Impermeability of Y3Al5O12ceramic against molten glassy calcium-magnesium-alumina-silicate
2018-12-26ZhaoluXUEYueMAShengkaiGONGHongboGUO
Zhaolu XUE,Yue MA,Shengkai GONG,Hongbo GUO
School of Materials Science and Engineering,Beihang University,Beijing 100083,China
Key Laboratory of High-Temperature Structure Materials and Coatings Technology(Ministry of Industry and Information Technology),Beihang University,Beijing 100083,China
KEYWORDS Calcium-magnesium-alu mina-silicate(CMAS);Ceramics;Thermal barrier coating;Yttrium aluminum garnet;Zirconia
AbstractDegradation of thermal barrier coatings(TBCs)caused by calcium-magnesium-aluminasilica(CMAS)glassy penetration is becoming an urgent issue for TBCs industrial applications.In this work,yttrium aluminum garnet(Y3Al5O12,YAG)nano-powders were synthesized through a chemical co-precipitation route.The resistance of YAG ceramic to glassy CMAS infiltration at 1250?C was evaluated.YAG ceramic bulk sintered at 1700?C for 10 h was comprised of a single garnet-type Y3Al5O12phase.The molten CMAS glass was suppressed on the surface of the YAG ceramic at 1250?C.A chemical reaction between YAG and the molten CMAS glass did not occur at 1250?C for 24 h,suggesting that YAG could act as an impermeable material against glassy CMAS deposits in the TBC field.
1.Introduction
Currently,yttria partially stabilized zirconia(YSZ)thermal barrier coating(TBC)is widely and successfully applied in gas-turbine engines to provide heat insulation protection for metallic components and prolong their service life.1–5The turbine inlet temperature(TIT)significantly increases with the thrust-weight ratio rising,which would bring about some harsh requirements for new materials.Especially,one failure mode caused by environment sediments on the surface of TBCs has increasingly brought people’s attention.These environment sediments ingested and deposited on the hotter TBC surface mainly include dust,sand,volcanic ash,and runway debris,and the primary melt constituents are calcium oxide,magnesium oxide,aluminum oxide,silicon dioxide(CMAS)and trace vanadium,sulfur,sodium,iron elements.3,6–8When the surface temperature exceeds the melting point of CMAS glass,these molten deposits infiltrate into the YSZ coating and the stabilizer of zirconia dissolves in the melt,leading to a phase transformation with a volume change,thermophysical and mechanical properties degradation,and eventually accelerating the failure of TBCs.8–10The damage mechanism under thermo-chemicaland thermo-mechanicalcouplingeffects between CMAS and TBCs has been investigated in detail.8–11Because of the frequent aviation business transactions around the world,ubiquitous dust and grit,and requirement for a higher turbine inlet temperature,the CMAS damage of TBCs is becoming one of the urgent problems for high-performance aero-engines.
Substantial attempts to mitigate the CMAS damage on TBCs have been made in some literature.Mohan et al.12and Song et al.13reported that Al2O3/YSZ double ceramic layer TBC could effectively inhibit the molten CMAS infiltration through forming a high-melting point anorthite phase(CaAl2-Si2O8).Similarly,Aygun et al.14and Drexler et al.15showed that 62.4ZrO2-5.8Y2O3-28.9Al2O3-2.9TiO2(wt%)TBC prepared by solution precursor plasma spray(SPPS)and air plasma spray(APS)had excellent CMAS corrosion resistance.Meanwhile,Drexler et al.16and Liu et al.17indicated that Y2Zr2O7and Y2O3were much more potential materials in the CMAS-resistant aspect due to rapid crystallization of the Y-apatite phase(Ca4Y6(SiO4)6O).It is basically concluded that compounds containing high levels of alumina and/or yttria may have good CMAS-resistant performance.Therefore,yttrium aluminum garnet(Y3Al5O12,YAG),as a reservoir of Al and Y elements,may also have excellent CMAS-resistant performance.
Garnet-type Y3Al5O12has been considered as one of the promising candidates for TBCs due to some excellent performances,such as good phase stability up to its melting point,relatively high thermal expansion coefficient,and extremely low oxygen diffusivity which is about 10 orders of magnitude lower than that of YSZ.18–21These excellent performances are determined by its garnet-type crystal structure.A YAG unit cell can be regarded as a bonding network of[AlO6]octahedral,[AlO4]tetrahedral,and[YO8]dodecahedron,where Y3+ions are located in the center of dodecahedron gaps which are formed through apex angles interlinkage between[AlO4]tetrahedron and[AlO6]octahedron.The crystal cell structure of YAG is shown in Fig.1,where a,b,and c in the cell are the lengths of the unit translation vectors in the directions of the three crystal axes,respectively.Padture et al.19and Zhou et al.20verified the potential of garnet-type rare earth aluminate compounds as TBC candidates by first principles and experiments,respectively.Su et al.21and Gu et al.22showed that Y3Al5O12could act as an oxygen barrier and obviously improve the phase stability of zirconia in the YAG/YSZ TBC and YAG-YSZ composite ceramic TBC,respectively.In our previous study,the thermal conductivity of YAG was reduced and its thermal expansion coefficient was increased by Gd3+ions substitution for Y3+ions.23However,the specifi c CMAS-resistant mechanism of YAG has rarely been reported.

