含肟基的Schiff碱Cuギ和Niギ配合物的合成、超分子结构和光谱性质
2018-12-10张宏佳贾浩然孙银霞
张宏佳 常 健 贾浩然 孙银霞
(兰州交通大学化学与生物工程学院,兰州 730070)
0 Introduction
Much attention has been focused on oxime-based ligands in recent years due to their high stability and playing an important role in the development of coordination chemistry[1-5].The design of new Schiff-base compounds has received long-lasting research interest because of not only their appealing structural and topological novelty[6-10]but also their potential wide application in the fields of biochemistry[11-16],catalysis[17-20]and optical[21-33],magnetic materials[34-40]and constructing supramolecular structures[41-52].Schiff-base compounds and its derivatives are very important as versatile ligands,properties of interest in materials science.Also,the Schiff base ligands with N-and O-group are strong donors and therefore the oximecontaining ligands were found to efficiently stabilize high oxidation states of metal ions and prepare complexes with different structures and functionalities like Cuギ and Niギ complexes[53-58].So,asan extension of our work[9-10,55,59],two new Cuギ and Niギ complexes,[Cu(L1)2]·(1,4-dioxane)(1)(HL1=8-((4-(1-(benzyloxy)imino)ethyl)phenyl)imino)methyl)-7-hydroxy-4-methyl-2H-chromen-2-one)and[Ni(L2)2] (2)(HL2=1-(4-((2-hydroxynaphthalen-1-yl)methylene)amino)phenyl)ethanone O-benzyloxime),have been synthesized and characterized.Complexes 1 and 2 are both mononuclear structures and the central metal Cuギ and Niギions are all four-coordinated with the slightly distorted square-planar geometry.In addition,the fluorescence property of HL1,HL2and their metal complexes 1 and 2 are discussed.
1 Experimental
1.1 Materials
7-Hydroxy-4-methyl-chromen-2-one,hexamethylenetetramine,4-aminoacetophenone,O-benzylhydroxylamine,2-hydroxy-1-naphthaldehyde were purchased from Alfa Aesar and used without further purification.The other reagents and solvents were analytical grade reagents from Tianjin Chemical Reagent Factory,and were used without further purification.
1.2 Instruments and methods
C,H and N analyses were carried out with a GmbH Vario EL V3.00 automatic elemental analyzer.FT-IR spectra were recorded on a VERTEX70 FT-IR spectrophotometer,with samples prepared as KBr(400~4 000 cm-1)pellets.UV-Vis absorption spectra were recorded on a Shimadzu UV-3900 spectrometer.Luminescence spectra in solution were recorded on a Hitachi F-7000 spectrometer.X-ray single crystal structure was determined on a Bruker Smart 1000 CCD area detector.Melting points were measured by the use of a microscopic melting point apparatus made in Beijing Taike Instrument Limited Company and the thermometer was uncorrected.
1.3 Syntheses of HL 1 and HL 2
HL1and HL2were synthesized according to the following steps shown in Scheme 1.
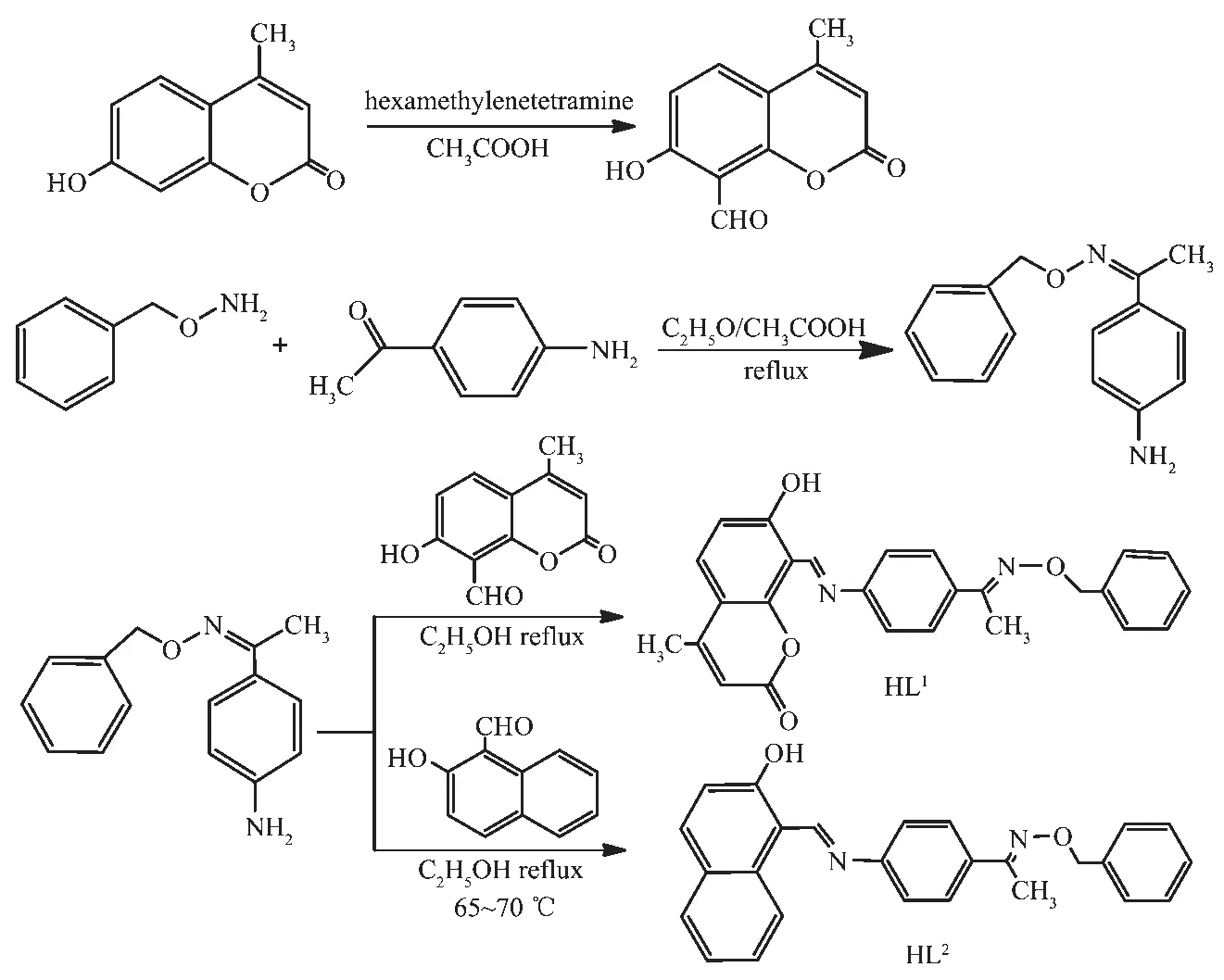
