重金属污染农田安全利用:进展与展望*
2018-10-11杨树深孙衍芹李小方
杨树深, 孙衍芹, 郑 鑫, 李小方**
重金属污染农田安全利用:进展与展望*
杨树深1,2†, 孙衍芹3†, 郑 鑫1, 李小方1**
(1. 中国科学院遗传与发育生物学研究所农业资源研究中心/中国科学院农业水资源重点实验室 石家庄 050022; 2. 中国科学院大学 北京 100049; 3. 河北地质职工大学 石家庄 050081)
我国耕地土壤污染面积广, 污染情况复杂, 农产品重金属超标问题已经关系到国计民生。常用的物理化学修复方法成本高, 不适用于大面积的中低污染农田。植物提取修复方法成本低, 环境友好, 但修复时间长, 推广困难。总的来讲, 基于重金属移除的诸技术在解决农田重金属污染方面还没有太大优势。相较而言, 农田安全利用在不移除或缓慢移除土壤重金属的条件下, 以生产安全农产品为目标, 具有更加坚实的现实意义和推广价值。种植低吸收农作物是安全利用的重要措施, 基因工程手段在低吸收农作物品种筛选中具有巨大的潜力, 但其可能带来的生态环境风险使得这些通过基因工程得到的低吸收作物的田间种植面临着巨大挑战。土壤添加剂可以改变土壤重金属形态, 降低重金属的生物有效性, 但会对土壤质量产生影响。微生物尤其是土著微生物的利用越发受到关注, 改变微生物的生存环境与基因工程手段能够强化微生物的钝化效果。施肥、水分管理、间作等农艺措施也能改变土壤重金属的形态, 抑制作物对重金属的吸收。未来以加强推广为目的, 多种技术手段的联合应用是重金属污染农田安全利用的重要发展方向, 其中以生物技术为核心的利用模式具有十分重要的意义。
重金属污染农田; 安全利用; 农作物; 添加剂; 微生物; 农艺措施; 重金属钝化
