Study on Early Warning Signals of Report on Adverse Reactions of Spontaneous Reporting System of Runzao Zhiyang Capsule
2018-10-30WngLingdi王领弟XieYnming谢雁鸣WngLinxin王连心ZhngChng
Wng Lingdi (王领弟), Xie Ynming (谢雁鸣), Wng Linxin (王连心)*, Zhng Chng (张 长)
a. Institute of Basic Research in Clinical Medicine, China Academy of Chinese Medical Sciences, Beijing 100700, China
b. Shandong University of Traditional Chinese Medicine, Jinan 250000, China
c. Renmin University of China, Beijing 100872, China
ABSTRACT Spontaneous reporting system (SRS) is an important way to monitor the adverse drug reaction (ADR) and discover the ADR signal for marketed drugs. It can detect adverse reaction signals timely and effectively, and prevent the occurrence of drug damage. Runzao Zhiyang Capsule is mainly composed of Radix Polygoni Multiflor, Radix Polygoni Multiflori Preparata, Radix Rehmanniae Recens, Radix Sophorae Flavescentis, Folium Mori and Urtica dentata Hand.-Mazz. It has the functions of nourishing blood, nourishing yin, expelling wind to relieve itching and moistening the intestines to relieve constipation. It is mainly used for skin itching, acne, constipation and other diseases caused by blood deficiency and wind dryness. The large, national SRS database of ADRs needs effective evaluation methods. We reported on the use of Bayesian confidence propagation neural network method (BCPNN) and reporting rate ratio method (PRR) with propensity score to control confounding variables. The tendency score method was used to control the hybrid bias produced by SRS data analysis. After the calculation of PRR and BCPNN, the score of "diarrhea", "rash" and "gastric dysfunction" showed that there was an early warning before and after matching. To sum up, it indicated that diarrhea,rash and gastric dysfunction were early warning signs.
KEYWORDS: Runzao Zhiyang Capsule; Spontaneous reporting system; Adverse reactions; Liver injury;Reporting rate ratio method; Bayesian confidence propagation neural network method
Spontaneous Reporting System (SRS) can help medical staff, other professionals and customers provide reports about adverse drug reaction (ADR)to manufacturing enterprises, organizations of ADR surveillance, and Food and Drug Administration[1,2].At present, SRS is the basic method of monitoring and detecting ADR. It is also one of reliable ways to evaluate the safety of drugs, so it can effectively prevent ADR[3]. Data mining has become the major method of analyzing and evaluating ADR all over the world. And the Bayesian belief propagation neural network in the Bayesian method and the reporting rate ratio method in the traditional frequency method are 2 commonly used pharmacovigilance analysis methods[4].
With the wide use of Traditional Chinese Medicine (TCM) preparations, its safety has been increasingly challenged. "Whether it is safe or not?" is a complicated question because of its mixed constituents,the patients' different conditions, their different habits to take medicine, and the various environment. As a TCM preparation, Runzao Zhiyang Capsule has the characteristic of causing allergy easily. What's more,the dose of Radix Polygoni Multiflor, Radix Polygoni Multiflori Preparata is great in Runzao Zhiyang Capsule.The reports on the ADR caused by Radix Polygoni Multiflor preparations have been increased[5], and the safety issue of Radix Polygoni Multiflor, especially its harm to liver, has received a great deal of attention from doctors and Food and Drug Administration. The SRS database has a large number of reports of TCM preparations. In this study, the reports of Runzao Zhiyang Capsule were selected for statistical analysis description. The data mining method was used to screen the early ADR signals of Runzao Zhiyang Capsule.It is an effective method which has important clinical significance for safer and more reasonable application of Runzao Zhiyang Capsule.
MATERIALS AND METHODS
Source of materials
A total of 377 ADR reports of Runzao Zhiyang Capsule in the SRS database of Center for Drug Reevaluation, China Food and Drug Administration(National Center for ADR Monitoring, China) from 2009 to 2016 were selected.