Fig.1Crystal cell structure of YAG.
The idea of this paper is to have YAG dissolved into the CMAS melt and make the CMAS composition shift toward the easy crystallization area or have YAG as a permeability barrier when YAG is subjected to the molten CMAS corrosion.In this work,the CMAS glass infiltration for YAG ceramic and the high-temperature interactions between CMAS glass and YAG were investigated,providing a basis for YAG coating against CMAS glass.
2.Experimental
Y3Al5O12powders were synthesized through a chemical coprecipitation route using Y2O3and Al(NO3)3?6H2O as the raw materials.The co-precipitation synthesis process of YAG was described in our previous study.23The acquired powders were calcined at 1000?C for 3 h.The synthesized powders were cold-pressed and then isostatically cold-pressed at 200 MPa for a holding time of 30 min to form pellets and subsequently sintered at 1700?C for 10 h in vacuum to form the final YAG bulks.
CMAS with a composition of 31CaO-8MgO-12Al2O3-49SiO2in mass ratio was used in this work.CaO,MgO,Al2O3,and SiO2powders were suspended in ethyl alcohol and fully mixed by planetary ball milling(QM-3SP4)for 12 h at a speed of 400 r/min.The melting point of the used CMAS was determined using a differential scanning calorimeter(DSC,NETZSCH STA 409C/CD)at a heating rate of 10?C/min.The CMAS powders were coated onto the surface of the YAG ceramic bulks at around 40 mg/cm2.The YAG bulks with CMAS were heat-treated in a box electric furnace at 1250?C for 12 h and 24 h in air atmosphere,respectively.
The phase compositions were identified by X-ray diffraction(XRD,Rigaku Diffractometer D/max 2500PC,Cu Ka radiation k=0.15418 nm)at 40 kV and 200 mA with a scanning speed of 6(?)/min.The microstructures of the YAG ceramic and the cross-sectional morphologies of the CMAS attacked samples were characterized by a scanning electron microscope(SEM,FEI Quanta 600)equipped with an energy dispersive spectrometer(EDS)at 20 kV with a working distance of 10–13 mm.The morphologies of the synthesized YAG powders and the interaction between YAG and CMAS were observed by a transmission electron microscope(TEM,JEM-2100F,Japan)at 200 kV equipped with a SUTW(spectrometer ultra-thin telescopic window)-Sapphire detector EDS.
3.Results and discussion
Fig.2 shows the XRD patterns of the synthesized YAG powders and ceramic bulk.The phase compositions of YAG powders and ceramic bulk were comprised of a single garnet-type Y3Al5O12phase(JCPDS NO.33-0040),and diffraction peaks of other impurities were not observed.This result indirectly suggested that YAG had good stability till up to 1700?C.Additionally,Fig.3 shows the TEM and high-resolution TEM(HR-TEM)images of the synthesized YAG powders,where Z.A stands for zone axis and SAED selected-area electron-diffraction.It was observed from Fig.3 that the grain size was about 40 nm and the crystal plane spacing was 0.45 A˚ which was consistent with the(2 2 4)crystal plane spacing of YAG standard card(JCPDS NO.33-0040).The sintered YAG ceramic bulk was relatively compact,with very few spherical and spherical-like micro-pores appearing in the fracture surface of the sintered ceramic bulk,and the average grains size was about 2–3 mm,as seen in Fig.4.The percent density of the sintered YAG ceramic bulk calculated by the theoretical density(4.55 g/cm3)and the actual density(4.19 g/cm3)was about 92%.

