Electrolytic deposition of super-smart composite coating of Zn-V2O5-NbO2on low carbon steel for defence application
2018-10-18FyomiKnynePopoolOyedepo
O.S.I.Fyomi,L.R.Knyne,A.P.I.Popool,S.O.Oyedepo
aDepartment of Chemical,Metallurgical and Materials Engineering,Tshwane University of Technology,P.M.B.X680,Pretoria,South Africa
bDepartment of Mechanical Engineering,Covenant University,P.M.B 1023,Ota,Nigeria
Keywords:Electrodeposition Low carbon steel Micro-hardness Thermal stability corrosion behavior Rare earth metal(REM)
ABSTRACT Despite the massive usages of low carbon steel in automobile for engineering components,its corrosion and high friction coefficient in aggressive environment make it limited in service.This paper is aimed at modifying low carbon steel structural component with thin film composite for enhanced mechanical and corrosion properties.The steel structure was electrodeposited with Zn-V2O5and embedded with varied NbO2weight concentration of 6-12 wt%based electrolyte.Scanning electron microscope(SEM)and high optical microscope was used to study the microstructural evolution of the fabricated coatings.The thermal stability of the fabricated coatings was studied in an isothermal furnace at 300oC and 600oC and further characterized using a high tech optical microscope.Potentiodynamic polarization technique was used to investigate the corrosion behavior of the composites in 3.65%NaCl.From the result,the effect of NbO2on Zn-V2O5-NbO2was massive with improved crystal grain within the coatings lattices.The coating possesses strong metallurgical bonding and good corrosion resistance properties of about 0.315 mm/yr corrosion rate compare to 4.1mm/yr of as-received sample.No doubt the impact of thermal shock on the resilient characteristics of the composite coating was moderate owing to the stable adherent properties of the deposited coatings.
1.Introduction
The demand and applications of low carbon steel for different purposes especially in marine,construction and defence environment are mainly because of their excellent with resilient characteristic[1-3].These properties such as malleability,ductility and welder-ability provide good mechanical properties even for extended application.However,the impact of corrosion and thermal shock on steel mostly affects the surface phenomenon which limits their applications in service leading to breakdown[4,5].
Zinc and zinc based are considered as effective methods employed for the corrosion protection of low carbon steel and resilient mitigation of surface against mechanical deformation[6-8].In service,zinc deteriorates at steady chloride and acidified medium thereby devaluing its potential for stable protection[9-12].The need for enhancement of existing zinc deposition becomes necessary.Efforts on alternative materials have been carefully investigated by different researcher among which are Zn-SiO2,Zn-Al2O3,Zn-TiO2,Zn-Ni,Zn-Co alloy to mention but a few[13-16].This composite embedded zinc coating had been found to provide good anti-corrosion properties when used as coating material on low carbon steel[17].
Niobium is a rare earth metal(REM),and are known to posses is refractory characteristics.It is reported that Niobium forms a super ficial oxide film and therefore its in-corporation into a composite coating for electrolytic deposition has been attested a potential improve corrosion[18].Vanadium is used for high resistance dental implant.Vanadium oxides are capable of stabilizing a metal surface to prevent further oxidation when they form part of the coating[19-23].
Among many work done by different authors to improve the mechanical and corrosion properties of mild steel,there is no work open literature on Zn-V2O5-NbO2fabrication on mild steel via electrode position technique.Therefore,in this work,the microstructure evolution,mechanical properties and corrosion resistance properties of Zn-V2O5become necessary in the presence of NbO2rare earth metal.
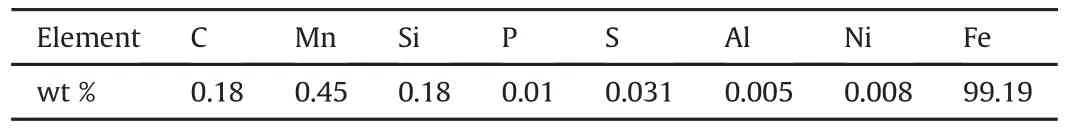
Table 1 Chemical composition of low carbon steel sample.
2.Experimental procedures
2.1.Sample preparation and bath formulation
Mild steel sheets(40mm×40 mm×2mm)were selected as the substrate(cathode)for the experiment and the chemical composition of the mild steel is presented in Table 1.Substrate preparation involved mechanical polishing using grit SiC paper from a grit size of 40μm-1200μm.The anode was pure 99.99%commercial zinc with dimension of(60 mm×60 mm×5 mm).
The electrolytic bath and activating solution were prepared 24h prior the experiment proper.The deposition was prepared with sulphate based constituent with activating solution of 0.5 MH2SO4.All deposition were done for 15s with pH of 4 as described in Table 2.The chemicals used are Analar grade which were prepared using de-ionised water.The coating designed structure is presented in Table 3.