Standardized data
The purpose of data standardization is to: (1) ensure the uniqueness of the patient being analyzed; (2) ensure the consistency of the doctor's advice and the diagnosis;(3) ensure the validity of the dosage unit. Based on the above principles, the information of the database is standardized to form complete and effective analytical data. ADR/ADE refers to The WHO Adverse Reactions Terminology. Western medicine diagnosis, combined diseases, and others refer to the International Classification of Diseases Code [ICD-10]. TCM disease name and syndrome refer to GB/T 16751.1-1997 Chinese Medicine Clinical Diagnosis and Treatment Terminology - Disease Section, GB/T 16751.2-1997 Traditional Chinese Medicine Clinical Diagnosis Terminology - Syndrome.
Statistical methods
The data of this study were standardized by SPSS 17.0 statistical software. The software of Matlab was used for programming. And the reporting rate proportional method in the traditional frequency method and the Bayesian belief propagation neural network method for signal monitoring in the Bayesian method were employed for transforming information into machine code. Then the early warning signal of drug is calculated and the early warning trend map was formed. Finally,the early warning signal was analyzed and verified based on the propensity score method. This study used Excel 2016 for mapping.
RESULTS
In the 377 case reports, 364 cases (96.55%) were mild ADRs, 13 cases (3.45%) were severe ADRs. 10 cases(71.4%) were mild liver injuries and 4 cases (28.6%) were severe liver injuries in the 14 case reports of liver injury.
General information
163 patients in the 18-44 age group accounted the most for 43.24% of 377 cases. 115 patients were 45-64 years old, accounting for 30.50%. Age distribution of patients with severe ADR, suspected liver injury, and patients with severe suspected liver injury were the same as all ADR patients. Considering the wide applications of Runzao Zhiyang Capsule, ADR may occur in a relatively wide age group, including the occurrence of suspected liver injury cases. The sex ratio of all ADR patients was close to 1:1. Among the total reports of ADR, patients with family medical history accounted for 0.27%, and those with previous medical history accounted for 1.06%with low number of patients. And the other information was shown in Table 1 below.
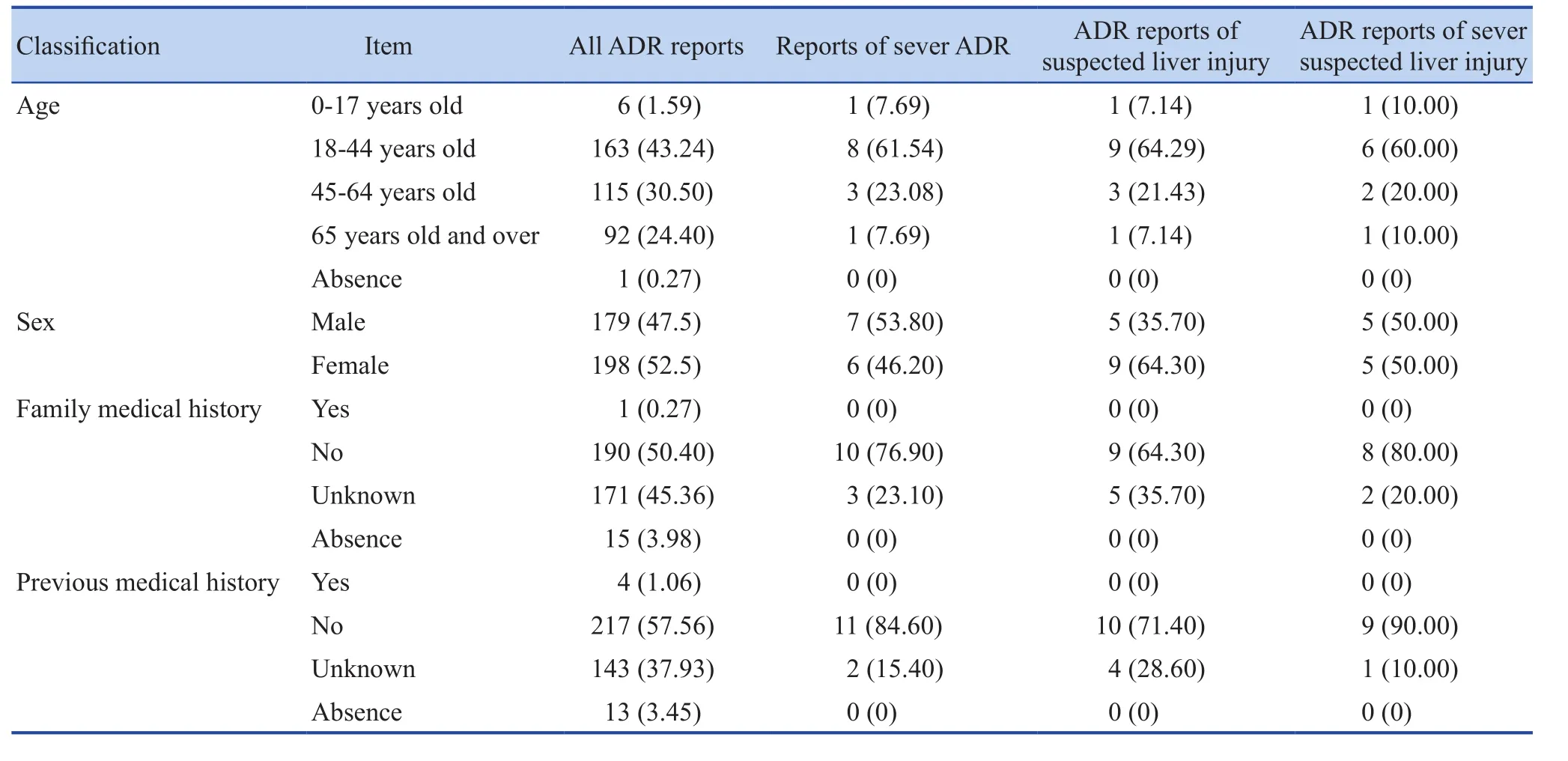
Table 1. The basic information n (%)
The information of doctor's advice