Scheme 1 Synthetic routes of HL1 and HL2
HL1:Firstly,7-hydroxy-4-methyl-chromen-2-one 8.0 g (50 mmol)and hexamethylenetetramine 14.0 g(100 mmol)were dissolved in 50 mL glacial acetic acid,and the mixed solution was stirred and refluxed for 5~6 h.A 5%hydrochloric acid solution was added to adjust the pH value to about 4.5,and then refluxed and stirred for 30 min.The mixed solution was allowed to come to room temperature,then extracted with ether,washed with sodium chloride solution and dried with anhydrous MgSO4. After removing solvent and recrystallizing from absolute ethanol,1.7 g crystalline solid of 7-hydroxy-4-methyl-2-oxo-2H-chromene-8-carbaldehyde was collected.Yield:17%.m.p.178~180 ℃.Anal.Calcd.for C11H8O4(%):C,64.71;H,3.95.Found(%):C,65.04;H,4.02.
Secondly,1-(4-aminophenyl)ethan-1-one O-benzyl oxime was synthesized according to the reported method[53]as yellow crystals.Yield:874.3 mg,80.6%.m.p.80~81 ℃.Anal.Calcd.for C15H16N2O(%):C,74.97;H,6.71;N,11.66.Found(%):C,75.07;H,7.35.N,12.43.
At last,a solution of 1-(4-aminophenyl)ethan-1-one O-benzyl oxime (480.6 mg,2.00 mmol)in ethanol(7.5 mL)wasadded toa solution of 7-hydroxy-4-methyl-2-oxo-2H-chromene-8-carbaldehyde (404.4 mg,2.00 mmol)in ethanol(7.5 mL)and the mixture was heated to reflux at 65~70 ℃ for 7~8 h.After being cooled by ice-water bath,washed with an anhydrous ethanol solution and dried under reduced pressure,orange-red powder was obtained.Yield:569.1 mg,64.3%.m.p.229~230 ℃.Anal.Calcd.for C26H22N2O4(%):C,73.23;H,5.20;N,6.57.Found(%):C,74.07;H,6.05.N,6.43.
HL2:The synthesis of HL2is similar to that of HL1except substituting 7-hydroxy-4-methyl-2-oxo-2H-chromene-8-carbaldehyde with 2-hydroxy-1-naphthaldehyde.The precipitate was filtered and washed successively with ethanol and ethanol/n-hexane (1∶4,V/V),respectively.The product was dried in vacuum to obtain yellow powder.Yield:79.43%.m.p.127~129 ℃.Anal.Calcd.for C26H22N2O2(%):C,79.17;H,5.62;N,7.10.Found(%):C,80.03;H,6.05.N,7.46.
1.4 Syntheses of[Cu(L 1)2]·(1,4-dioxane)(1)and[Ni(L 2)2](2)
Complex 1:A solution of copperギ acetate monohydrate (0.51 mg,0.002 5 mmol)in methanol(2 mL)was added dropwise to a solution of HL1(1.9 mg,0.005 mmol)in 1,4-dioxane (2 mL)at room temperature.The color of the mixing solution turned to pale green immediately,and then the solution was filtered and the filtrate was allowed to stand at room temperature for about one week.Brown block-shape single crystals suitable for X-ray structural determination were obtained.Anal.Calcd.for C56H50CuN4O10(%):C,67.13;H,5.03;N,5.60.Found(%):C,68.09;H,5.64;N,5.98.
Complex 2:The synthesis of complex 2 was same as above,and red-brown prismatic single crystals suitable for X-ray crystallographic analysis were obtained.Anal.Calcd.for C52H42NiN4O4(%):C,73.91;H,5.01;N,6.63.Found(%):C,73.46;H,4.68;N,6.85.
