Enhanced Solubilization and Stability of Resveratrol in Cosmetic Applications
2018-10-22CaoPing
Cao Ping
R&D Center, Shanghai Jahwa United Co., Ltd., China
Abstract A newly designed microcapsule system to dramatically enhance solubility and stability of resveratrol was reported through solvent screening, surfactant and composition optimization etc. Resveratrol solubility increased to about 550 times higher than that in pure water, the solution is colorless and transparent, and HPLC results indicated that the resveratrol was stable under different temperatures (4 ºC, 25 ºC, 40 ºC, 48 ºC): within four weeks, higher than 95.0% of resveratrol was retained after the thermal tests. It was also confirmed that with the addition of the selected protective active, resveratrol retained about 90.0% after light radiation exposure for 24 hours. This microcapsule system may find high potential application for unstable and undissolved ingredients in cosmetic products.
Key words resveratrol; solubilizing system; optimization design; microcapsule system
Introduction
Resveratrol (3,5,4’-trihydroxystibene) is a bioactive polyphenol found in grapes and other plants. Many recent studies reported that resveratrol performs multi biological and pharmacological functionalities, which include but are not limited to anti-oxidation, anti-aging and antiinflammatory, etc. These multi functionalities give it a bright future in the cosmetic field.[1-4]
Although Resveratrol offers numerous benefits to many aspects of human health in, the low solubility (about 0.003 wt% at 25 ºC) in water, as well as in most oils, photoinduced instability and high reactivity with other chemicals limit its application, particularly in cosmetics.[5]To date,many efforts have been made to improve its performance by using liposome encapsulation or by cyclodextrin coating method. However, most approaches are still in the early stages of research.[6,7]There was no better approach to enhance its photo-induced instability in cosmetic application except using opaque packaging or adding UV filters in the formulation.
In this work, a new microcapsule system was developed by using the optimal combination of nonionic surfactants to enhance solubilization of resveratrol and protective actives to reduce its reactivity with other chemicals and degradation under UV exposure in the formulation. It was found that the solubility and stability of resveratrol was significantly improved.
Materials and experimental methods
Resveratrol was kindly provided by Symrise. All other components used were of analytical grade and used as received. Double-distilled water was used throughout.
Resveratrol solubility determination in different solvents
At 25 ºC, a certain concentration of resveratrol indifferent solvents were prepared, mixed sufficiently, and allowed to equilibrate in the dark for one week. An optical microscope was used to detect if any crystal precipitated out of solution to determine Resveratrol solubility.

Table 1. Resveratrol dissolved state in different solvents
Optimization design for resveratrol solubilizing experiment
Optimal experiments were designed to select the solvents and surfactant composition and analyze the effect of preparation parameters on the improvement of solubility and stability. First, the solubilizing solvent and resveratrol were mixed together, then certain surfactant where added with different concentrations in the system.The mixtures were magnetically stirred for 2 hours, and water was added to the system to suspend the micelles.The samples were stored in darkness at 25 ºC for further investigation.
The stability test for the solubilizing system
The sample was stored in the dark at different temperature (4 ºC, 25 ºC, 40 ºC and 48 ºC) for one month.The resveratrol concentration was measured by HPLC and the color of the samples was recorded by digital photo.
Protective agent selection to improve resveratrol light stability
A certain protective agent with different concentrations was added to the resveratrol solubilizing system and mixed well. The stability test under UV beams and at different temperatures (4 ºC, 25 ºC, 40 ºC and 48 ºC) were carried out to select the suitable protective agent to enhance resveratrol’s light stability. The resveratrol concentration was monitored by HPLC, and the color was recorded by digital photo and colorimeter.
