Propolis modulates cellular biochemistry, antioxidants, cytokine profile,histological and ultra-morphological status against antituberculosis drugs induced hepatic injury
2018-12-15NishaSahuGitaMishraHemeshwerKumarChandraSatendraKumarNiralaMonikaBhadauria
Nisha Sahu, Gita Mishra, Hemeshwer Kumar Chandra, Satendra Kumar Nirala, Monika Bhadauria✉
1Toxicology and Pharmacology Laboratory, Department of Zoology, Guru Ghasidas University, Bilaspur 495009(CG), India
2Laboratory of Natural Products, Department of Rural Technology and Social development, Guru Ghasidas University, Bilaspur 495009(CG), India
Keywords:Antituberculosis drugs Propolis Biochemical Hepatic injury
ABSTRACT Objective: To evaluate hepatic injury induced by antituberculosis drugs (ATDs) when administered orally for 2, 4, 6 and 8 weeks and the therapeutic potential of propolis (bee hive product) against ATDs induced hepatic injury. Methods: The ATDs were administered for 8 weeks as well as propolis extract at three different doses (100, 200, 400 mg/kg) conjointly for 8 weeks in rats. Silymarin (50 mg/kg) was given as positive control. Animals were euthanized after 8 weeks; blood and liver samples were collected to perform various biochemicals,serological and histopathological and ultramorphological studies. Results: Significant increase(P < 0.05) in aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase,triglyceride and cholesterol along with reduction in glucose and albumin level were noted after ATDs induced hepatic injury. Significant increase (P < 0.05) in lipid peroxidation, triglyceride,cholesterol and CYP2E1 activity; decline in reduced glutathione, catalase, superoxide dismutase, glutathione reductase, glutathione peroxidase, glucose-6-phosphatase dehydrogenase activity were observed after ATDs intoxication. Due to presence of a wide range of flavonoids and polyphenols in propolis extract, its administration reduced hepatic injury and maintained biochemical indices towards control. Histopathological and electron microscopic observations indicated hepatoprotective potential of propolis at cellular level whereas, TNF-αα, IL-6 and IGF-1 confirmed therapeutic potential of propolis at molecular level. Conclusions: It can be concluded that propolis possess hepatoprotective potential against ATDs induced hepatic injury that may prove itself as a clinically useful natural product in management of drug induced liver injury.
1. Introduction
The tuberculosis (TB) has been declared by World Health Organization as a public health emergency globally because it is the second leading reason for death from an irresistible ailment after the HIV worldwide[1]. For treatment of tuberculosis, generally 6-9 months course of antibiotics is recommended that include four medications (isoniazid, rifampicin, pyrazinamide and ethambutol)in serious stage for first two months, and then two medications(isoniazid and rifampicin) for four months during maintenance stage[2]. Hepatotoxicity is one of the severe adverse effect of antituberculosis drugs (ATDs) medications that remains a significant problem during treatment as it may reduce treatment efficacy by compromising treatment regimens[3]. The incidence rate of hepatic injury is 2.6% with isoniazid and rifampicin when given in combination but isoniazid and rifampicin individually could produce hepatic injury up to 1.6% and 1.1% respectively[4]. Liver is the most complex internal organ, which involves in most of the biochemical or metabolic pathways related to fight against diseases, supply nourishment and metabolism of carbohydrates, lipids and proteins.Being a multifunctional organ, liver cells are exposed to significant concentration of harmful drugs and chemicals, which may result in cell injury, liver dysfunction and even organ failure.
The age old system of herbal medicine and natural products is being resuscitated by day to day preparation for its long-term curative effect, simple accessibility, natural way of healing and lesser side effects[5]. A complex, resinous, sticky product propolis,is mainly collected by honeybees from several plants and bee wax pollen well known to have 300 or more compounds that includes flavonoids, phenolic acids which play major role in various biological activities[6,7]. It is widely used in traditional medicine and is extensively used in food due to its potent antioxidant activity against free radical induced damages[8]. It has a large amount of antioxidative compounds like ferulic acid, caffeic acid phenethyl ester (CAPE) and caffeic acid[9] and has an extensive variety of biological activities such as immunomodulatory[10], antibacterial[11],anti-carcinogenic[12], antioxidative[13], antifungal[14] as well as hepatoprotective effect[15] against galactosamine[16],paracetamol[17], carbon tetrachloride[18] and aluminium[19] toxicity.Administration of ATDs causes severe hepatic injury. Therefore,present study attempted to examine toxic consequence of ATDs at different durations i.e., 2, 4, 6 and 8 weeks. Since propolis has various biological activities, it was hypothesized that it may alleviate ATDs induced hepatic injury. To validate this hypothesis, the entire study was undertaken to investigate whether propolis treatment enhances ATDs induced hepatic injury considering various indicators of oxidative stress, antioxidant status (enzymatic and non-enzymatic)and inflammatory pathway, optical and ultra-structural observations.