Days of the occurrence of ADR after taking the capsules: the average days in all cases were 3.64 days,with the shortest 1 day, and the longest 63 days. Of the 377 cases reported, 125 patients were taking the capsules for 1 day (accounting for 33.16%), 101 patients for 2 days(26.79%), 59 patients for 3 days (15.65%), 58 patients for 3-7 days (15.38%), 20 patients for 8-14 days (5.31%),and 14 patients for 15 days and longer (3.71%). Of the 13 severe cases reported, 2 patients were taking the capsules for 1 day (15.38%), 1 patient for 2 days (7.69%), and also 1 patient for 3-7 days (7.69%), 3 patients for 8-14 days(23.08%), 6 patients for 15 days and longer (46.15%). Of the 14 cases with suspected liver injury, 1 patient is taking the capsules for 3-7 days (7.14%), 4 patients for 8-14 days(28.57%), 9 patients for 15 days and longer (64.29%).Of 10 cases with severe suspected liver injury, 1 patient was taking the capsules for 3-7 days (10%), 3 patients for 8-14 days (30%), 6 patients for 15 days and longer (60%).In conclusion, the occurrence of ADR of many reports was in 3 days. And the occurrence of severe ADR in a lot of reports was over 1 week. However, the patients with occurrence of suspected liver injury accounted for the most with over 14 days. Possibly the occurrence of drug damage was related to the accumulation of time.
The frequency of taking the capsules daily: of the 377 reports, 17 patients took the capsules once a day(4.51%), 13 patients twice a day (3.45%), 342 patients 3 times a day (90.72%), 5 patients 4 times a day (1.33%).Of the 13 cases with severe ADR, 1 patient took the capsules twice a day (7.69%), 12 patients 3 times a day(92.31%). Of the 14 reports with liver injury, 1 patient took the capsules twice a day (7.14%), 13 patients 3 times a day (92.86%). Of the 10 cases with severe liver injury,1 patient took twice a day (10%), 9 patients 3 times a day(90%). In conclusion, 1.33% patients exceed the stated dose, but patients with suspected liver injury followed the instructions.
Dosage (each time): of the 377 ADR reports,359 patients took 1-4 capsules each time (95.23%), 2 patients 4-6 capsules each time (0.53%), 8 patients more than 8 capsules each time (2.12%), and the 8 patients'information was absent (2.12%). 13 patients with severe ADR took 1-4 capsules each time. The average dosage of the 14 patients with suspected liver injury was 3.04,and the highest dosage was 4 and the lowest dosage was 1.5. Those numbers were within the maximum dose. The recommended dosage of Runzao Zhiyang Capsule was 4 capsules each time and 3 times a day. In all case reports,2.65% of patients exceed the recommended dose, while patients suspected of having liver injury followed the instructions.