1.5 X-ray crystallography of complexes 1 and 2
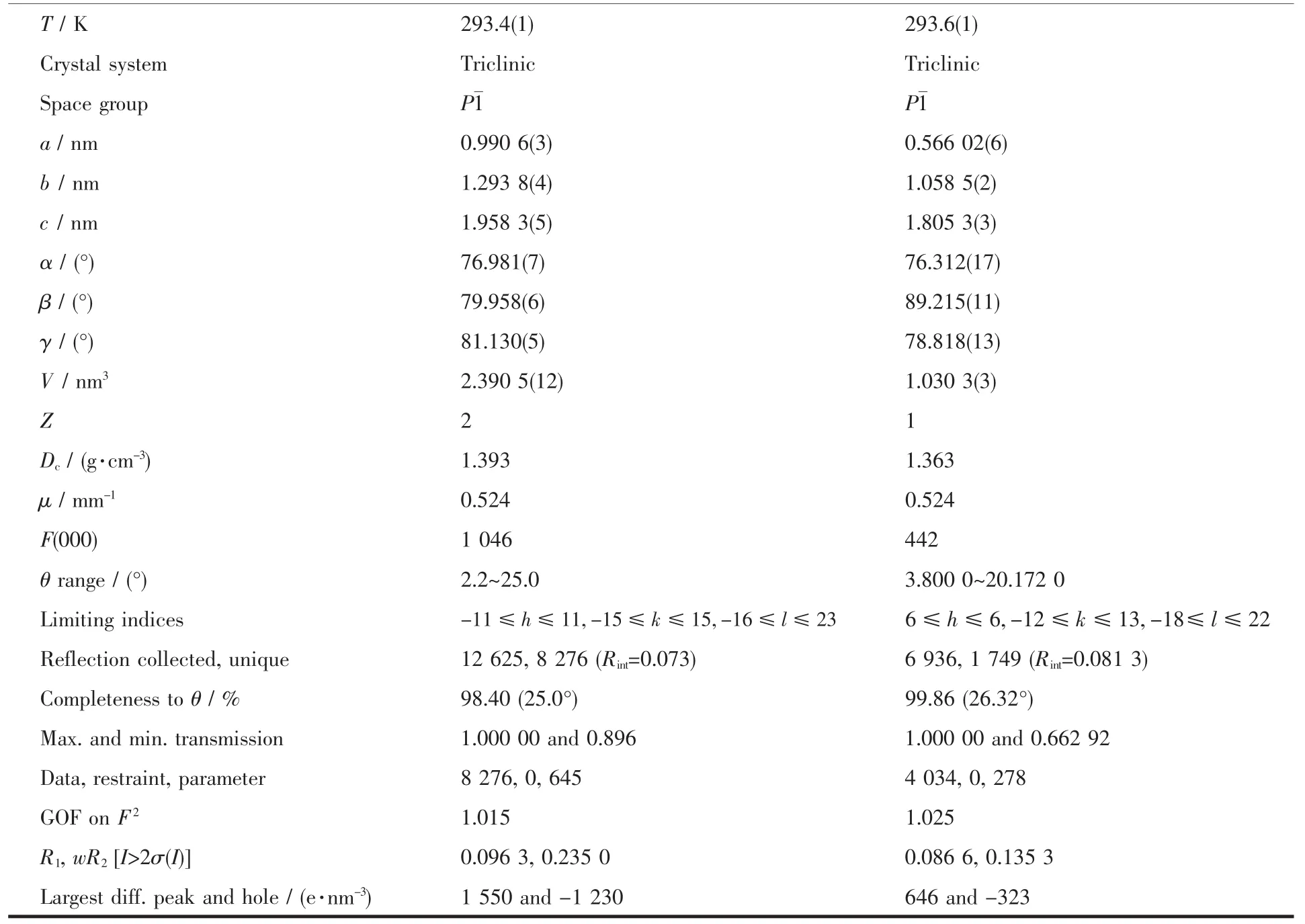
The single crystals of complexes with approximate dimensions of 0.11 mm× 0.16 mm× 0.21 mm (1)and 0.28 mm×0.03 mm×0.02 mm (2)were placed on a Bruker Smart 1000 CCD area detector,respectively.Thediffraction data of complexes 1 and 2 were collected using a graphite monochromated Mo Kα radiation (λ=0.071 073 nm)at 293.4(1)and 293.6(2)K,respectively.The Lp corrections were applied to the SAINT program[60]and semi-empirical correction were applied to the SADABSprogram[61].The crystal structures were solved by the direct methods (SHELXS-2014)[62].All nonhydrogen atoms were refined anisotropically.All the hydrogen atoms were generated geometrically and refined isotropically using the riding model.Details of the data collection parameters and crystallographic information for complexes 1 and 2 are summarized in Table 1.
CCDC:1542901,1;1857932,2.