2. Materials and methods
2.1. Animals and chemicals
Albino Wistar strain female rats [(150±10) g body weight] were accommodated under standard husbandry conditions [(25±2) ℃temp, 60%-70% relative humidity, 14 h light and 10 h dark]. The animals were nourished on standard pelleted diet and drinking water ad libitum. All the experiments were held as per the rules set by the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) India. All the protocols of present study were endorsed by institutional animal ethics committee(994/Ere/Go/06/CPCSEA).The antituberculosis drugs isoniazid,rifampicin, ethambutol and pyrazinamide were liberally acquired from Chhattisgarh Institute of Medical Sciences, Bilaspur (C.G.).Propolis (crude) was gathered from artificial hives of Apis mellifera from Jiwaji University campus, Gwalior and was prepared as an aqueous suspension in 1% gum acacia. Silymarin procured from Sigma-Aldrich was used as positive control as it is well known hepatoprotective agent and was given at the dose of 50 mg/kg[18].About 1% gum acacia in water was used as vehicle for better delivery of therapeutic agent.
2.2. Duration dependent assessment of hepatic injury induced by ATDs
The rifampicin (65 mg/kg), isoniazid (85 mg/kg), ethambutol(170 mg/kg) and pyrazinamide (210 mg/kg) were given orally as per the directions provided in anti-TB kit after converting human doses into animal dose[20]. For this purpose, animals were distributed into five groups of six animals each. Group Ⅰ served as control.Group Ⅱ to Ⅴ were administered with ATDs for 2, 4, 6 and 8 weeks respectively. Animals were euthanized after 24 h of the last treatment. Just before euthanasia of animals, blood was drawn from retro orbital venous sinus[21] and left in EDTA coated and EDTA free vials for 45 minutes. Collected blood sample of EDTA free vials were centrifuged at 3 000 rpm for 10 min to obtain serum and stored at -20 ℃ until analyzed. Various hematological and serological parameters were assessed. Liver samples were fixed in Bouin’s fixative for histopathology.
2.3. Therapeutic potential of propolis against ATDs induced injury
Rats were allocated into seven groups of six animals each.Group Ⅰ served as control given vehicle only (1% aqueous gum acacia suspension). Group Ⅱ was given propolis per se at the dose of 400 mg/kg p.o. Group Ⅲ to Ⅶ were administered with above mentioned doses of ATDs for 8 weeks (3 alternative days in a week)and group Ⅲ served as experimental control. Animals of groups Ⅳ, Ⅴand Ⅵ were given 100, 200 and 400 mg/kg dose of propolis, p.o., for 8 weeks (3 alternative days in a week considering every next day to ATDs exposure) respectively. Group Ⅶ was given silymarin 50 mg/kg, p.o., as positive control. Animals were euthanized after 24 h of last treatment; blood and tissues were collected, processed and stored at -20 ℃ until analyzed.
2.3.1. Assessment of hepatic biochemical markers in serum
The serum was used for estimation of aspartate aminotransferase(AST), alanine aminotransferase (ALT), alkaline phosphatase(ALP), bilirubin, albumin, triglycerides, cholesterol and glucose level in serum using diagnostic kits according to the manufacturer’s instructions (ERBA diagnostics Mannheim GmbH Mallaustr,Germany).
2.3.2. Assessment of oxidative stress markers and antioxidant status
Liver samples were processed to determine lipid peroxidation,which were evaluated by estimating thiobarbituric acid reactive species (TBARS)[22] and reduced glutathione was measured by its reaction with 5-5’-dithiobis 2-nitrobenzoic acid to give a yellow colored product[23]. Superoxide dismutase was assayed by assessing inhibition of rate of adrenochrome formation[24], catalase was examined by assessing decomposition of hydrogen peroxide/min[25],glutathione peroxidase was assayed by measuring the reduction of NADP+to NADPH[26], glutathione reductase was assessed by measuring the amount of NADPH utilized[27] and glucose-6-phosphatase dehydrogenase was assessed by measuring the rate of conservation of NADPH to NADP+[28].
2.3.3. Tissue biochemical assay
Hepatic tissues were processed to determine cholesterol by assessing amount of reddish colored complex with ferric chloride[29]and triglyceride were analyzed by measuring intensity of yellow product with acetylacetone[30]. Protein was assayed by measuring concentration of blue colored substance of Folin’s reagent with copper bound protein complexs in the hepatic sample[31].
2.3.4. Assessment of aniline hydroxylase for CYP2E1 activity
Microsomal CYP2E1 activity was measured in terms of aniline hydroxylase by assessing concentration of blue colored conjugate of phenol with p-amino phenol (PAP)[32]. Microsomal lipid peroxidation and protein were also estimated[22, 31].
2.3.5. Histopathological observations
In Bouin’s fixative, liver samples were fixed. Then, samples were dehydrated with ethanol of different graded series. Samples were cleared with xylene and embedded in paraffin wax (60 ℃ melting point). Liver sections of five micrometer thickness were cut,spreaded on glass slides, deparaffinized and processed routinely for hematoxylin-eosin (H & E) staining. DPX mounted slides were examined under a light microscope.
2.3.6. Transmission electron microscopy
For ultra-structural studies, 1 mm3pieces of liver were fixed in Karnovsky’s fixative at 4 ℃ for 18 h. Following this, samples were washed in phosphate buffer (pH 7.2) and post fixed in osmium tetraoxide (1%). Then dehydration was performed in acetone series and subsequently inclusion was done in eon embedding resin and polymerized at 70 ℃ for 20 h. The tissue blocks were cut into ultra-thin sections using glass knives on Reichert Jung ultra-cut-E Microtome. The sections were placed on uncoated grids, doubly stained with solution of uranyl acetate and lead citrate and analyzed with Technai transmission electron microscope.
