The inhibitive study of egg shell powder on uns n08904 austenitic stainless steel corrosion in chloride solution
2018-10-18SanniPopoolaFayomi
O.Sanni,A.P.I.Popoola,O.S.I.Fayomi
Tshwane University of Technology,Department of Chemical,Metallurgical and Materials Engineering,Pretoria,South Africa
Keywords:Austenitic stainless steel Corrosion Inhibition Egg shell Chloride Langmuir
ABSTRACT The effect of egg shell powder(ES)as an environmental friendly inhibitor was studied for its corrosion inhibitive tendency on N08904 austenitic stainless steel in simulated saline(3.5%NaCl)solution using potentiodynamic polarization,weight loss,and SEM/EDX at room temperature.The experimental data explained the effective performance of ES with values of 57%-100%inhibition efficiency,at 2 g-10 g inhibitor concentration from weight loss tests due to the inhibition of stainless steel.The electrochemical action was as a result of the ionized particles which inhibit the compound influencing the redox reaction mechanism causing surface corrosion.ES's best performance was achieved when 6 g of the inhibitor concentration was added to the saline medium.Corrosion rate value decreased progressively with the presence of inhibitor because of anions adsorption at the interface of the metal film.Corrosion potential(Ecorr)value was found to decrease from-0.3991V to-0.3447 V in the presence of inhibitor at 2 g concentration,decreasing gradually to-0.2048 at 6g inhibitor concentration.The compounds identified in the ES completely adsorbed onto the surface of stainless steel as observed from the EDX analysis.The ES adsorption on stainless steel surface obeyed Langmuir adsorption isotherm.A corroded morphology with pits was observed in the SEM results without ES which contrast the images obtained with the presence of ES.
1.Introduction
Corrosion is degradation of material properties as a result of interactions with their environments,leading to huge amount of money lost yearly.One of the major causes of stainless steel corrosion is the presence of acids and chlorides which is a fundamental concern in academics and industries[1].Seawater environments are extensively used in industries such as gas production and offshore oil,shipping,coastal industrial plants mainly for cooling and power plants,desalination plants and oil field water injection.The presence of chloride anions is the main cause of corrosion in these environments.Corrosion cause damage in the marine industry due to exposure of vessels to chloride medium,corrosion cost has geometrically increased yearly with an approximately total cost of$50-80 billion worldwide[2].The electrochemical behaviour and corrosion resistance of stainless steel in this environment is of great interest because of its diverse application in different industry,this account for its being selected for use in this research.
UNS N08904 generally regarded as 904 L is a high alloy austenitic stainless steel with low carbon,used widely in situations where the corrosion property of stainless steel Type AISI 317 L and 316L provides undesirable results.Some of the major uses of stainless steel 904L includes;wiring in electrostatic precipitators,oil refinery components,gas scrubbing plants,seawater cooling devices,pulp and paper processing industries,sulphuric,phosphoric and acetic acid processing plants owing to their good corrosion resistance,excellent stability,high strength and weldability.Nevertheless,under critical environments corrosion of stainless steel 904 L is unavoidable.Its resistance to corrosion can be improved greatly with the use of corrosion prevention technique which can enhance its life span.One of the most reliable and cost proven way of preventing/minimizing corrosion is through the consistent application of corrosion inhibitors[3-10].Diverse studies have been carried out in order to understand stainless steel electrochemical characteristics in acidic,chloride and basic environments[1,6,8,9,14-17].There is a lot of research attention on the behaviour of different inhibitors on steel corrosion but there are limited researches on the use of ES as a corrosion inhibitor in research related to steel corrosion.Eggshell corresponds to 11%of the total weight of an egg and has been largely studied since 1964.Eggshell is mainly made up of 95%calcium carbonate,1%calcium phosphate and other organic matters[19,20].Egg shell is regarded as a non edible product with very limited use,mostly disposed as a waste.Due to high disposal costs and increasing environmental concerns[18],it is necessary to find a way of transforming the waste eggshells into a valuable one leading to low disposal costs.Egg shell has been previously studied as an inhibitor for Type 316 stainless steel corrosion in H2SO4solution by our research group[6].We have found that this compound is efficient inhibitor in 0.5 MH2SO4for Type 316 stainless steel and the corrosion inhibition is mainly controlled by a physisorption process.The aim of this research is to study the inhibitive tendency of ES on stainless steel UNS N08904 in 3.5%NaCl environment through weight loss and potentiodynamic polarization tests.The chemistry and structure of ES is anticipated to offer a significant amount of electrochemical actions for stainless steel corrosion inhibition in the chloride medium studied.It is expected that the outcome from this study will be of economic and technological benefits.
