult to rapidly improve litter size. In recent years, with the advent of molecular marker technology, researchers have discovered some major genes that affect the fertility of sheep, such as,,and other genes, afterwards, researches began to use conventional breeding methods combined with these molecular markers to cultivate new sheep breeds with high fecundity. Studies have shown that in addition to these major genes that have been discovered, there are still some genes that have a certain regulatory effect on the fecundity of sheep. Based on this situation, the purpose of this study was to explore the differential expression of candidate genes which may affect the fertility of sheep. 【Objective】,,,and, in the tissues associated with the gonadal axis (brain, cerebellum, hypothalamus, pituitary, uterus, ovary and oviduct) in Small Tail Han sheep and Sunite sheep, which would provide a reference for clarifying the mechanism of high fecundity of sheep. 【Method】 Polytocous Small Tail Han sheep and monotocous Sunitesheep were used as the experimental animals, and real-time fluorescence quantitative PCR was performed to detect the expression difference of these five genes in seven gonadal-related tissues in two sheep breeds. 【Result】 The results showed thatgene was expressed in all seven tissues of the gonadal axis. The expression of<0.05). The expression of<0.01), however, the expression difference of this gene in the brain and uterus of the two sheep breeds was not significant (>0.05). The expression of<0.01), although the expression level of this gene in the hypothalamus and uterus of Small Tail Han sheep was higher than that of Sunite sheep, however, the expression difference was not significant (>0.05). The expression of<0.05), the expression of<0.01), but there was no significant difference in the expression of this gene in the cerebellum and uterus of Small Tail Han sheep and Sunite sheep (>0.05). The trace expression ofwas found in hypothalamus and pituitary, and the higher expression in other tissues in two sheep breeds, the expression ofgene in oviduct and uterus of Small Tail Han sheep was higher than that of Sunite sheep (<0.01), however, the expression levels of this gene in the brains of Small Tail Han sheep and Sunite sheep were almost the same (>0.05). Thewas highly expressed in hypothalamus of two sheep breeds. The expression ofgene in pituitary of Sunite sheep was higher than that of Small Tail Han sheep<0.05), and the expression ofin brain of Small Tail Han sheep was higher than that of Sunite sheep (<0.01), whereas, there was no significant difference in the expression of this gene in other tissues used in this experiment of two sheep breeds (>0.05). 【Conclusion】These results implied that the five genes may have some regulatory roles on sheep fertility.
sheep; litter size; candidate genes; gonadal axis; tissue expression
2018-04-18;
2018-07-07
国家自然科学基金(31772580);国家转基因科技重大专项(2016ZX08009-003-006和2016ZX08010-005-003);国家肉羊产业技术体系专项(CARS-38);中央级公益性科研院所基本科研业务费专项(Y2017JC24、2017ywf-zd-13);中国农业科学院科技创新工程(ASTIP-IAS13、CAAS-XTCX2016010-01-03、CAAS-XTCX2016010-03-03、CAAS-XTCX2016011-02-02);宁夏农林科学院科技创新先导资金(DWJLC-2016001);内蒙古自治区科技重大专项;农业科研杰出人才及其创新团队项目(农办人[2015]62号);国家万人计划科技创新领军人才项目(W02020274);天津市科技计划项目(16ZXZYNC00050);天津市农业科技成果转化与推广项目(201704020)
张壮彪,E-mail:zhangzhuangbiao18@163.com。
储明星,E-mail:mxchu@263.net
10.3864/j.issn.0578-1752.2018.24.011
(责任编辑 林鉴非)