2.3.7. Assessment of tumor necrosis factor-alpha,interleukin-6 and insulin like growth factor-I in serum
ATDs induced effect on inflammatory pathway was assessed through tumor necrosis factor-αα(TNF-α), interleukin-6 (IL-6)and insulin like growth factor-1 (IGF-1) in serum using ELISA kits (Ray Biotech, Inc; Norcross, GA 30092 USA) and the results were expressed as pg/mL. All the processes in kit were performed according to manufacturer’s instruction given in manual.
2.4. Statistical analysis
The results were expressed as mean±SE of six animals used in each group. Mean of various parameters were compared by one-way analysis of variance (ANOVA) followed by student’s t-test computed at P< 0.05.
3. Results
3.1. Assessment of hepatic injury by ATDs
Table 1 shows the effect of ATDs induced alterations on hematological variables. Exposure to ATDs for 2, 4, 6 and 8 weeks cause slight declined in hemoglobin (Hb), RBC level and significant(P<0.01) drop in WBC whereas, significant (P<0.01) increase in platelets as compared to control group. Significant elevation was observed in hepatic marker enzymes ALT, AST, ALP, cholesterol,triglyceride, bilirubin along with decrease in albumin and glucose level (Table 2) in duration dependent manner after 2, 4, 6 and 8 weeks. Significant (P<0.01) alterations were noted in almost all the parameters only at 8 weeks duration, thus, 8 weeks duration was considered as most suitable toxic duration for liver that could be carried forward for evaluation of therapeutic potential of propolis against ATDs induced hepatic injury.
Histopathological examinations of liver are shown in Figure 1(A-J) respectively. The control group of liver showed regular normal hepatic cells, sinusoids and central veins (Figure 1A and B).The ATDs treated groups for 2, 4, 6 and 8 weeks duration showed liver injury in duration dependent manner. The 2 and 4 weeks of ATDs administered animals showed slight degeneration in hepatocytes when compared to control liver histology (Figure 1C-F). The ATDs administered animals showed moderate toxicity at 6 weeks duration(Figure 1G and H), whereas, 8 weeks duration showed more degenerated alterations (Figure 1I and J).
On the basis of liver function tests and histopathological observations, a conclusion was drawn that exposure to ATDs for 8 weeks caused severe hepatic injury and this duration may be used for further studies on treatment aspects against ATDs induced hepatic injury.
3.2. Therapeutic potential of propolis against ATDs induced hepatic injury
On the basis of pilot experiment, 8 weeks duration of exposure to ATDs was selected for induction of hepatic injury. Therapeutic potential of propolis against ATDs induced hepatic injury was assessed considering biochemical and molecular markers as well as optical and ultra-structural changes in liver.
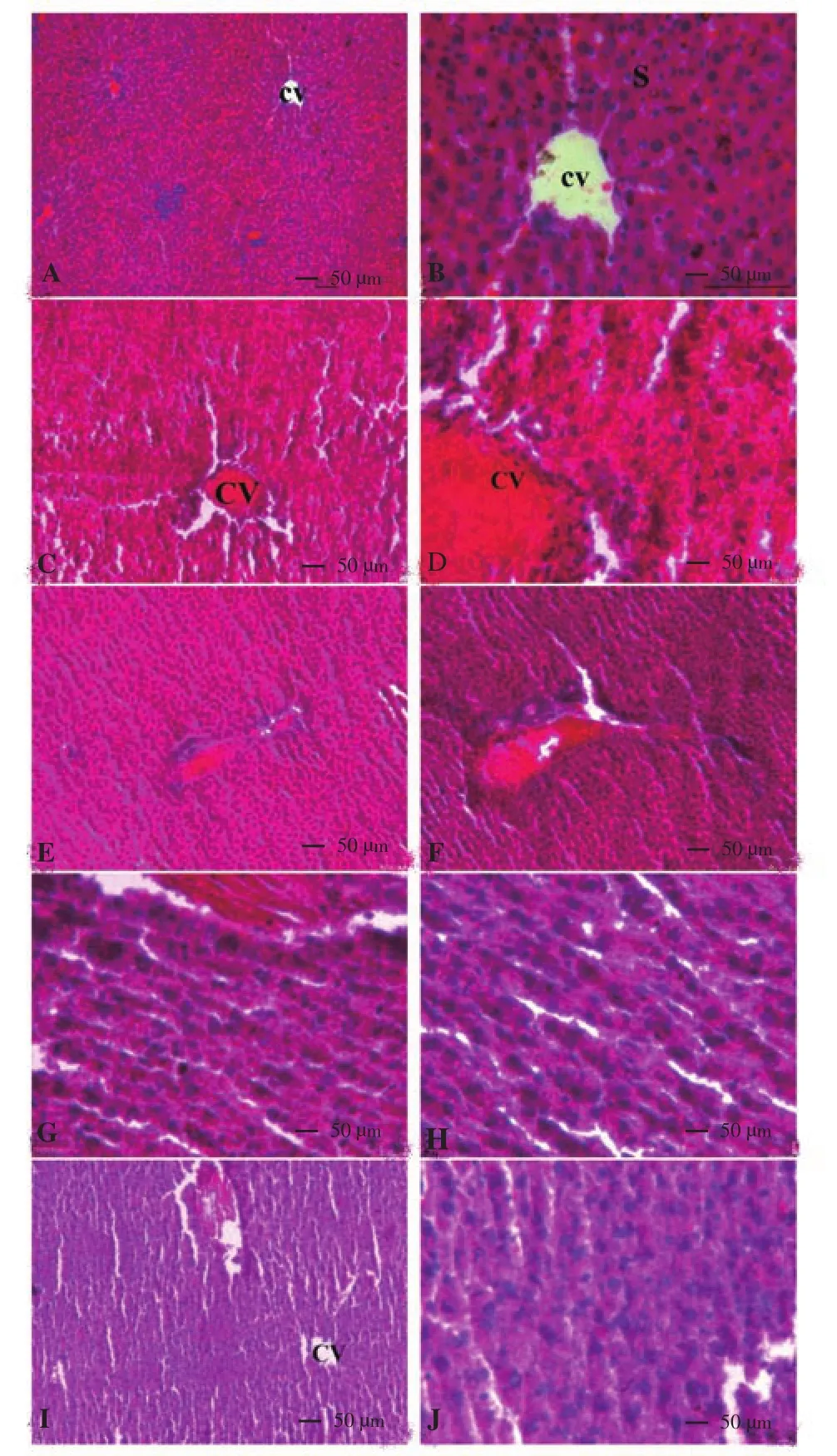
Figure 1. (A-J) Light micrographs of liver of rat (H & E stain).