Reasons for taking the capsules: The reasons for taking the capsules in 377 cases reports were pruritus (141 patients), dermatitis (50 patients), eczema (48 patients),acne (29 patients), constipation (23 patients), urticarial (18 patients), anaphylaxis (11 patients), and rash (11 patients).The reasons for taking the capsules after TCM syndrome differentiation were clearing heat and removing toxin (4 patients), nourishing yin and blood (3 patients), expelling wind (1 patient), killing parasites and relieving itching(1 patient). Of the 13 patients with severe ADR, the reasons were pruritus (5 patients), urticarial (2 patients),anaphylaxis (1 patient), acne (1 patient), parapsoriasis(1 patient), dermatitis (1 patient), rash (1 patient), and eczema (1 patient). Of the 14 cases with suspected liver injury, they took capsules because of pruritus (4 patients),eczema (3 patients), dermatitis (2 patients), anaphylaxis(1 patient), acne (1 patient), parapsoriasis (1 patient),rash (1 patient), urticaria (1 patient). Of the 10 cases with severe suspected liver injury, the reasons were pruritus(3 patients), and anaphylaxis, acne, psoriasis, dermatitis,rash, eczema, urticaria of 1 case each. In conclusion,the major reasons for taking the capsule were pruritus,dermatitis, eczema, acne, constipation, and urticaria.
Adverse drug reaction
Clinical manifestations and involved systems:A total of 477 ADR events were involved in 377 case reports. The main clinical manifestations of the top 10 ADR were nausea, diarrhea, rash, dizziness, vomiting,abdominal pain, itching, gastrointestinal dysfunction,headache, palpitations and other symptoms, as shown in Table 2. 477 adverse drug reactions affected multiple systems and organs, mainly involving gastrointestinal system (257 times), sympathetic and parasympathetic nerve (121 times), skin and accessory structure (90 times), the whole body (65 times), central and peripheral nerve (61 times), hepatobiliary system (18 times), etc.The clinical manifestations of the 13 cases with severe ADR were liver dysfunction, hepatitis, hepatocellular damage, jaundice, abnormal renal function, belonging to the damage of hepatobiliary system. There were 16 events about liver injury in 14 suspected liver injury reports.The main clinical manifestations were liver dysfunction,hepatocellular damage, hepatitis, jaundice, abnormal renal function, involving hepatobiliary system and urinary system damage. There were 12 events about liver injury in the 10 reports of severe liver injury. And the clinical manifestations and systemic damage were consistent with all patients with suspected liver injury.
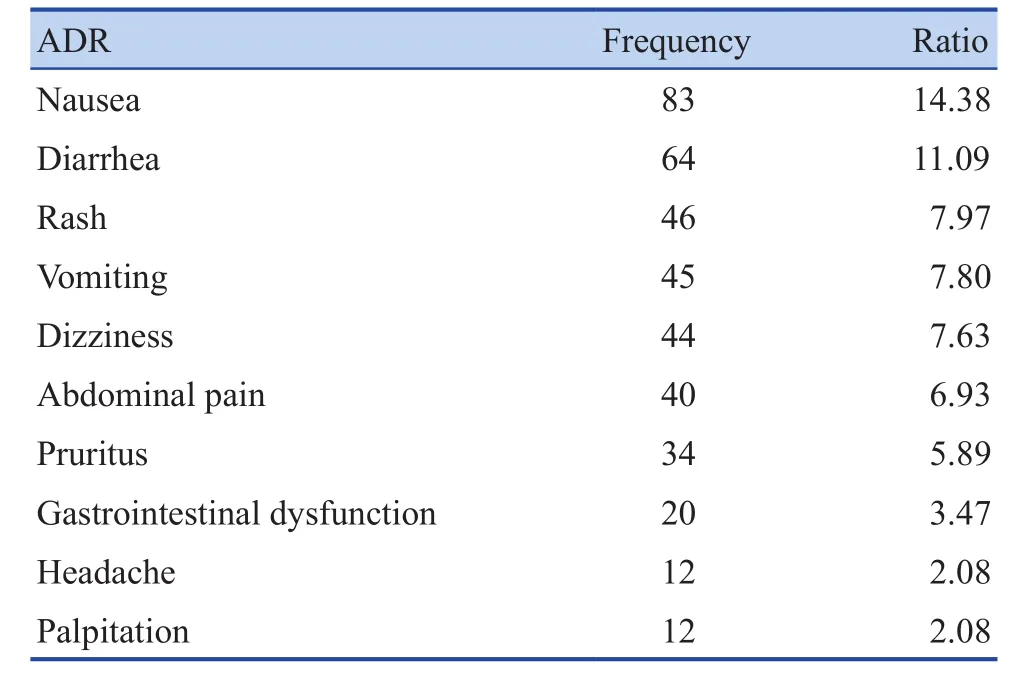
Table 2. Clinical manifestations of top 10 ADR
Impact on the original disease: of the 377 reports,the process of the disease of 16 patients was prolonged(4.24%), 9 patients' conditions were aggravated (2.39%),351 patients were in stable condition (93.10%), and 1 patient got sequela (0.27%). Of the 13 cases with severe ADR, the process of the disease of 4 patients was prolonged (30.77%), a patient got worse (7.69%), 7 patients were in stable condition (53.85%), and 1 patient got sequela (7.69%). Of 4 reports with liver injury, 10 patients were in stable condition (71.43%), the process of the disease of 3 patients was prolonged (21.43%), 1 patient got sequela (7.14%). In conclusion, ADR has no significant influence on the majority of patients. 