Results and discussion
Resveratrol solubility determination in different solvents
Eight representative solvents were selected for study based on the physical-chemical properties of resveratrol and the results listed in Table 1. The outcome indicated that resveratrol has low solubility in polyols including glycerin and butylene glycol, and limited solubility in propylene glycol, propanediol and pentylene glycol. It has good solubility in the ingredients containing the PEG group,such as PEG-8 and PEG-12. More than 15% resveratrol can dissolve in PEG-8 and PEG-12 at room temperature as shown in Table 1.
From the resveratrol solubility experiment, alcohol and the ingredients containing PEG group are good solvents for resveratrol, and we speculate that the solubilizer-solvent interaction(s) through the hydrogen bond which the hydrogen atom of the OH group in resveratrol can associate with -O- groups in PEG groups, which could be the driving force to solubilize the resveratrol around the solvent structure as shown in Figure 1. In addition, PEG-8 and PEG-12 also were known as excellent moisturizers in cosmetic application,alcohol could be used as a coolant and solvent but it has the potential risk to make human skin sensitive or irritated.
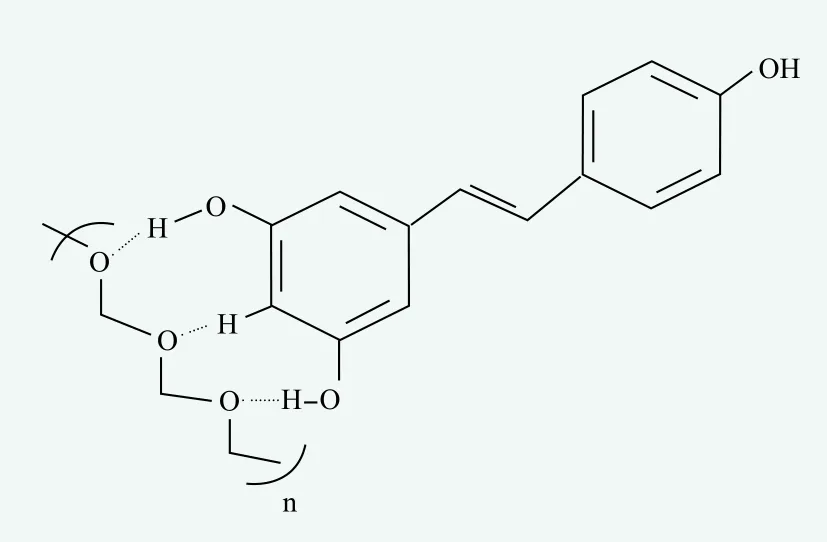
Figure 1. The mechanism of PEG group dissolving resveratrol
Optimization design for Resveratrol solubilizing experiment
Based on the knowledge obtained from the solubility experiments, at the 5.0% concentration of solubilizing agents, nonionic surfactants with polyglycerol ester,glycolipid, PEG group at the concentration of 1.0%were screened to build microcapsule systems to further enhance resveratrol solubility. The results in Table 2 show that the polyglycerol ester surfactant and glycolipid surfactant do not solubilize the resveratrol, no matter what the hydrophobic groups; however, the surfactant with the PEG group can solubilize resveratrol dramatically.
The solubilizing agent played an important role in resveratrol microcapsule systems. Although resveratrol can solubilize in PEG-40 Hydrogenated castor oil instantly, one week later the resveratrol crystalized from the sample. If polyols, such as glycerin and butylene glycol, worked as the solubilizing solvent, the resveratrol crystalized from the PEG-40 hydrogenated castor oil solution instantly. But if the solubilizing solvent with the PEG group was used, the resveratrol solubilized in the systems very well and the systems were stable for more than one week.
As known, the chain length of PEG groups decides the HLB value of surfactants (hydrogenated castor oil)directly. An increase in the chain length of the PEG portion of the surfactant generally results in increased solubilization capacity for resveratrol in the interior of the micelle in aqueous media. The appearance of the solutions for resveratrol in different chain lengths of PEG hydrogenated castor oil changes from opaque to transparent as shown in Figure 2. With PEG length increasing, resveratrol solubility shows a trend of rising first and then down listed in Table 3.