3.2.1. Hepatic markers and biochemical variables in serum
Exposure to ATDs for 8 weeks resulted in hepatic injury as indicated by significantly (P<0.01) elevated concentration of ALT, AST, ALP, bilirubin, triglyceride, cholesterol along with significant (P<0.01) fall in albumin and glucose level (Table 3) as compared with control group. Treatment with propolis extract at 100,200 and 400 mg/kg doses refurbished biochemical variables towards control in a dose dependent manner. The AST and glucose were not reversed significantly towards control at 100 mg/kg dose of propolis. However, doses of 200 and 400 mg/kg of propolis showed significant (P<0.05 or 0.01) effects in restoring all the serological variables towards control. Effectiveness of propolis was paralleled with silymarin positive control group and results evidently showed therapeutic potential of propolis similar to silymarin. The per se propolis extract group (400 mg/kg) showed no adverse effect in various serological parameters as the values remained near to control.
3.2.2. Oxidative stress markers and tissue biochemical status
The ATDs intoxication for 8 weeks significantly (P<0.01)increased hepatic lipid peroxidation (Figure 2A). Increased TBARSis a flawless indication of disproportionate formation of free radicals, enhancement of oxidative stress and subsequent hepatic damage. All the three doses of propolis extract diminished oxidative stress significantly by reducing lipid peroxidation (P<0.01). Level of glutathione (GSH) (Figure 2B) was significantly decreased in liver after exposure to ATDs for 8 weeks. The propolis therapy at 100 mg/kg dose did not reverse reduced GSH significantly; however,200 and 400 mg/kg dose significantly (P<0.05) turned-up hepatic reduced GSH level near to control. Cholesterol and triglyceride levels (Figure 2C, D) were significantly (P<0.01) raised in liver after exposure to ATDs for 8 weeks in comparison to control group. No significant reversal was found in hepaticcholesterol level at 100 mg/kg dose of propolis extract; whereas,200 and 400 mg/kg doses of propolis extract showed significant reversal in cholesterol and hepatic triglyceride level towards control. The results were almost similar to silymarin group. No adverse effect was noticed in biochemical parameters in per se group of propolis extract at 400 mg/kg dose. Total protein in liver (Figure 2E) was decreased significantly(P<0.01) due to ATDs induced injury that was recovered towards control significantly with propolis at the dose of 400 mg/kg.Data are mean±S.E ofn= 6; ▽ATDvs.Control atP<0.05, φATDvs.Control atP<0.01,*ATD+Therapyvs.ATD atP<0.05,**ATD+Therapyvs.ATD atP<0.01.Abbreviations: ALT=Alanine aminotransferase; AST=Aspartate aminotransferase; ALP=Alkaline phosphatase; ATD=Antituberculosis drugs; Pro 100=Propolis 100 mg/kg; Pro 200=Propolis 200 mg/kg; Pro 400=Propolis 400 mg/kg; Sily 50=Silymarin 50 mg/kg.