Fig.2XRD patterns of synthesized YAG powders and ceramic bulks.

Fig.3TEM and HR-TEM images of the synthesized YAG powders.

Fig.4Fracture surface SEM image of the sintered YAG ceramic bulk.

Fig.5TG-DSC curves of CMAS from room temperature to 1250?C.

Fig.6Cross-sectional SEM images of YAG ceramic with CMAS at 1250?C and corresponding EDS Si,Ca,and Y elemental maps.

Fig.7XRD patterns of CMAS(1#)and mixtures(1:1,by weight)of YAG and CMAS(2#)after heat-treated at 1250?C for 24 h.
Fig.5 shows the TG-DSC curves of CMAS from room temperature to 1250?C.There are two endothermic peaks at around 380?C and 640?C on the DSC curve of CMAS powders,and in the same condition,the TG curve corresponds to the weight reduction.The reason might be that a small amount of water was mixed during the CMAS ball-milling process,resulting in a small amount of Mg(OH)2and Ca(OH)2generation.Therefore,two endothermic peaks could correspond to the thermal decomposition of Mg(OH)2and Ca(OH)2,respectively.There is an obvious exothermic peak at around 870?C,suggesting that the crystal phase would be precipitated here.What is more,the endothermic peak and the constant TG curve at about 1170?C indicated the CMAS glass began to melt.Therefore,the melting point of the used CMAS was round 1170?C.
According to the literature,8–11the failure of YSZ TBCs would occur along with Si and Ca elements completely infiltrating into the YSZ coating when YSZ was subjected to the molten CMAS glass.Fig.6 shows the cross-sectional image and corresponding EDS Si,Ca,and Y elemental maps of YAG bulks after interaction with CMAS glass at 1250?C for 12 h and 24 h,respectively.Surprisingly,the molten CMAS glass was suppressed on the surface of the YAG ceramics at 1250?C,and an interaction zone between YAG and CMAS could not be found.Accidentally,there were tiny Si and Ca elements in YAG and tiny Y element in CMAS.The reason was that the sintered YAG ceramic bulk was not completely dense and there were still a small number of pores which might provide a channel for CMAS penetration.Compared with the molten CMAS penetration for YSZ,the amounts of Y elements in CMAS and Si elements in YAG could be ignored.Generally speaking,Si and Ca signals were hardly found in YAG,and a Y signal was also hardly found in CMAS glass,as shown in Fig.6.As a result,the molten CMAS could scarcely infiltrate into YAG at 1250?C.This is clearly different from Y2O3or Al2O3against the molten CMAS glass.12,13,17When Y2O3or Al2O3was exposed to the molten CMAS glass,an interaction zone,whose main component was a highmelting point anorthite phase(CaAl2Si2O8)or Y-apatite phase(Ca4Y6(SiO4)6O),would generate on the interface between CMAS and Y2O3or Al2O3.
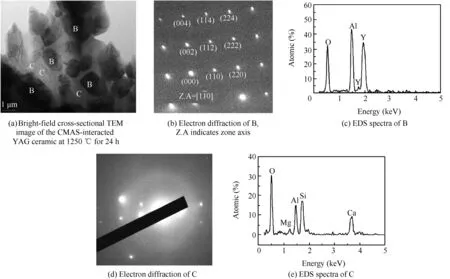
Fig.8Bright-field cross-sectional TEM micrograph of the CMAS-interacted YAG ceramic at 1250?C for 24 h,and corresponding electron diffraction and EDS spectra.
To find out the reason of YAG ceramic against the molten CMAS glass infiltration,the products of the interaction between YAG and CMAS(at 1:1 ratio,by weight)at high temperature were characterized by using XRD and TEM,as shown in Figs.7 and 8.Fig.7 shows the XRD patterns of mixtures(1:1,by weight)of YAG and CMAS and individual CMAS after heat-treated at 1250?C for 24 h.The diffraction peaks from Ca(Mg,Al)(Si,Al)2O6(JCPDS NO.41-1370)and CaSiO3(JCPDS NO.42-0547)could be observed in the XRD patterns of individual CMAS glass.Although YAG ceramic is rich in Y and Al elements,the diffraction peaks of new phases,such as anorthite(CaAl2Si2O8)and/or Y-apatite(Ca4Y6(SiO4)6O),were not detected except