2.Experimental procedure
2.1.Material
Austenitic stainless steel(Type 904L)sourced commercially with elemental composition shown in Table 1 was used for this investigation.The steel was made into electrodes measuring 2mm×2 mm×1mm.The stainless steel samples after machining were abraded with different grits of silicon carbide papers(80-1000 grits),followed by polishing with 6 to 1μm diamond paste.The steel samples were later washed with de-ionized water,rinsed with acetone,dried and stored in desiccators for potentiodynamic polarization and weight loss tests according to[11].
2.2.Methods
2.2.1.Inhibitor formulation
Commercially available chicken eggshell powder was used as inhibitor in this study due to its high calcium content and the presence of other elements.Its structural formula is given in Fig.1.Fig.2 illustrates the EDX spectra of the egg shell powder(ES)with the chemical properties of the inhibiting compound.The choice of this inhibitor is based on molecular structure considerations,that is;the type of substituent's and number of active centers present in the compound.The concentrations of ES used in the test medium are 2,4,6,8 and 10 g.
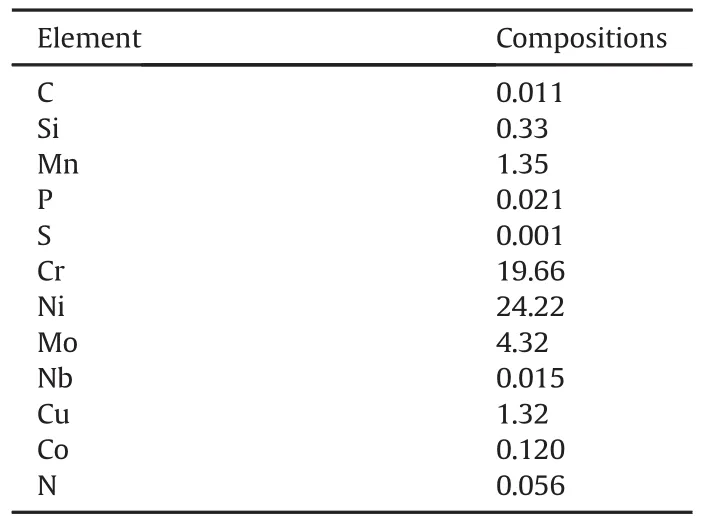
Table 1 Stainless steel Type 904 chemical composition(%wt).
2.2.1.1.Test medium.Sodium chloride of 3.5%concentration was used as the corrosive medium in this study.
2.2.2.Corrosion measurements
2.2.2.1.Weight loss test(168 h).This technique involves exposure of sample material to a process medium for a particular period of time,taking the weight of the sample at a time interval.Triplicate experiments at room temperature were performed by separately immersing pre-weighed samples in 250 ml of 3.5%NaCl beakers.After each immersion time,the specimens were washed in running distilled water at the same time scrubbing the steel surface with a soft brush.After drying in air,the electrodes were reweighed.The test samples were taken out every 24h washed with de-ionized water,rinsed with acetone,dried and weigh again.Plots of inhibition efficiency(%),degree of surface coverage(ɵ)corrosion rate(mm/year)and weight loss(mg)against time of exposure(hours)were calculated from the recorded readings.
2.2.2.2.Potentiodynamic polarization tests.Stainless steel(UNS N08904)samples embedded in resin with an unconcealed surface area of 1 cm2were prepared following[12].Potentiodynamic polarization plots of stainless steel specimens in the test medium with different concentrations of(ES)were measured with Potentiostat/Galvanostat(model Reference-668)by NOVA software,1.8 Version.The linear potentiodynamic polarization methods were carried out on the prepared samples immersed in sodium chloride medium with and without diverse concentrations of inhibitor.Three electrode systems were used for this method.The Type 904 stainless steel plate embedded in resin was used as working electrode.Platinum rod as counter electrode and Ag/AgCl(silver chloride electrode)as reference electrode,this was connected to the cell with the aid of Luggin capillary according to[12].LSV staircase parameter;step potential(0.001 m/s),stop potential(+1.5 v)and start potential(-1.5 v)was used according to[13].The test solution was maintained at a constant temperature by stirring with a magnetic stirring bar.Prior to the test,the specimens were polished,degreased in acetone and rinsed carefully.Potential applied against current density was plotted.Corrosion current was obtained by extrapolating the corrosion potential to the linear part.In cathodic and anodic curves,the linear slope part gives Tafel cathodic and anodic constants(bc and ba)respectively.