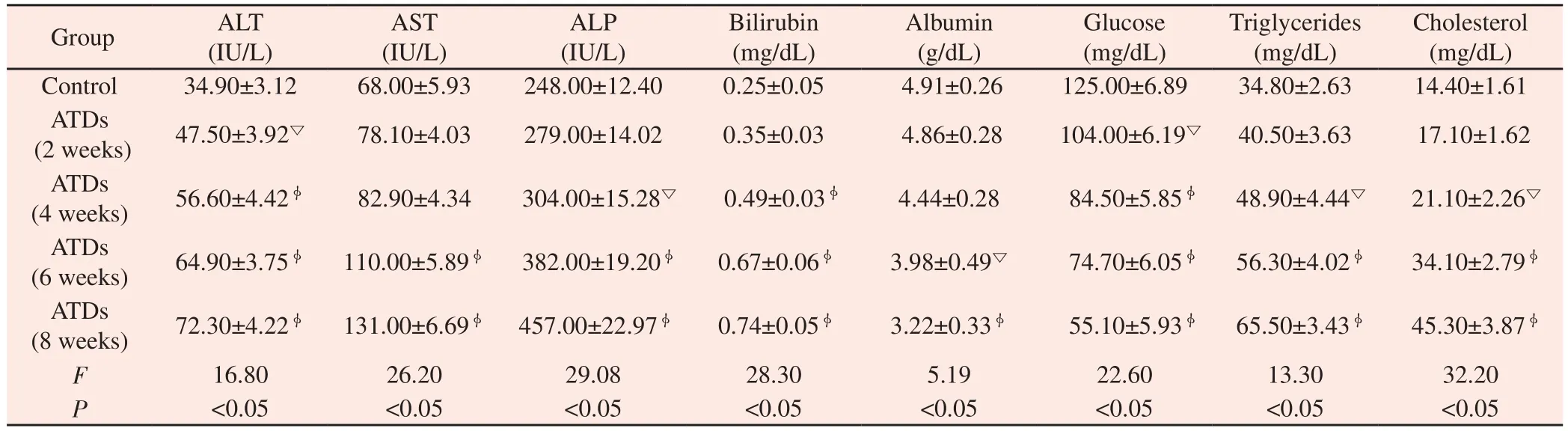
Table 2 Anti-tuberculosis drugs induced alterations on serum biochemical parameters.
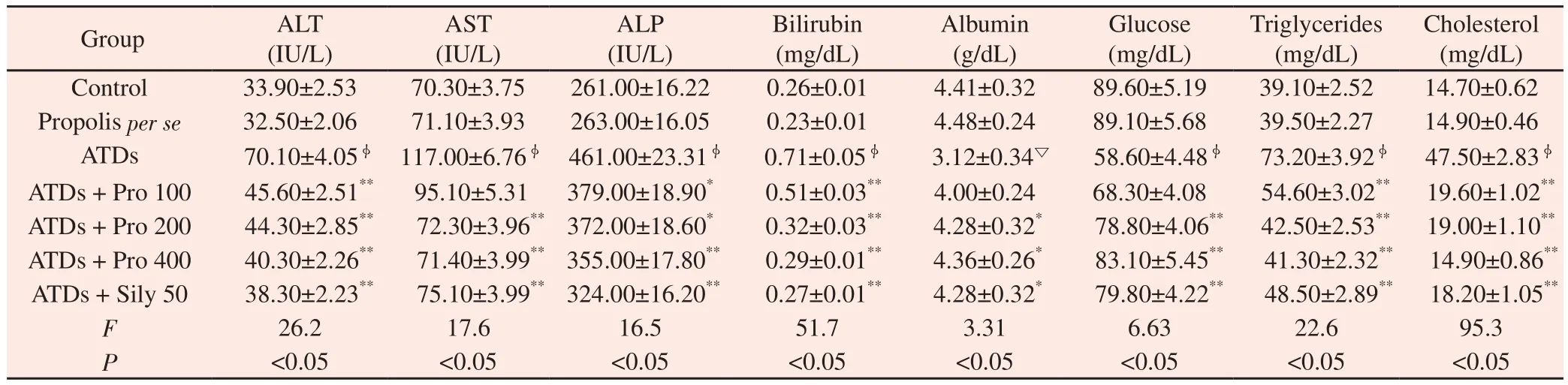
Table 3 Therapeutic effect of propolis on serum biochemical variables against antituberculosis drugs induced injury.
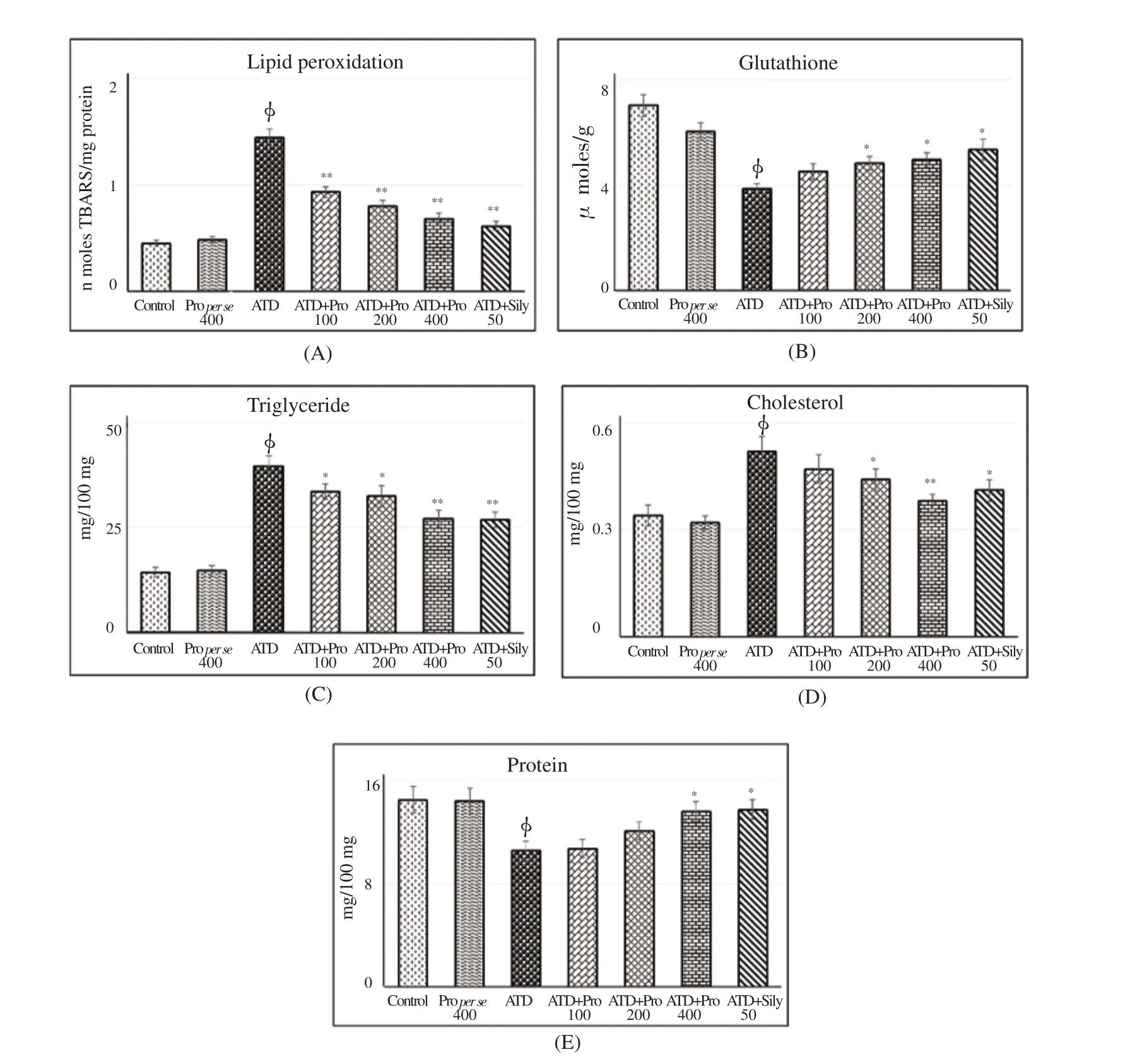
Figure 2. Therapeutic potential of propolis on ATD induced oxidative stress and tissue biochemical status.
3.2.3. Antioxidant status
Level of superoxide dismutase and catalase (Figure 3A, B) were significantly (P<0.01) decreased in liver after exposure to ATDs for 8 weeks. All the three doses of propolis therapy exhibited significant retrieval in superoxide dismutase and catalase levels in dose dependent manner, which could help in diminishing oxidative stress. Pharmacological action of propolis extract was equally effective in maintaining antioxidant status as positive control silymarin. Propolis extract per se (400 mg/kg) showed no adverse effects on cellular antioxidant status. The ATD administration caused significant (P<0.01) inhibition in activities of glutathione peroxidase,glutathione reductase and glucose-6-phosphatase dehydrogenase enzymes in liver (P<0.01) (Figure 3C-E). Oral administration of propolis revealed asignificant (P<0.01) protection in activities of glutathione dependent enzymes. Results conquered that propolis treated groups were nearly effective as silymarin treated groups.
3.2.4. Assessment of aniline hydroxylase for CYP2E1 expression
Eight weeks exposure to ATDs significantly (P<0.01) increased microsomal lipid peroxidation, protein (Figure 4A and B) and activity of drug metabolizing enzyme aniline hydroxylase(Figure 4C). Higher doses of propolis therapy significantly decreased microsomal lipid peroxidation, protein and aniline hydroxylase.Propolis therapy at 200 and 400 mg/kg doses showed more noticeable therapeutic effects in reversal of aniline dehydroxylase towards control.
3.2.5. Histopathological observations