1 patient got sequela because of the severe ADR. He was 12 years old and his weight was 36 kg. He was taking Runzao Zhiyang Capsule for dermatitis for 36 days, 4 capsules per time, 3 times a day. After 30 days, fatigue, abdominal distension and yellowing of the skin were observed.The liver function test showed that direct bilirubin was 139.7 Umol/L, alanine aminotransferase was 701 U/L,and aspartate aminotransferase was 641.3 U/L. He was treated at the hospital, where his condition was improved.The causes of ADR awaited further investigation, and the drug was not ruled out. This ADR was reported by a pharmacist. And the pharmacist and the hospital believed that the Runzao Zhiyang Capsule probably caused this event, because the patient had no previous medical history and family medical history and he felt better after suspending the capsule.
ADR/ADE outcome: for the general condition of processing and prognosis in 377 reports, 168 patients recovered (44.56%), 198 patients got better (52.52%),3 patients did not show any improvement (0.80%), 8 patients' information was unknown (2.12%). Of the 13 cases with severe ADR, 3 patients recovered (23.08%), 8 patients got better (61.54%), 1 patient did not show any improvement (7.69%), and 1 patient's information was unknown (7.69%). In conclusion, most patients who got ADR after taking the Runzao Zhiyang Capsule recovered or improved after suspending the capsule or receiving treatment.
Whether ADR/ADE stopped when the patient suspended the capsule or decreased the dose: of the 377 cases, 352 patients felt better when they suspended the capsule or decreased the dose (93.37%). 2 patients did not improve (0.53%). 8 patients did not suspended the capsule or decreased the dose (2.12%). 15 patients' information was unknown (3.98%). Of the 13 cases with severe ADR,10 patients felt better when they suspended the capsule or decreased the dose (76.92%). 1 patient did not improve(7.69%). 2 patients' information was unknown (15.38%).In conclusion, the majority of patients felt better when they suspended the capsule or decreased the dose.
Whether ADR appeared when the patient took the capsule again: of the 377 cases, 22 patients had ADR after they took the capsule again (5.84%). 15 patients did not have ADR although they took the capsule again (3.98%).301 patients suspended the capsule (79.84%). 39 patients'information was unknown (10.34%). Of the 13 cases with severe ADR, 1 patient had ADR again (7.69%), and 12 patients did not take the capsule again (92.31%). In conclusion, most patients did not take the capsule again.
Study on early warning signals of all ADR of Runzao Zhiyang Capsule
Since 2009, the PRR method and BCPNN method were used to caculate the early warning signals of top 10 ADR every quarter. PRR value1 or BCPNN value0 means the appearance of early warning signals. PRR results showed that the vomitting in the fourth quarter of 2010, the diarrhea in the third quarter of 2011 and the nausea in the fourth quarter of 2013 showed the early warning signals. BCPNN results showed that every quarter had early warning signals except the first quarter of 2011. For example, the nausea and dizzeness of the first quarter of 2009, the vomiting and palpitation of the second quarter of 2009, the rash, pruritus and headache of the second quarter of 2011 all had early warning signals. Other results were shown in the Figure 1.