2.2.2.3.Scanning electron microscopy and energy dispersive X-ray spectroscopy(SEM/EDX).The SEM and EDX images of 904 L stainless steel samples with the presence and absence of ES were carried out after weight loss test.
3.Results and discussion
Weight loss and potentiodynamic polarization techniques were used to study the corrosion inhibition of stainless steel in 0.5 MH2SO4solution in the absence and presence of ES.
3.1.Weight loss technique
The results obtained from weight loss test for the samples dipped in 3.5%sodium chloride solution with the presence and absence of ES with different concentrations were shown in Fig.3.The test samples without ES demonstrated highest weight loss values throughout the experiment.The weight loss value was found to decrease with the presence of egg shell concentration which ranges from 2,4,6,8 and 10g.Lowest value of corrosion rate was obtained with 6 g of ES concentration in 250mL-3.5%NaCl.In general,the ES could be concluded as an effective inhibitor for stainless steel corrosion in the 3.5%sodium chloride medium.Fig.4 explains the reduction in corrosion rate values with time of exposure for the specimens tested at studied concentrations.The plot further indicates that the specimen with 6g concentration of ES had the highest inhibition efficiency and lowest corrosion rate values.In addition passivation on the electrode surface could be as a result of corrosion deposit from the corroded electrode.Shownin Figs.5 and 6 are degree of surface coverage and inhibition efficiency(IE)graphs against time of exposure respectively.The results from Fig.6(IE)show that after 24h of exposure,6 g of egg shell revealed highest inhibition efficiency of 100%.Similar trends were observed after 48h of exposure time in the sodium chloride solution for 8g and 10 g of ES.92.45%inhibitive performance was recorded at the end of the experiment in the presence of 6g concentration of egg shell powder.These results imply that adsorption took place first but slows down with time as a result of decrease in concentration of ES.Moreover,it is important to point out that the redox process of the ES studied was affected,thus influencing the stainless steel surface cathodic regions by physiochemical interaction between the chloride solutions and the molecules of the inhibitor.
3.2.Potentiodynamic polarization technique
Cathodic and anodic potential polarized was measured in the presence and absence of ES.Electrochemical values and parameters such as cathodic Tafel constant(bc),corrosion potential(Ecorr),anodic Tafel constant(ba),corrosion current(icorr),polarization resistance(PR)and corrosion rate(mm/year)are shown in Table 2.Fig.7 explains the cathodic and anodic linear polarization curves for ES in 3.5%sodium chloride medium at various inhibitor concentrations.The generated plots for stainless steel at various ES concentrations in the studied medium are shown in Fig.7.As observed from Table 2 and 6g ES concentration in 250 mL-3.5%sodium chloride had minimum current density value of 2.38E-05(A/cm2)exhibiting best inhibitive characteristics.In addition,it shows the highest polarization resistance and lowest corrosion rate values revealing it to be the most effective concentration.Without ES,maximum corrosion magnitudes were observed as shown from the overall potentiodynamic polarization result.The potentiodynamic polarization technique result showed that ES alters drastically the electrochemical process initiating corrosion.Furthermore,the inhibitive behaviour of ES is related to its formation and adsorption of compact barrier film on the metal electrode surface.This was further shown by the corrosion potential values of the samples with the ES when compared to values of corrosion potential samples without the presence of ES.It was obvious from Fig.7 that cathodic and anodic plots were both polarized.The inhibitor molecules adsorption on the stainless steel surface blocks the active sites thus slows down the corrosion rate.The obtained results from weight loss and polarization techniques were in good agreement(see Fig.8).