Histological examination of control liver (Figure 5A, B) showed normal lobular architecture with well-formed hepatocytes amiably arranged nearby hepatic central vein, sinusoidal spaces uniformly terminating into central vein. ATDs administered group for 8 weeks showed liver damage with irregularly arranged and damaged hepatocytes, degenerated nuclei, vacuolation, lymphocytic infiltration(Figure 5C, D). Treatment with 100 mg/kg dose of propolis showed slight recovery with lesser inflammatory cells around portal triad(Figure 5E, F). Treatment with 200 mg/kg dose of propolis showed improvement in hepatocytes, absence of necrosis, cordially arranged hepatocytes (Figure 5G, H). Treatment with 400 mg/kg dose of propolis showed uniform cord arrangement of hepatic cells with clear central vein (Figure 5I, J). Treatment with silymarin at 50 mg/kg dose showed fine hepatocytes arranged around intact central vein (Figure 5K, I).
From the above mentioned variables, it was observed that 200 and 400 mg/kg doses of propolis were effective against ATDs induced hepatic injury. So, group of only 200 mg/kg dose of propolis was further processed for electron microscopy, TNF-α, IL-6 and IGF-1 determination.
3.2.6. Electron microscopy observation
Transmission electron micrograph of control liver showed welldefined plasma membrane, numerous regular shaped mitochondrion of various size as well as clusters of rough endoplasmic reticulum(Figure 6A). ATDs administered rat for 8 weeks showed hepatic injury with lipid droplets, damaged mitochondria, congested deformed nucleus, deformed and lesser endoplasmic reticulum(Figure 6B, C). ATDs administered rat for 8 weeks with propolis therapy at 200 mg/kg dose showed hepatocyte structure with normally appeared numerous mitochondria, hepatocyte have binucleated nucleus with nucleolus, which indicated regeneration process (Figure 6D, E). ATDs administered rats for 8 weeks when treated with silymarin at dose of 50 mg/kg were well compared with control group having plenty of normal mitochondrion and well formed nucleus (Figure 6F).