Table 1 Crystal data and structure refinement for complexes 1 and 2
Continued Table 1
2 Results and discussion
2.1 IR spectra analyses
The FT-IR spectra of HL1,HL2and complexes 1 and 2 exhibited various bands in the 400~4 000 cm-1region.The most important FT-IR bands for HL1,complex 1 and HL2,complex 2 are given in Table 2.HL1and HL2exhibited characteristic stretching bands of C=Ngroup at 1 610 and 1 612 cm-1,respectively[63-69].While those of complexes 1 and 2 were observed at the 1 580 and 1 597 cm-1,respectively.Compared to the ligands,the C=N stretching frequencies of complexes 1 and 2 were both shifted to lower frequencies by ca.30 and 15 cm-1,respectively,which indicated that the Cuギ and Niギ ions coordinate with the oxime nitrogen lone pair[70-72]in the C=N group,lowering the bond energy of the C=N bond.The stretching bands at 3 053 cm-1(in HL1)and 3 453 cm-1(in HL2)were assigned to the characteristic vibration of O-H in phenolic hydroxyl groups,respectively,which disappeared in complexes 1 and 2,indicating that the phenolic hydroxyl groups in HL1and HL2are deprotonated and coordinate with Cuギ and Niギ ions to form coordination bonds,respectively.The Ar-O stretching bands at 1 076 and 1 140 cm-1of complexes 1 and 2 shifted toward lower frequencies by ca.85 and 24 cm-1compared with those of HL1and HL2at 1 165 and 1 164 cm-1,respectively.Thereason maybe is due to that coordination of Cuギ and Niギ ions with the phenolic oxygen atoms of ligands result in reducing the Ar-Obond energy[73].The FT-IR spectrum of complex 1 showed ν(M-N)and ν(M-O)vibration frequencies at 578,517 and 458 cm-1(or 463 and 427 cm-1for complex 2),respectively.These assignments are consistent with the literature[49].
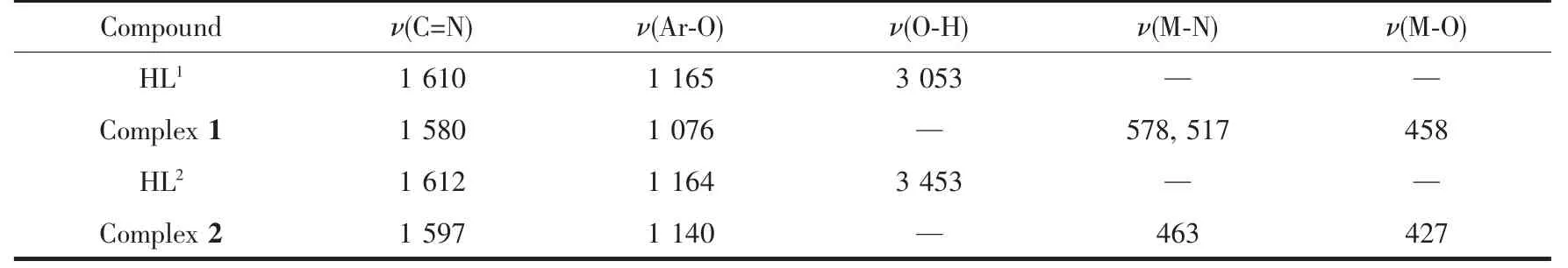
Table 2 Main bands in IR spectra of H 2L 1,H 2L 2 and their Cuギ and Niギ complexes cm-1
2.2 UV-Vis absorption spectra analyses
The absorption spectra of HL1and its corresponding Cuギcomplex 1,ligand HL2and its corresponding Niギcomplex 2 were determined in diluted DMSO solution as shown in Fig.1 and 2,respectively.UV-Vis spectrum of HL1exhibited two absorption peaks at ca.281 and 328 nm.The former at 281 nm can be assigned to the π-π*transition of the benzene rings,which blue-shifted to high energy region by ca.23 nm in complex 1,indicating Cuギion coordinated with the O and N atoms of deprotonated (L1)-ligand.The later at 328 nm attributed to the intra-ligand π-π*transition of C=N bonds[74]was absent in complex 1,indicating the oxime nitrogen atom is involved in the coordination with Cuギion[75].However,a new absorption peak observed at 410 nm in complex 1 can be ascribed to the d-d forbidden transition of Cuギion.

Fig.1 UV-Vis absorption spectra of HL1 and complex 1 in diluted DMSO solution at room temperature
It can be seen that the absorption peaks of complex 2 are obviously different from those of HL2upon coordination (Fig.2).Compared with complex 2,an important feature of the absorption spectrum of HL2was that three absorption peaks were observed at 390,446 and 470 nm attributed to the intra-ligand π-π*transition of C=N bonds and conjugated aromatic chromophore,which are absent in the spectrum of complex 2.A new absorption peak owing to L→M charge-transfer transitions[75]was observed at 426 nm in complex 2,which are characteristic of the transition metallic coordination compounds with N2O2coordination spheres.And the absorption peak at 324 nm assigned to the π-π*transitions of phenyl rings in HL2was shifted to 323 nm in complex 2 indicating the coordination of Niギion with HL2.