Figure 2. Photos for the Resveratrol/ Hydrogenated castor oil system with different PEG length from left to right, PEG-20, PEG-40, PEG-60, PEG-80 and PEG-100 respectively
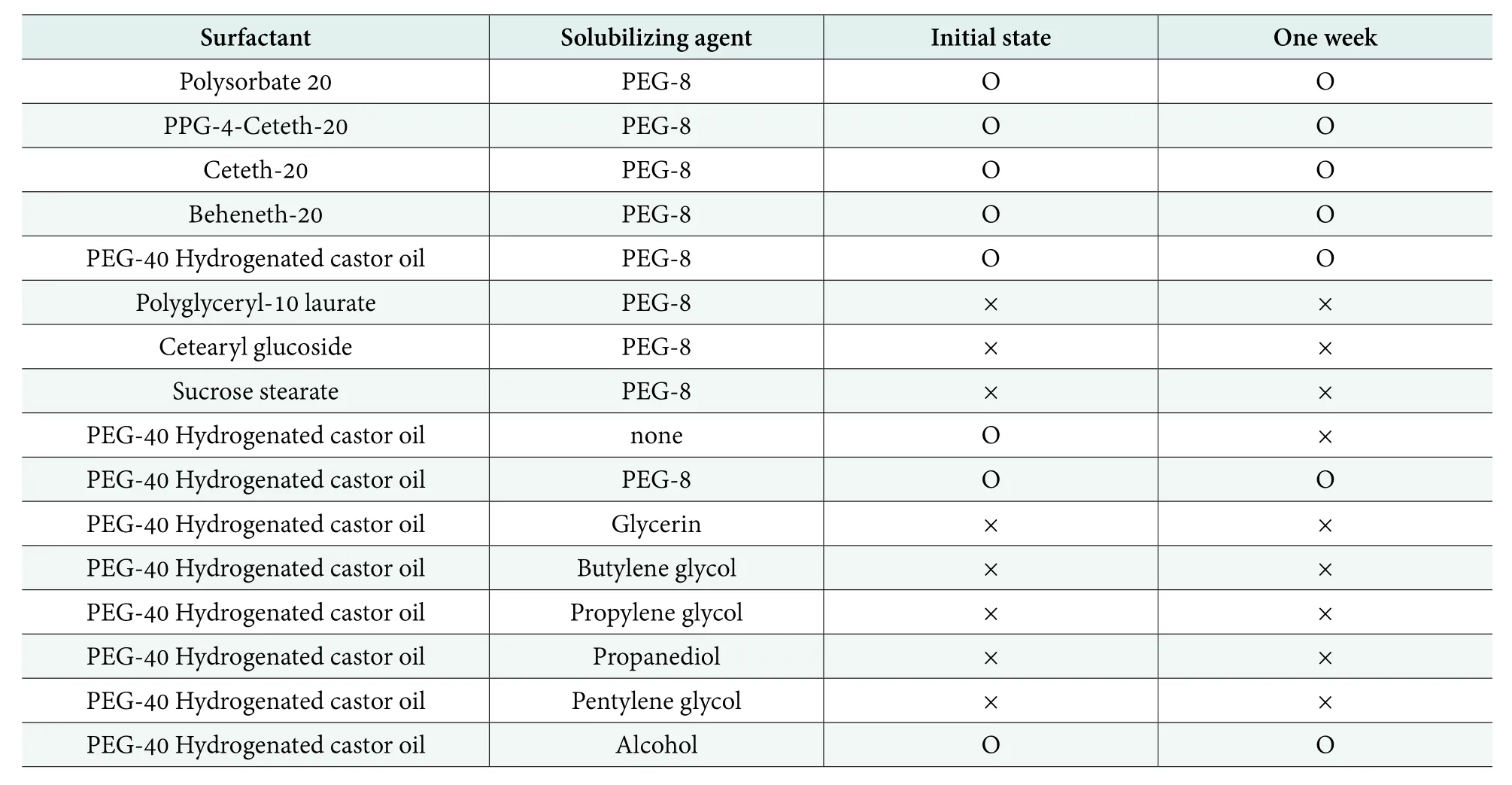
Table 2. Resveratrol solubilizing experiments with different surfactant and solubilizing agents

Table 3. Resveratrol solubilization with different chain length of PEG hydrogenated castor oil at the concentration of 1.0%
From the solubilization features and mechanism, the solubilizing system was optimized and works as below. When the surfactant containing PEG group worked as the solubilizing system, the PEG chain organized toward the outside of the micelles where resveratrol associated with the PEG chain and makes the solubilization possible. With PEG-8 or EPG-12 as the solubilizing solvent, they make the higher concentration of PEG chain on the surface of the micelles, so the difference of concentration can be driving force to solubilize the resveratrol at the sites around the micelle surface and anchor them tightly. When the PEG chain length is short, the HLB value of surfactant is low, which leads to a bigger micelle, so that the solubilization system was not transparent. When PEG chain became longer, the HLB value of the surfactant became higher, the smaller micelle can be formed to obtain transparent resveratrol solution. Thus the higher the PEG chain number,the higher the density of PEG for resveratrol dissolution.Once the PEG chain number is longer than 60, the resveratrol solubility decreased. The possible reason is that longer PEG chain made the micelle solid, the flexibility of micelle decreases,which make less space for resveratrol solubilization. The schematic of solubilization system is shown as Figure 3.

Figure 3. The mechanism schematic of the Resveratrol solubilization
The stability test for the solubilizing system