3.2.7. TNF-α, IL-6 and IGF-1
Administration of ATDs for 8 weeks caused significant (P<0.01)rise in TNF-α, IL-6 level and decreased IGF-1 level in serum(Figure 7A-C). The 200 mg/kg dose of propolis was significantly(P<0.01) effective in reversing deviation in these cytokines towards control and the efficacy was more or less same as with positive control silymarin treatment.
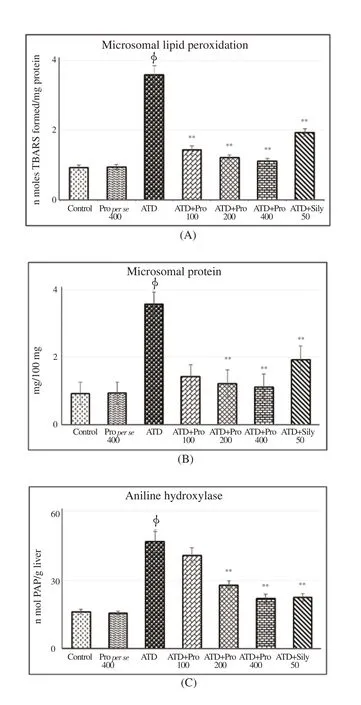
Figure 4. Therapeutic effect of propolis on microsomal lipid peroxidation,protein and aniline hydroxylase.

Figure 5. Photo micrographs of liver (H & E stain).

Figure 6. Transmission electron micrograph of liver.

Figure 7. Efficacy of propolis on ATDs induced alterations in serum TNF-α, IL-6 and IGF-1.
4. Discussion
Hepatic injuries are the severe concern during treatment of tuberculosis with ATDs and still remain a challenge to medicine practitioners. The present study attempted to evaluate hepatic injury during 2, 4, 6 and 8 weeks of exposure to ATDs. Therapeutic potential of propolis extract at 100, 200 and 400 mg/kg doses were evaluated against ATDs induced hepatic injury. Doses of isoniazid, rifampicin, ethambutol and pyrazinamide are effective in treatment of tuberculosis but also develop multidrug resistance,immunodeficiency and cause aberrations and dysfunction in liver.As these drugs are biotransformed in liver and generate more toxic products like free radicals, which lead induction of oxidative stress,inhibition of antioxidant defense and inflammation of cells[33].Isoniazid undergo acetylation in presence of liver enzyme N-acetyl transferase 2 and form acetyl hydrazin and isonicotinic acid. These acetylhydrazin on hydrolysis produce toxic metabolite hydrazine and diacetyl hydrazine, which cause cellular toxicity[34].
Exposure to ATDs for 2, 4, 6 and 8 weeks caused hepatic injuries in a duration dependent manner because ATDs prompted excessive sensitive reactions resulted in eosinophilia, hypoalbuminemia and hypoglycemia condition, which signify fatal hepatic damage[35].Isoniazid might be the main cause for cellular leakage and damage of cell membrane in liver[36]. Rifampicin cause hyperbilirubinemia by hampering excretion of bilirubin through obstructing bile salt exporter pump[37]. ATDs exposed group showed maximum damage to liver at 8 weeks duration of exposure. Thus, 8 weeks duration was taken into consideration for further experiments to evaluate therapeutic potential of propolis extract against ATDs induced injury. Propolis has been used as therapeutic agent because of its expansive range of biological properties and diverse uses against various toxic conditions. Silymarin was used as positive control in respect to propolis as it is one of the major active principle of an ancient medicinal plant Silybum marianum which has been used for centuries for treatment of different diseases including liver disorders and protecting liver against various toxicants. Due to its antioxidative and membrane-stabilizing properties, it has been shown to protect liver against a number of insults and oxidative stress[38].
Elevated level of ALT, AST and ALP are well known hepatic marker enzymes in serum indicated damage in liver[39]. Bilirubin is a good indicator of liver functionality, abnormal rise in concentration of bilirubin in serum indicates damage in hepatic cell or bile duct[40].Administration of both propolis extract and silymarin was able to decrease marker enzymes in circulation by stabilization of plasma membrane and improved the secretary ability of hepatic cells. It was previously reported that presence of flavonoids and phenolic acids in propolis is responsible for recuperating damage caused by ATDs[41].Serum triglycerides and cholesterol level were additionally observed to be aberrantly increased due to hepatic impairment. These results corroborated with other studies[42]. Treatment with propolis extract brought triglycerides and cholesterol level towards control that was an indication of improvement in lipid metabolism. The hepatic damage due to ATDs exposure was assessed by lipid peroxidation,which is a multistep reaction that resulted destruction in structure and function of cellular membranes[43]. Animals administered with ATDs for 8 weeks showed increased TBARS as a result of cell deformity in liver. The conjoint treatment of propolis at different therapeutic doses reversed these changes by decreasing TBARS level. Flavonoids present in propolis are capable of defending lipids and others compounds being oxidized or demolished due to oxidative stress[44].Propolis improved lipid profile, catalase and superoxide dismutase activity and reduced levels of chemically reactive species like H2O2that might be responsible for its anti-inflammatory effects[45].Propolis could modulate antioxidant enzymes in a constructive way and decrease in triglycerides and cholesterol level in a dose dependent manner. Declined level of GSH after ATDs instigated damage could be an after effect of lessened synthesis or expanded use of GSH because of increased oxidative stress. Propolis reduced oxidative stress by free radical scavenging potential and induction of antioxidant enzymes[18]. Propolis at 200 mg/kg and 400 mg/kg doses reestablished GSH level towards control, which consecutively amplified the detoxification of dynamic metabolites of ATDs.