Fig.2 UV-Vis absorption spectra of HL2 and complex 2 in diluted DMF solution at room temperature
2.3 Structural description of complexes 1 and 2
The molecular structures of complexes 1 and 2 are shown in Fig.3 and 4,respectively,and selected bond lengths and angles are listed in Table 2.Complexes 1 and 2 are all mononuclear structures and crystallize in the triclinic system,P1 space group.Both of complexes 1 and 2 consist of one metal ion Mギ(M=Cu or Ni),two bidentate L-units,in which the difference is that complex 1 contains a crystallizing 1,4-dioxane molecule.In the molecular structures of complexes 1 and 2,the metal centers Mギ (M=Cu for 1 and Ni for 2)are tetra-coordinated in a trans-MN2O2slightly distorted square-planar geometries,with two phenolic O and two imino N atoms from two N,O-bidentate oxime-type Schiff ligands (HL1and HL2).In complex 1,the four atoms of the donor set(N1,N3,O1,O5)and Cu1 approximately lie in a plane with the distance being 0.000 7 nm and 0.017 3~0.017 4nm of Cuギand N/O atoms to the square plane.The dihedral angle between the coordination plane of N1-Cu1-O1 and that of N3-Cu1-O5 is 14.22°,indicating slight distortion toward tetrahedral geometry from the square planar structure.And the four atoms of the donor set (N1,N1a,O1,O1a)and Ni1 are completely coplanar.In addition,the bond lengths of M-N(0.203 3(6)and 0.202 1(6)nm for Cu-N,0.189 0(5)nm for Ni-N)are longer than M-O bond (0.188 4(6)and 0.189 3(5)nm for Cu-O,0.182 8(4)nm for Ni-O)in complexes 1 and 2,which is probably due to the weakening of the coordination abilities of coordinating nitrogen atoms by the larger electronegativity of oxygen atoms of phenolic hydroxyl groups.The significant elongation has been observed in other metal complexes with Schiff ligands[47].