2.2.Morphological characterization
The structural evolution of the electrodeposited samples was characterized on Optical Microscope(OM)and Joel JSM6510 Scanning Electron microscopy(SEM).
2.3.Linear polarization resistance
The polarization resistance and corrosion rate measurements were taken using aμAutolab Type III Pontentiostat/Galvanostat.The polarization measurements were from a potential of-1.5 V to 1.5 V with scanning rate of 0.01V/s.The circuit consisted of a saturated calomel electrode as a reference,graphite as a counter electrode and the coated samples as the working electrode.
2.4.Microhardness properties
The microhardness of the fabricated coatings layers were evaluated by means of micro vickers hardness tester under diamond based indentation loads of 100 gf.The indentation time was 10s with four separate indentations taken at different measurement location within the surface interface.
3.Results and discussion
3.1.Morphological study
The surface micrographs of Zn-20V2O5and Zn-20V2O5-12NbO2matrix were characterized by SEM to obtain the structuralevolution of the developed alloy.Fig.1 clearly shows that the Zn-20V2O5coating surface evolution presented has small grains size with non-homogeneous and crystals at the interface.Although[11,12]attested that zinc coatings often possess inherent pores and cracks along the crystal buildup but this is invariably different from result obtained.The incorporation V2O5particles on Zn electrolyte improved the surface morphology of Zn based composite coatings.Moreover,Fig.2 represent SEM image of Zn-V2O5with the addition of NbO2at different weight percent.Within the surface matrix of all embedded NbO2coating was an improved crystal evolution with compact grains noticed.The surface modification of the coatings can be credited to the incorporation of NbO2particulate which occupied up the gaps and the micro-holes of the coating lattice.Malatji et al.[11]stated that,the incorporation of nano particles in a coating stimulates increase in number of nucleation sites and crystal growth bringing about small-sized grains.According to[15],the impact of the rare earth metal(REM)inform of multigrain always result into a cohensive hexagonal crytal which is in par with a noticeable observation in this study as presented in Fig.2(a)and(b).Although Fig.2(b)gave a vivid coating with a superb uniform array of deposit compare to coating develop 6NbO2induced.
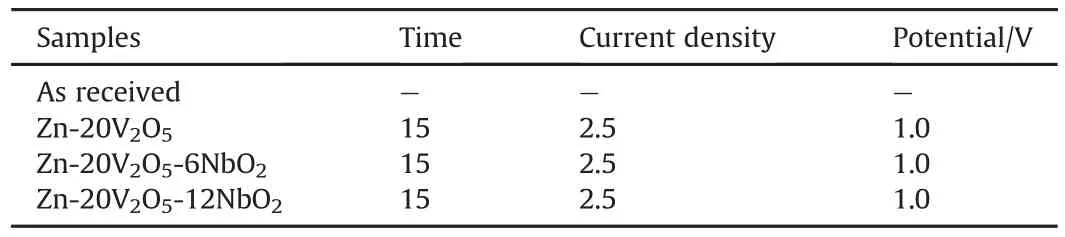
Table 3 Deposition pattern of Zn-V2O5/NbO2alloy of fabricated samples.
3.2.Microhardness examination
The hardness values of the substrate and zinc composite fabricated coatings are presented in Fig.3.Improvement in microhardness can be noticed on all the composite coating samples compared to the control sample.An addition in NbO2nano-particle concentration in the bath solution on Zn-V2O5developed electrolyte resulted in enhancement of hardness potential.Although study by Ref.[13]mentioned that,metal matrix composite particles can lead to development of new microstructure and refinement in grain structure;hence causes maximum improvement in microhardness.This noticeable result is in par with the result obtained by Zn-V2O5-NbO2alloy developed.The presence of NbO2could be seen to contribute forcefully to the surface hard tendency of the substrate thereby resulting into appreciable hardness properties.No doubt Zn-V2O5displayed an appreciable coating performance in all regards but apparently not as ternary coating system with significant effect of about 190HVN.Surprisingly an increase in REM does not affect the microhardness performance of the coated samples positively,rather at 12 wt%,the microhardness properties of the fabricated samples decreased which is expected.Although[17]stated that multidoped coating often result into stable crystal refiner and not necessary on performance characteristics.