3.3.Adsorption studies
Molecular adsorption can be used to further explain inhibition of corrosion.The surface charge and nature of metal,organic compounds chemical structure,type of aggressive media and molecule charge distribution are identified factors influencing process of adsorption.This was carried out by setting the obtained experimental data to different adsorption isotherms.ES consists of,oxygen(O)and nitrogen(N)which are known to be heteroatom present in the ES structure.These elements are envisaged to have notable inhibitory effect aiding its adsorption on the surface of the metal.From this research,ES can be regarded as cheap and environmental acceptable compound having a significant inhibition tendency for stainless steel in 3.5%sodium chloride medium.The investigated result as shown in Table 2 from the Tafel slope parameter also shows that ES affects both the cathodic and anodic reactions.Data obtained for(θ)degree of surface coverage were tested with diverse adsorption isotherm equations which include Freundlich,Temkin,El-Awady,Langmuir,Frumkin,Florry-Huggins and Bockris-Swinkel.The obtained results show that the isotherm that best describes the stainless steel surface adsorption characteristics of ES is Langmuir adsorption.
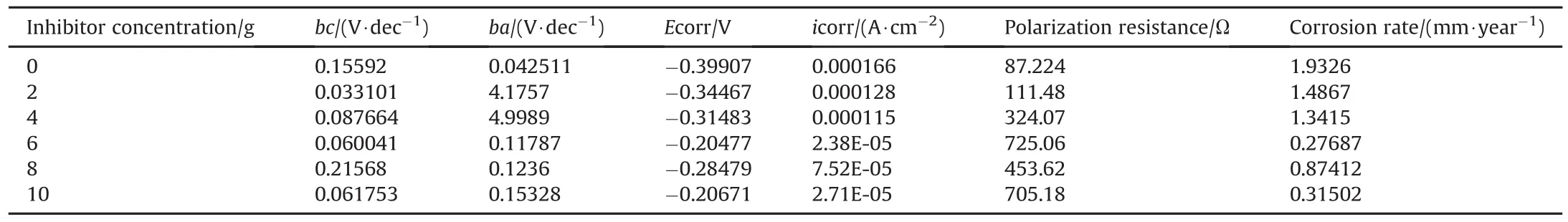
Table 2 Potentiodynamic polarization parameter for stainless steel with the presence and absence of ES in 3.5%NaCl medium.
3.4.Scanning electron microscope and energy dispersive X ray spectroscopy(SEM/EDS)
Figs.9 and 10 shows the SEM/EDX surface morphology analysis of stainless steel in chloride medium with the presence and absence of ES.Fig.9(a)and(b)is the SEM images of the stainless steel in chloride solution without and with inhibitor,Fig.10(a)and(b)is the EDX of stainless steel specimen after weight loss experiment in sodium chloride medium with and without inhibitor,a surface corroded sternly was noticed in this sample after immersion without the presence of ES due to corrosive damage of the sodium chloride.The stainless steel surface corrosion product layer in the absence of inhibitor was porous and as a result gives no corrosion protection.With the presence of ES,corrosion damage was minimized,with an evidence of ES present on the metal surface as shown in Fig.10(b)
4.Conclusions
The result presented in this manuscript supports the possibilities of using eco-friendly waste products as new type of corrosion inhibitors for stainless steel in saline medium based on the following deductions:
(1)Egg shell is an efficient and excellent inhibitor for stainless steel Type 904 in 3.5%sodium chloride solution for 168 h with inhibition efficiency of100% at6g egg shell concentration.
(2)Inhibition by ES increases with increased inhibitor concentration and decreased time of exposure,which indicates that physical adsorption was the predominant inhibition mechanism because the quantity of adsorbed inhibitor decreases with increasing exposure time.
(3)The adsorption of ES on stainless steel 904 L surface in 3.5%NaCl medium fits the Langmuir adsorption isotherm.
(4)The obtained results from linear potentiodynamic polarization and weight loss techniques are in good agreement.
杂志排行
Defence Technology的其它文章
- Overview of Al-based nanoenergetic ingredients for solid rocket propulsion
- Implications of fine water mist environment on the post-detonation processes of a PE4 explosive charge in a semi-confined blast chamber
- Systematic research on the performance of self-designed microwave plasma reactor for CVD high quality diamond
- cis-1,3,4,6-Tetranitrooctahydroimidazo-[4,5-d]imidazole(BCHMX)as a part of explosive mixtures
- Joining and machining of(ZrB2-SiC)and(Cf-SiC)based composites
- Structural evolution,optoelectrical and corrosion properties of electrodeposited WO3integration on Zn-TiO2electrolyte for defence super application