Figure 4 is the HPLC results that showed the resveratrol concentration under different stability conditions. The optimized solubilization system was very stable for resveratrol for 4 weeks, no matter whether stored at high temperature or low temperature. Formulated with this system, 1.70% stable resveratrol solution was achieved, the solubility of resveratrol increases over 550 times compared with that in water.
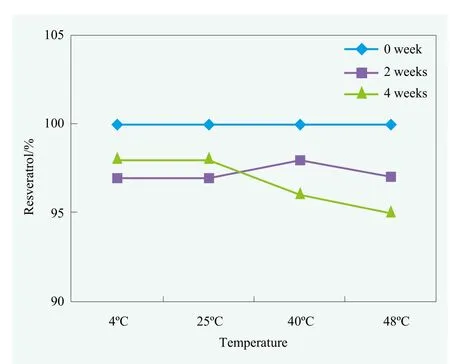
Figure 4. Resveratrol concentration in the stability test
The color of the resveratrol system didn’t change during the stability test, no matter at high temperature or low temperature, which showed that resveratrol was stable in this system as well (Figure 5). Testing results further supported the assumption that resveratrol was locked at the PEG group of the micelle tightly to achieve the target of solubilization and stability enhancement with the microcapsules system.

Figure 5. The appearance of microcapsules system after different temperature of stability test
Protective agent selection to improve resveratrol light stability
As for solubilizing system, the micelle aggregation structure didn’t prevent degradation by UV light. Four common water-soluble protective agents were tested to study their effects on the improvement of resveratrol light stability. Table 4 shows the results of resveratrol concentration change with and without a protective agent at 0.5%. The resveratrol concentration dramatically dropped to 40% after 24 hours UV exposure. With ascorbic acid and niacinamide as protective agents, the resveratrol concentration also dropped significantly, which meant that ascorbic acid and niacinamide didn’t protect the resveratrol from UV exposure very well. In sharp contrast, with grape extract or disodium phenyl dibenzimidazole tetrasulfonate(DPDT), the resveratrol concentration remained more than 90% after 24 hours UV exposure. These two ingredients improved the resveratrol photo stability significantly.