Figure 1. Runzao Zhiyang Capsule's 10 kinds of ADR pharmacovigilance trend
SRS data has certain confounding factors (such as age, gender, etc.), which may have a certain impact on the outcome, containing false positive results, or missing the true signs. However, propensity (PS) can balance the baseline and combine multiple confounding covariates into one value for analysis, which can avoid the collinearity of the independent variables, and avoid over-matching at the same time, making results closer to the real situation. Therefore, the propensity was used for the correction of the data. The applicability and effect of the data would also be discussed in this study, that is, the obtained early warning signs would be verified. Gender,age, and weight were used as matching factors for further exploration of ADR. Then the data was processed by PRR and BCPNN and was corrected by propensity. It was found that the headache was not a warning sign after the application of PRR, while diarrhea, rash and stomach dysfunction still were. The top 10 ADR were still warning signs after the processing of BCPNN. In conclusion,diarrhea, rash and stomach dysfunction were still warning signs after the processing of PRR and BCPNN. Details were shown in Table 3.
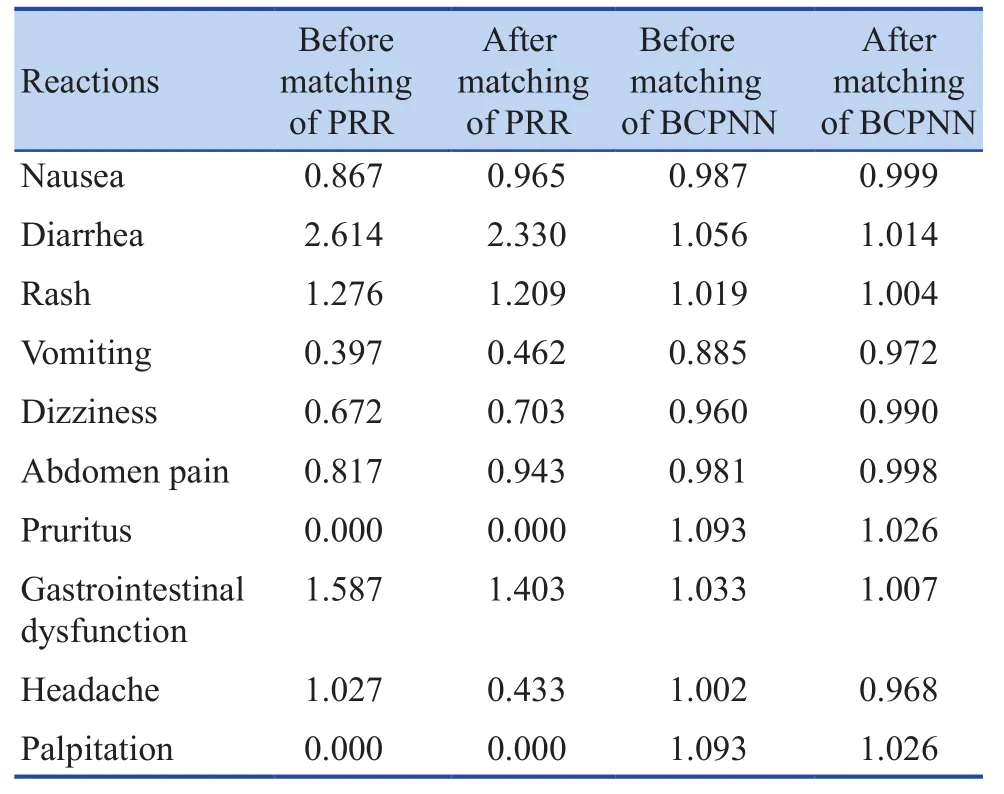
Table 3. Results of propensity
DISCUSSION
PRR method and BCPNN method are common data mining methods for early warning signs of ADR. PRR method is applicable to large-sample-size data, while BCPNN method can be applied to large-sample-size data,and can also be used for a sample of less than 3 ADR cases. What's more, some literature finds discrepancies between the two methods. The PRR method has higher sensitivity than BCPNN, while the BCPNN has high specificity than the PNN method. Therefore, theoretically more warning signs will be gotten when using the BCPNN method. However, this conclusion is in conflict with the findings of that literature. The reason is that in fact, the number of people of each symptom in per quarter is only 0-2 in account of small sample size, resulting in A + B=0 or C + D=0 in the formula when using PRR for calculation, so few early warning signs are obtained. In contrast, BCPNN method is applied to small-samplesize data, and more warning signs are obtained. There is no gold standard for monitoring ADR signals. And every method has its own advantages and limitations. For example, all data models cannot fully simulate the real world, because real-world contains a lot of confounding factors, and it is difficult to build simple models, so the appearance of fake signs are unavoidable. The propensity is used in this study for accuracy, though it cannot be 100%accurate. In addition, due to the lack of reporting and the absence of the denominator in the spontaneous reporting system, all data mining methods will produce certain deviations, causing false positive or false negative results.