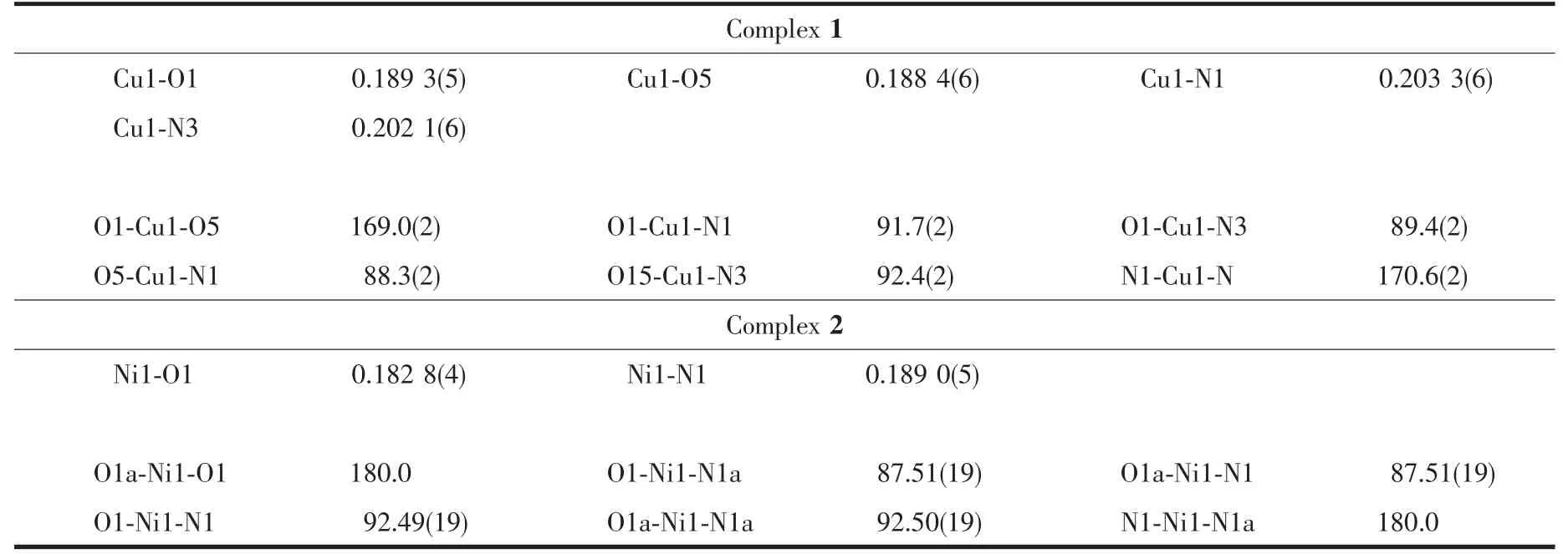
Table 3 Selected bond lengths(nm)and bond angles(°)for complexes 1 and 2
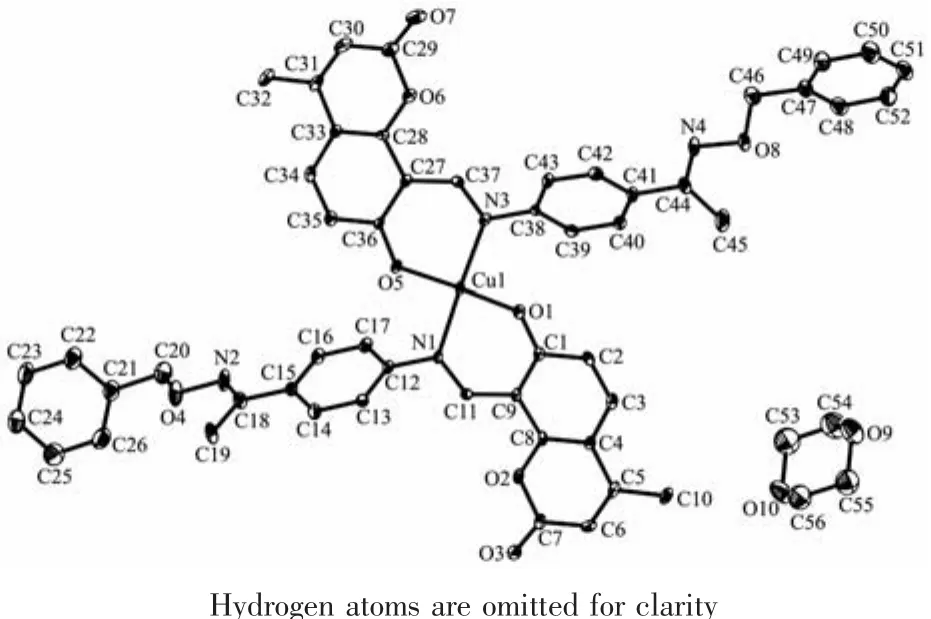
Fig.3 Molecular structure of complex 1 showing 30%probability displacement ellipsoids

Fig.4 Molecular structure of complex 2 showing 30%probability displacement ellipsoids
2.4 Supramolecular interaction of complexes 1 and 2
The main hydrogen bond andπ…πstacking interactions parameters of complexes 1 and 2 are given in Table 4 and Table 5.As shown in Fig.5,a pair of intermolecular non-classical hydrogen bonds C13-H13…O7a have stabilized a pair of complex 1 molecules to form a dimer unit.Synchronously,this dimer unit is further stabilized via two hydrogen bonds C22-H22…O9 and C34-H34…O10 between the crystallizing 1,4-dioxane molecule and complex 1 molecule (Fig.6).Furthermore,this linkage is linked via two pairs of intermolecular C6c-H6c…O3 and C51d-H51d…O3 hydrogen bonds to form an infinite 1D band-like supramolecular structure (Fig.6).Thus,complex 1 molecules are linked together into an infinite 2D-layer supramolecular structure via intermolecular non-classical C-H…O hydrogen bonds interactions (Fig.7).In addition,this adjacent 2D-layer are further held together by the intermolecular complicatedπ…πstacking interactions to form 3D network supramolecular structure (Table 