我国耕地资源十分紧缺, 2016年底耕地面积为1.35亿hm2(20.24亿亩)[1], 人均占有量不及世界平均水平的1/2, 且总体质量不高, 中低产田达到了2/3[2]。此外, 由于建设占用、灾毁、生态退耕、农业结构调整等原因, 我国耕地面积保有量近年总体有所下降。与此同时, 土壤污染加剧了耕地资源的紧张。据2014年发布的《全国土壤污染调查公报》, 我国农田土壤受到了广泛的重金属污染, 其中轻微、轻度、中度和重度污染点位比例分别为11.2%、2.3%、1.5%和1.1%[3]。重金属污染不仅减少了可利用耕地面积, 而且降低了耕地质量。另有研究发现, 我国部分地区已经出现农产品重金属含量超标现象, 威胁到了人体健康[4-5]。由此可见, 我国重金属污染耕地土壤修复刻不容缓。
土壤重金属污染修复分为两种技术路线, 一是将重金属从土壤中提取出来, 减少其在土壤中的含量, 称为提取修复; 二是降低土壤重金属的生物有效性, 抑制其进入生物链, 称为固化修复。提取修复方法主要包括物理修复、化学修复与生物修复(主要为植物提取修复)[6]。
物理、化学修复方法(表1)能够有效地去除土壤中的重金属, 且修复时间短, 但由于其对修复设备与技术的要求高, 修复成本高, 且会对土壤质量产生影响, 故其主要适用于污染严重、面积小的场地污染。

表1 重金属污染土壤主要物理、化学修复方法的原理和不足
植物提取利用超积累植物或累积能力较强的植物吸收富集污染土壤中的重金属并在地上部积累, 收割植物地上部分从而达到去除重金属的目的, 具有环境友好、修复成本低的特点。但其修复周期长(表2)。用于修复的植物往往没有经济产出, 在没有政府补贴的情况下, 农民的参与度不高, 不利于推广应用。
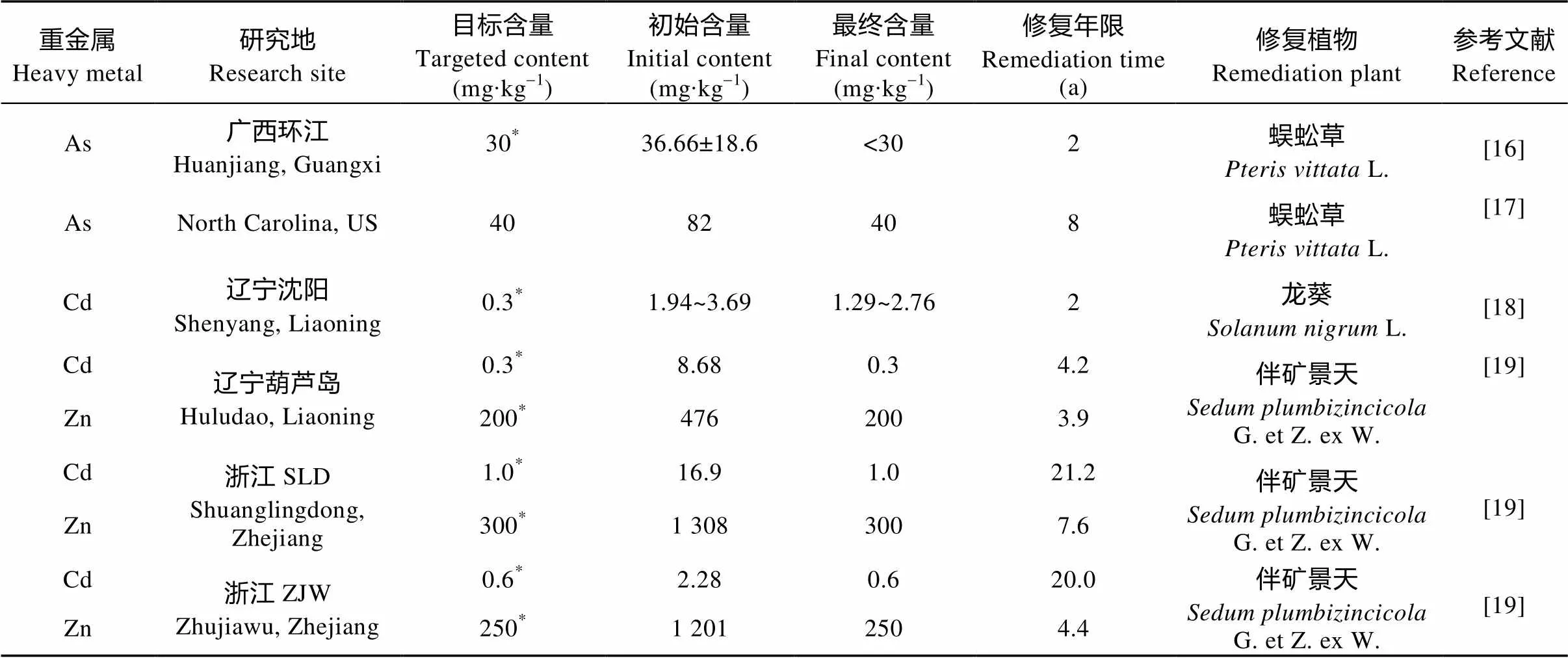
表2 部分植物提取修复重金属污染土壤所用时间
*土壤环境质量标准(GB 15618—1995)。* indicates the Soil Environmental Quality Standard (GB 15618—1995).