Table 4. Resveratrol content change with different protective agents after 24 hrs UV exposure
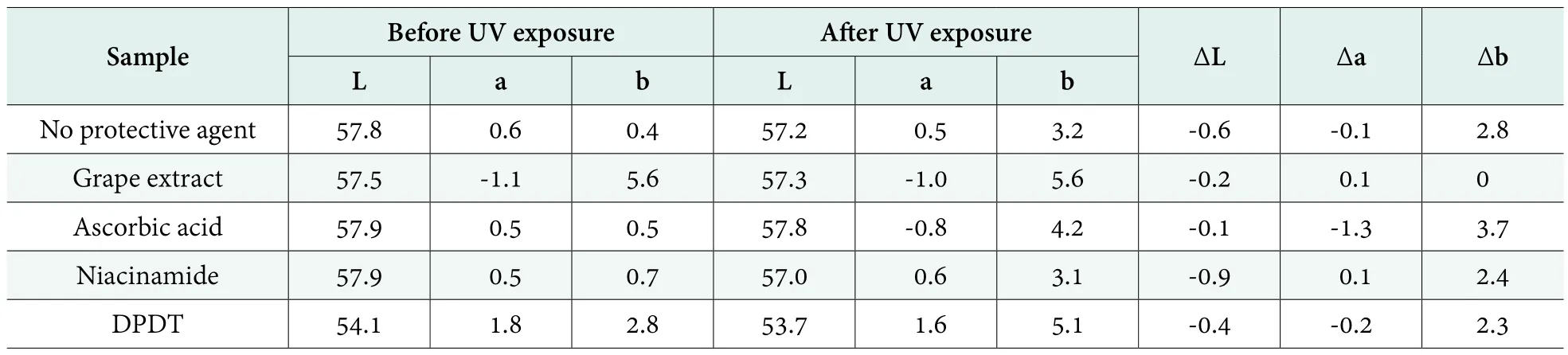
Table 5. The colorimetry changed with different protective agents before or after UV exposure
Furthermore, the colorimetry (L★a★b) data presented in Table 5, except for Jahwa patented technology, b value increased for all others as protective agents after UV exposure, which indicated the color of samples becomes yellow. Visible color changes of the samples before and after UV exposure showed in Figure 6, which proved the same color change direction.With DPDT, after UV exposure, the sample color changes to dark yellow eventually. Only if the system was protected by grape extract, the L,a,b value remained unchanged.
UV light can break down the structure of resveratrol significantly,[8]which made the sample change color. The DPDT is a well-known UV filter by absorbing 320 ~ 360 nm UV rays to protect the resveratrol photo stability. But the color of the sample still changes to dark yellow (Figure 6) which might be explained by the alkaline environment caused by adding DPDT accelerating the resveratrol degradation. Further studies will be conducted to prove the hypothesis, but right now, grape extract is a better choice to protect the resveratrol solubilizing system.

Figure 6. The color changed with different protective agents before and after UV exposure (1) No protective agent (2)Grape extract (3) Ascorbic acid (4) Niacinamide (5) DPDT
Conclusions
It can be concluded that by using the newly designed microcapsule the resveratrol solubility is dramatically increased to about 550 times higher than it is in pure water.This finding enables the resveratrol to deliver the required bio efficacy in cosmetic applications. Furthermore, the protective active has showed significant enhancement of resveratrol stability, which makes resveratrol applications more feasible.
杂志排行
China Detergent & Cosmetics的其它文章
- Major Industry Events
- Formula Development for Baby Sunscreen Products
- Simultaneous Determination of Six UV-Filters in Cosmetics by HPL C
- Clinical Study on the Anti-Aging Effect of A Cream Containing Ginseng Root Extract and L-Carnosine
- Application of the Quantitative Descriptive Analysis in Cosmetics Sensory Evaluation
- New process for the synthesis of a sun-screening agent isooctyl P-methoxy cinnamate