YAG,Ca(Mg,Al)(Si,Al)2O6,and CaSiO3during the interaction of YAG and CMAS at 1250?C.The absence of new phases indicated that YAG did not react with the molten CMAS glass at 1250?C.The reason might be that the activation energy of forming a YAG crystal phase was very high and YAG possessed good phase stability till its melting point,so that CMAS glass could not make the Al-O and Y-O bonds of[YO8]dodecahedron,[AlO4]tetrahedral,and[AlO6]octahedral breakage at 1250?C.24Tsai et al.25reported that the solid-state reaction temperature of the generated YAG was above 1400?C.In this case,YAG could also not promote the CMAS glass to shift toward the crystallization area of the anorthite phase and/or the Y-apatite phase.This result was basically consistent with those of Song et al.24and Nishi et al.26who reported that the YAG phase was not broken down in the H3BO3-SiO2-Al2O3-Na2CO3and CaO-Y2O3-Al2O3-SiO2glass at 1300?C,respectively.It was inferred that YAG might possess good chemical inertness with CMAS glass at 1250?C.
Fig.8(a)is a bright-field TEM micrograph of the CMAS-interacted YAG ceramic(1:1,by weight)at 1250?C for 24 h.This micrograph shows two significantly different regions marked as B and C,as shown in Fig.8(a).To identify the phase compositions of the two regions,selected area diffraction patterns(SADPs)and EDS spectrums were characterized,and results are presented in Fig.8(b)–(e).Fig.8(b)and(c)show an indexed SADP and Al and Y elements EDS spectra from region B indicating the Y3Al5O12phase,respectively.The other regions of CMAS glass were also observed,as determined by the SADP and EDS spectra(Fig.8(d)and(e))showing obviousamorphous ‘‘halo”.The observation was consistent with the result of XRD.Additionally,it was worth noting that Y elements could not be detected in CMAS glass,and neither Y-apatite phase nor anorthite phase could be observed in the bright-field TEM micrograph of the CMAS-interacted YAG ceramic,further confirming that a chemical reaction between YAG and the molten CMAS glass did not occur at 1250?C.In other words,YAG had good chemical stability in the molten CMAS environment at 1250?C,implying that YAG could serve as an impermeable material in the TBC field to prevent molten CMAS glass infiltration.
4.Conclusions
Single-phase Y3Al5O12(YAG)nano-powders were synthesized through a chemical co-precipitation route.YAG ceramic sintered at 1700?C for 10 h was comprised of a single garnettype Y3Al5O12phase.The molten CMAS did not infiltrate into the YAG ceramic,and was completely arrested on its surface.A chemical reaction between YAG and the molten CMAS glass did not occur at 1250?C for 24 h,suggesting that YAG remained excellent chemical stability in the molten CMAS glass.Y3Al5O12appears to be one of the promising candidates in TBCs systems to prevent molten CMAS infiltration.
Acknowledgement
This research was sponsored by the National Natural Science Foundation of China(NSFC)No’s.51590894,U1537212,51425102,51231001,51471019,and 51271011.
杂志排行
CHINESE JOURNAL OF AERONAUTICS的其它文章
- Aeroservoelastic stability analysis for fiexible aircraft based on a nonlinear coupled dynamic model
- Experimental research on rotating detonation with liquid hypergolic propellants
- A model-scale test on noise from single-stream nozzle exhaust geometries in static conditions
- Investigation of straightforward impedance eduction method on single-degree-of-freedom acoustic liners
- Experimental characteristics of a two-electrode plasma synthetic jet actuator array in serial
- Aircraft robust multidisciplinary design optimization methodology based on fuzzy preference function