After analysis of the existing 2009-2016 SRS data from the National Center for ADR Monitoring, it can be found that ADR of the capsule mainly include nausea,diarrhea, rash, dizziness, vomiting, which mainly are skin and accessory structure damage, systemic damage,gastrointestinal damage, respiratory damage. And after data mining, the results show that diarrhea, rash and gastrointestinal dysfunction may be the ADR warning signs of the drug, suggesting that the occurrence of those symptoms should arise our attention, especially for people who are more prone to ADR, such as children, the elder,the people with liver and kidney dysfunction. As a Chinese medicine preparation, Runzao Zhiyang Capsule contains Radix Polygoni Multiflori. Whether the adverse drug reaction is related to the Radix Polygoni Multiflori, there is no corresponding evidence at the current research stage to indicate the relationship between the two, but ADR of the capsule relates to liver damage. This still requires the attention of medical institutions and drug regulatory authorities at all levels. Enterprises should perfect drug facts in the future, warning the users to pay attention to the possible adverse reactions/events caused by the drugs. In this way, the ADR caused by disobeying the direction could be prevented. At present, due to the limitations of SRS itself,the incidence of ADR of Runzao Zhiyang Capsule cannot be calculated. Therefore, multi-center monitoring, large-sample monitoring, prospective hospital centralized monitoring can make up for this defect. They can record ADR and the relevant drug utilization situation in detail, and calculate the relative incidence of ADR, consequently the occurrence rules and risk factors can be known. At the same time,literature research, HIS analysis and other multi-faceted and multi-angle studies are also the next research step. Those methods can assure rational drug use and provide reliable data for post-marketing surveillance of TCM.
ACKNOWLEDGEMENTS
This work has been funded by National Science and Technology Major Project of New Important Drug Creation (2015ZX09501004-001-002); The National Natural Science Foundation of China (81473798): Study on allergic reaction mechanism of Chinese medicine injections with active monitoring and network target monitoring;Specialized Project of "One Belt, One Road" for Traditional Chinese Medicine of China Academy of Chinese Medical Sciences (GH2017-04); Construction of China-US Major Disease Clinical Research Center (GH2017-04-01).
猜你喜欢
杂志排行
World Journal of Integrated Traditional and Western Medicine的其它文章
- World Integrated Medicine Master Shen Ziyin
- Effects of Zhenren Yangzang Decoction Combined with Modified Shenling Baizhu Powder on Immune Function and Intestinal Microecology of Diarrhea Patients with Deficiency and Cold Syndrome
- Effects of Chaihu Guizhi Ganjiang Decoction Combined with Standard Triple Therapy on Reducing Serum CagA and Hp-NAP in Patients with H.pylori-Related Gastritis
- Clinical Efficacy of Modified Erchen Decoction on Cervical Spondylotic Vertebral Arteriopathy with Stagnation and Blockade of Phlegm-dampness Syndrome and Effects on Cerebral Blood Flow Parameters
- Effect of Shuxuening Injection Combined with Alprostadil on Healing of Diabetic Foot Ulcers and Hemodynamic Indexes of Dorsal Artery of Foot
- Clinical Analysis of Erzhi Mingmu Decoction in the Treatment of Non-exudative Age-related Macular Degeneration