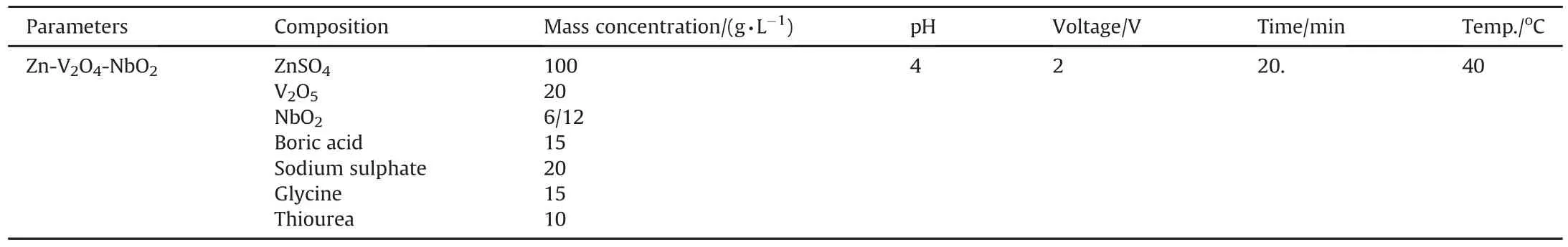
Table 2 Bath Composition of Zn-V2O4-NbO2and operating condition.
More so in Fig.3,examination of the thermal stability of the deposited coating were considered after an exposure to annealing process for 300oC and 600oC respectively for 3 h nanocomposite via electrodeposits have been evaluated by following microhardness changes of the co-deposited samples as revealed by Fig.4.The coatings were exposed to heat treatment at temperature of 300oC and 600oC respectively for 3h.All coating maintain stability with high homogeneity which lead to slight increase in hardness tendency except for Zn-V2O5at 300oC.
Figs.4 and 5 shows the optical microstructures of samples after annealing at 300oC and 600oC respectively.It was seen that the microstructure appearance at 300oC is finer and uniform with no evident of cracks at the interface.At 600oC,stress initiation was noticed but not excessive.Although,an increased wt%of NbO2(12 wt%),Zn-V2O5-6NbO2there is no cracks observed at both temperatures.This is invariably indicated stable crystal growth and resilience to repeated thermal shock[18].In general,there is a little increase in micro-hardness properties as compare to the samples without heat-treatment.
3.3.Linear polarization resistance
Figs.6 and 7 display the polarization resistance(Ω)and corrosion rate(mm/year)respectively.The performance of the fabricated coatings on mild steel in the 3.65%NaCl solution was examined.The results achieved from Fig.3 indicate that an increase in addition of NbO2elevates the polarization resistance of the Zn-20V2O5composite samples toward more positive region.Low carbon steel samples(uncoated sample)possess lowest polarization resistance of 27.6Ω.Moreover,the highest polarization(Rp)of 215.9Ω is attained at for Zn-V2O5-12NbO2,followed by Zn-20V2O5-6NbO2and Zn-20V2O5respectively.Material corrosion can be correlated to important factors such as chemical composition of the phase formed and grain size of the microstructural evolution.It is obvious that the maximum polarization resistance associated with 12wt%NbO2is due to the fact that NbO2is more resistance to corrosion[20].As expected,the substrate sample(uncoated mild steel)shows highest corrosion rate of 4.1mm/year as compared to all fabricated samples.The addition of NbO2at varying content shows a greater impact on the corrosion rate of the matrix.At a point where NbO2is 12wt%,the corrosion rate of the sample was lowest(0.315 mm/year).
The micrographs of the deposits after corrosion are shown in Fig.8.The most distinct difference between the initial coatings is the pitting effect.The presence of pits across in Zn-V2O5shows that there is a tendency of erosion propagation over time.Fig.8(b)also shows no significant corrosion product at the interface as expected with Zn-20V2O5-12NbO2.The better resilient corrosion propagation of Zn-20V2O5-12NbO2coating presented is attributed to good adhesion and compact grains for the deposited coatings[24].
4.Conclusions
(1)Zn-V2O5/NbO2was successfully electrode posited on low carbon steel.
(2)NbO2proved to be good grain refiner and potential for usage for extended application
(3)Fabricated composite coating matrix of Zn-V2O5-12NbO2revealed improved corrosion resistance as compared to the substrate
(4)Electrode posited composite coatings resulted in increased microhardness properties of over 190 HVN.
Acknowledgements
The authors gratefully acknowledge Surface Engineering Research Centre(SERC),the Tshwane University of Technology,Department of Chemical Metallurgical and Materials Engineering,Pretoria,South Africa.
杂志排行
Defence Technology的其它文章
- Overview of Al-based nanoenergetic ingredients for solid rocket propulsion
- Implications of fine water mist environment on the post-detonation processes of a PE4 explosive charge in a semi-confined blast chamber
- Systematic research on the performance of self-designed microwave plasma reactor for CVD high quality diamond
- cis-1,3,4,6-Tetranitrooctahydroimidazo-[4,5-d]imidazole(BCHMX)as a part of explosive mixtures
- Joining and machining of(ZrB2-SiC)and(Cf-SiC)based composites
- Structural evolution,optoelectrical and corrosion properties of electrodeposited WO3integration on Zn-TiO2electrolyte for defence super application