5)[76-80].Consequently,the intermolecular non-classical hydrogen-bonding plays a very important role in the construction of supramolecular networks structure[81-85].Whereas,in complex 2,the molecules linked only via a intermole-cular πcentroid(C21~C26)…πcentroid(C21e~C26e)stacking interactions of benzene rings of the neighboring molecules to form a 1D infinite chain parallel to the a axis (Fig.8,Table 5).

Fig.5 View of dimer unit stabilized by hydrogen bonds of complex 1
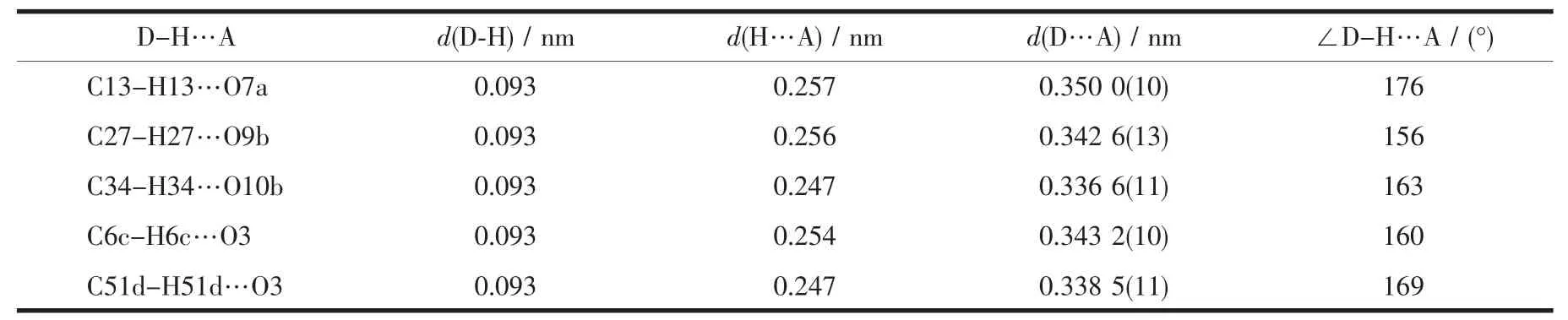
Table 4 Hydrogen bonds parameters for complex 1
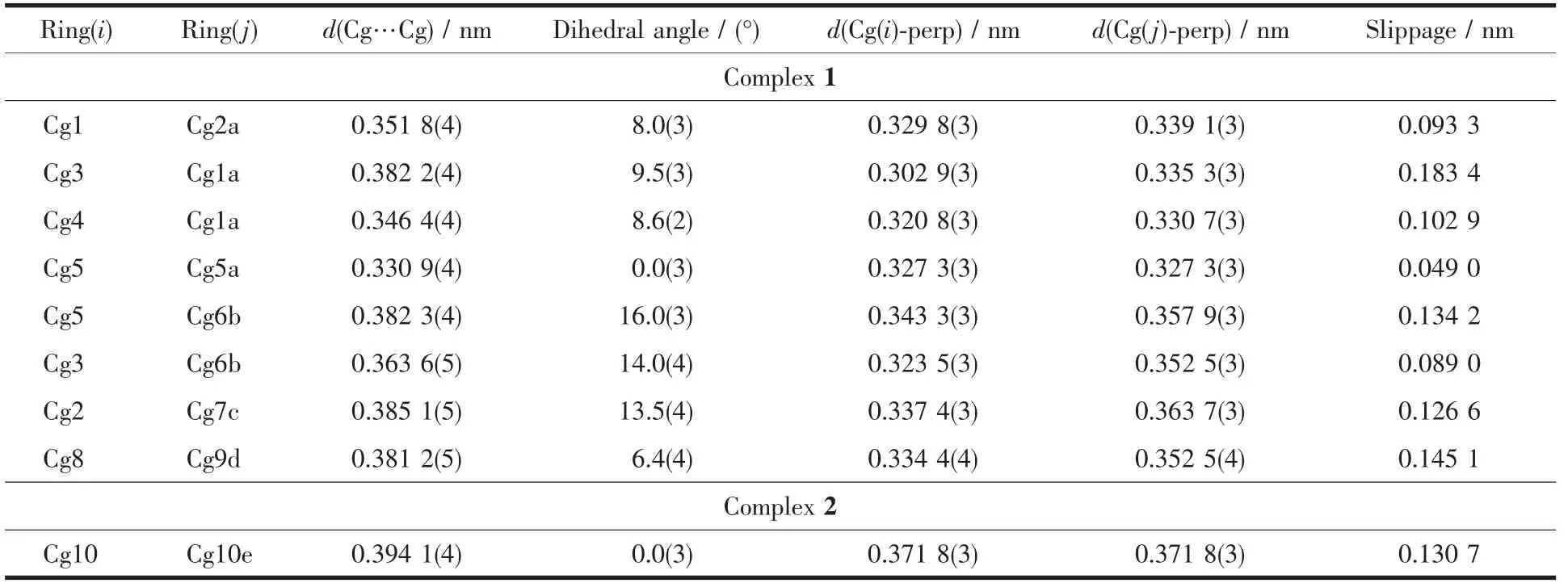
Table 5 π-π stacking interactions parameters for complexes 1 and 2
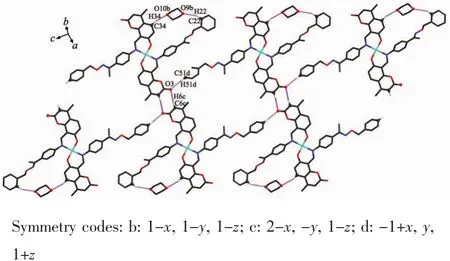
Fig.6 View of infinite 1D band-like supramolecular structure linked by hydrogen bondsof complex 1
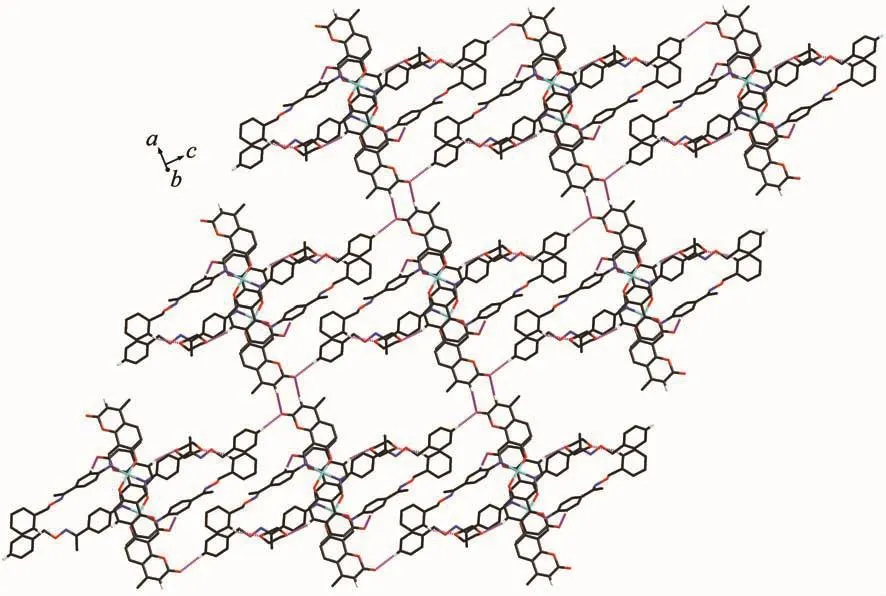
Fig.7 View of 2D-layer supramolecular stabilized by hydrogen bonds of complex 1

Fig.8 View of 1D infinite supramolecular chain linked byπ…πstacking interaction of complex 2
2.4 Fluorescence spectra
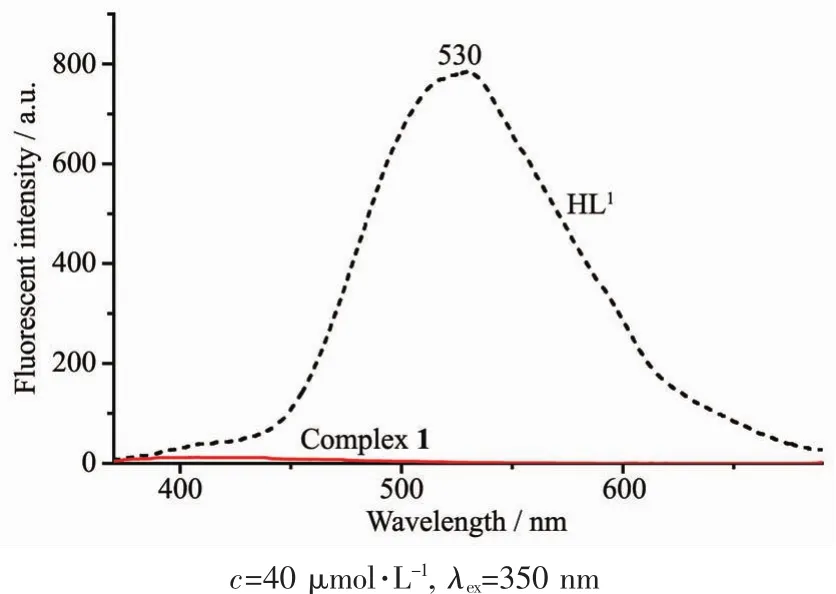
Fig.9 Emission spectra of HL1 and complex 1 in diluted DMSOat room temperature
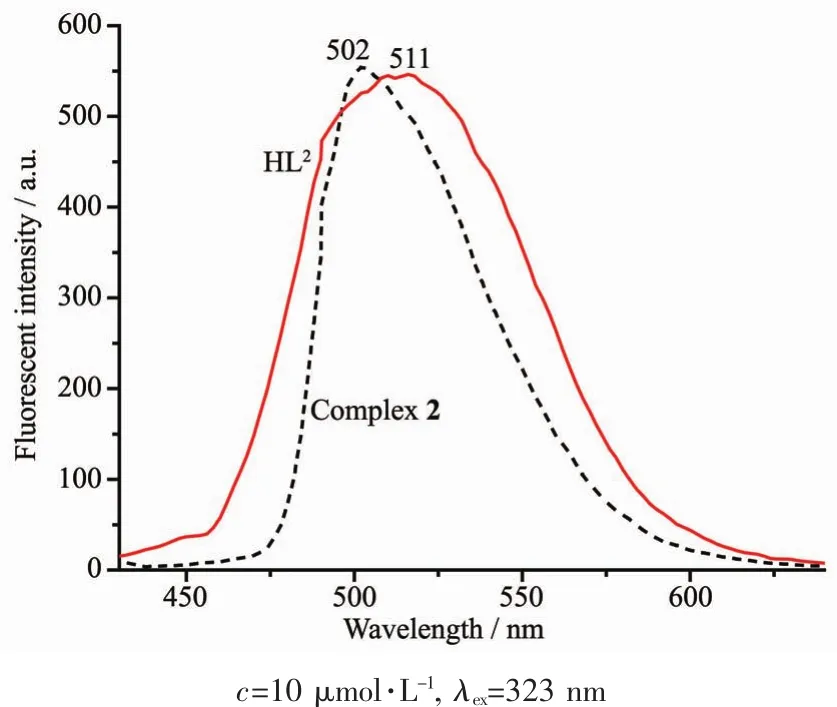
Fig.10 Emission spectra of HL2 and complex 2 in diluted DMF at room temperature
The fluorescence emission spectra of HL1,HL2and complexes 1 and 2 in diluted DMSO and DMF solution at room temperature are shown in Fig.9 and Fig.10,respectively.With excitation at 350 nm,HL1exhibited an intense emission at 530 nm and show a strongyellow-green fluorescence,which may