Reactive metabolite of ATDs is noxious to tissues through generation of free radical that diminished the activity of GSH,superoxide dismutase, catalase, glutathione reductase, glutathione peroxidase and glucose-6-phosphate dehydrogenase. Catalase and superoxide dismutase are vital enzymes involved in elimination of chemically reactive free radicals as superoxide dismutase catalyzes dismutation of superoxide into oxygen and hydrogen peroxide whereas, catalase catalyzes conversion of hydrogen peroxide to oxygen and water[46]. In the current study, reduction in superoxide dismutase and catalase activities was likely connected with augmented oxidative stress caused by ATDs in liver tissues.These observations are inconformity with other reports[47]. Radical scavenging property of flavonoids and phenolic present in propolis protect the liver from oxidative stress by reversing superoxide dismutase and catalase activities in dose dependent manner.Antioxidant GSH-dependent enzymes, such as glutathione reductase,glutathione peroxidase and glucose-6-phosphate dehydrogenase prevent and defuse free radical induced damage[48]. In the present investigation, activities of GSH-dependent enzymes were lessened altogether after exposure to ATDs. Decline in activities of these enzymes made the cells powerless against toxic compounds, which prompt over production of chemically reactive species[49] as also indicated by elevated level of lipid peroxidation by ATDs. Treatment with different doses of propolis significantly elevated activities of glutathione reductase, glutathione peroxidase and glucose-6-phosphate dehydrogenase to normalcy indicating capability of propolis to react with free radicals and reduced damage in liver caused by reactive metabolites of ATDs.
Isoniazid and its metabolite hydrazine mainly induce CYP2E1 activity[50]. The damage inflicted by ATDs caused a loss of drug biotransforming and metabolizing ability of the liver due to less number of endoplasmic reticulum, damaged mitochondria which is well presented in ultrastructural observations. Rifampicin with other ATDs lead to severe toxicity to liver as it is a strong inducer of cytochrome P450[51]. The ATDs increased the activity of aniline hydroxylase leading to excessive buildup of free radicals within the hepatic tissues. Previous findings also suggested that the ATDs reactive metabolites such as hydrazine severely affect the CYP2E1 activity and cause hepatotoxicity[34]. Propolis induced activity of aniline hydroxylase near to control by overturning and reducing the free radicals imposed by ATD in dose dependent manner confirmed its hepatoprotective property[52].The results suggested that propolis suppress over-expression of CYP2E1 and reduce reactive metabolites formation, and thus, led to a rapid recovery from ATDs induced hepatic injury.
The histopathological and ultrastructural observations strongly supported biochemical findings of this investigation. Enzymes are located in cytoplasm so increased level of marker enzymes can be ascribed to damage in hepatic cell, which lead to release of these marker enzymes into circulation. The ATDs administered liver showed marked cellular degeneration, damaged nuclei and heavy lymphocytic infiltration[53]. Propolis therapy at different doses caused lesser inflammatory cells, absence of necrosis and conservation of hepatocytes.
The TNF-αααand IL-6 well known pro-inflammatory cytokines,their production are increased due to infection, stress and injury[54]and decrease in IGF-1. In the present study, ATDs administration for eight weeks amplified TNF-αα, IL-6 and decrease IGF-1 production as compared to control group. Propolis contains numerous active components that have been displayed to exert a wide variety of protective effects including anti-inflammatory activity[55]. Propolis has ability to reduce pro-inflammatory cytokine production suggesting its anti-inflammatory effect. The 200 mg/kg dose of propolis reversed the level of TNF-αα, IL-6 and IGF-1 and caused a subsequent recovery in function of organs towards normalization.Result of this study are in confirmation to previous studies[56].
To conclude, propolis extract at 200 and 400 mg/kg was excellently effective in maintaining all the serological, tissue biochemical,histological and ultrastructural indices towards control. It can be concluded that propolis has potential to protect ATDs induced hepatic injury and can be used as an excellent hepatic protective agent when concomitantly given with ATDs during ATDs treatment regimen. Thus, these therapeutic agent have potential to be developed as promising strategy to reduce hepatic damage during TB treatment,so that the person suffering from TB worldwide may get relief from ATDs induced liver injury for better and effective TB treatment.
Conflict of interest statement
The authors declare they have no conflict of interest.
Acknowledgments
Authors are thankful to Chhattisgarh Institute of Medical Science,Bilaspur for antituberculosis drugs and All India Institute of Medical Science, New Delhi for extending transmission electron microscope facility.
Foundation project
The work was financially supported by UGC Major Research Project [(F42-520/2013(SR)].
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Brucellosis: Pathophysiology and new promising treatments with medicinal plants and natural antioxidants
- Larvicidal, pupicidal and oviposition deterrent activities of essential oils from Umbelliferae plants against house fly Musca domestica
- Plants used in traditional medicine for treatment of malaria by Tetun ethnic people in West Timor Indonesia
- Knowledge, attitudes and practice survey on Zika virus infection among pregnant women in Brunei Darussalam