beassigned to the intra-ligand π-π*transition[86-87].In comparison with HL1,an extremely weak fluorescence intensity of complex 1 was observed,indicating that the change of fluorescence is due to the coordination of Cuギion to HL1.However,both HL2and complex 2 show strong fluorescence emission at 502 and 511 nm with the excitation at 323 nm,respectively.Compared with HL2,the emission peak of complex 2 is slightly red shifted ca.9 nm,indicating that Niギion coordinates with the N and O atoms and occurs electron transition.
3 Conclusions
Two Schiff base mononuclear complexes 1 and 2 have been synthesized and characterized structurally.Complexes 1 and 2 are tetra-coordinated by two nitrogen atoms and two oxygen atoms of two deprotonated (L1)-units defining the N2O2basal plane.The coordination environment around the metal ions Mギ(M=Cu for 1 and Ni for 2)are best regarded as the slightly distorted square-planar geometries.Complex 1 forms a 3D network supramolecular structure by intermolecular non-classical C-H…O hydrogen bonds and π…πstacking interactions.Whereas,complex 2 just forms a 1D infinite chain held together by intermolecularπ…πstacking interaction.The fluorescence of complex 1 was quenched by Cuギbut that of complex 2 was red-shifted ca.9 nm upon complexation compared to HL1and HL2,respectively.
Acknowledgements:This work was supported by Gansu science and technology plan program (Grant No.18YF1GA054)and the Program for Excellent Team of Scientific Research in Lanzhou Jiaotong University (Grant No.201706),which is gratefully acknowledged.