综上, 农田土壤重金属污染修复是一个长期工程, 农田土壤将长期处于污染状态。考虑到我国耕地资源紧张与全面建设小康社会的要求, 在污染农田土壤上生产重金属含量不超标的安全农产品成为了适合我国当前实际情况的治理方法。相较于提取修复, 重金属污染农田安全利用通过降低土壤中重金属的生物有效性来减少重金属进入食物链的可能性, 从而保障农田的安全生产与人体的身体健康, 在我国现阶段具有十分重要的意义。进行重金属污染农田安全利用的措施主要包括低吸收作物种植、土壤添加剂与微生物调控以及农艺管理等。
1 低吸收作物种植
1.1 现有低吸收品种
对于重金属吸收富集能力的差异, 既存在于植物物种之间, 也存在于同一物种的不同品种之间, 即同时具有种间差异和种内差异。自低吸收品种的概念提出以来, 国内外已经开展了众多相关的试验筛选研究, 且大多数来自于亚洲尤其是中国(表3)。已筛选的作物涵盖小麦(L.)、水稻(L.)、玉米(L.)、大麦(L.)等粮食作物, 白菜(L.)、芹菜(L.)、辣椒(L.)等蔬菜作物, 大豆(L.)等油料作物以及烟草(L.), 针对的重金属包括Cd、Pb、Zn、Cu、As、Cr、Hg等。部分研究发现在污染农田种植低吸收品种使农产品重金属含量达到了国家安全标准[20]。
1.2 低吸收作物筛选
寻找新的低吸收作物品种一直是污染农田安全利用的主要发展方向。低吸收品种除了保障对重金属的低吸收外, 还应该具有如下特征:
1)当地适应性。由于农田土壤的类型、理化性质、污染程度、气候等存在差异, 同一作物品种在不同地区之间可能存在重金属吸收能力的差异。因此, 当在某地区种植低吸收作物时, 需要对已知的低吸收品种进行重新验证或重新筛选适合当地的低吸收品种。
2)多金属抗性。我国土壤多为重金属复合污染。若某Pb-Cd复合污染地区的小麦品种仅对Pb低吸收, 而Cd含量超标, 那此地出产的小麦仍不能进入食物链流通。此外, 我国部分地区土壤还存在着有机污染、干旱、盐碱、低温等胁迫, 寻找新的作物品种时还应考虑其对多种胁迫因素的耐受性。
3)产量不受太大影响。我国人口数量巨大, 耕地资源稀缺, 必须要保障农产品高产; 高产带来的高经济收益也有利于新的低吸收品种在农民中的推广应用。此外, 由于人们对生活质量的要求不断提高, 生产营养物质更加丰富的农产品也是寻找低吸收作物品种的目标之一。
当前, 我国低吸收作物品种的筛选主要集中在水稻、小麦等粮食作物上。除了粮食, 重点经济作物如烟草、油料、中草药等也是重金属进入人体的重要途径, 且我国拥有大面积种植这些农作物的产区, 因此对油料、中草药、棉麻等作物进行低吸收品种的筛选也十分必要。

表3 我国筛选出的部分用于重金属污染土壤修复的重金属低吸收作物及品种
1.3 基因工程应用
基因工程改造是另一个获得具有优良性状的低吸收品种的途径。目前, 生物体内的部分与重金属抗性相关的基因已经被确认和验证(表4)。这些基因表达产生植物鳌合肽合成酶(phytochelatins, PCs)、转运蛋白、还原酶等, 通过鳌合、转运、区隔化等作用降低重金属的毒性和调节对重金属的积累。通过基因工程技术改变这些基因在植物体内的表达量可以显著影响植物对重金属的抗性和吸收[51-54]。例如, 转入基因或提高了大麦对Cd和As的抗性[55]; 过表达基因提高了水稻对Zn的抗性[56]; 转入基因提高了烟草对As的抗性并降低了地上部As的浓度[57]; 过表达基因或者敲除、或降低了水稻地上部或籽粒对Cd的积累量[58-61]。
通过基因工程技术可以使农作物对重金属的积累量显著降低, 但复杂的田间因素与转基因作物可能带来的生态环境风险以及公众对转基因作物安全性的争议, 使得这些通过基因工程得到的低吸收作物的田间种植面临着巨大挑战[62-64]。
值得注意的是, 有些作物品种地上部能够积累大量的重金属, 而可食用部分如籽粒中的含量不超过安全标准, 这样的农作物被称作cropaccumulator[62]。种植cropaccumulator既可以减少土壤重金属的含量, 也能够保证农产品的产量与安全, 是一种很有前途的污染土壤的安全利用方式。通过基因工程技术将外源基因转入到作物中, 增强作物对重金属的抗性和积累能力被认为是一种获得cropaccumulator的方法[62]。Luo等[65]鉴定出了影响水稻Cd积累量的数量性状基因位点(quantitative trait locus, QTL)。能够调节叶片中Cd的积累, 而对Fe、Zn、Cu等其他营养元素和籽粒中的Cd无显著影响。这为cropaccumulator的寻找提供了有力的基础。
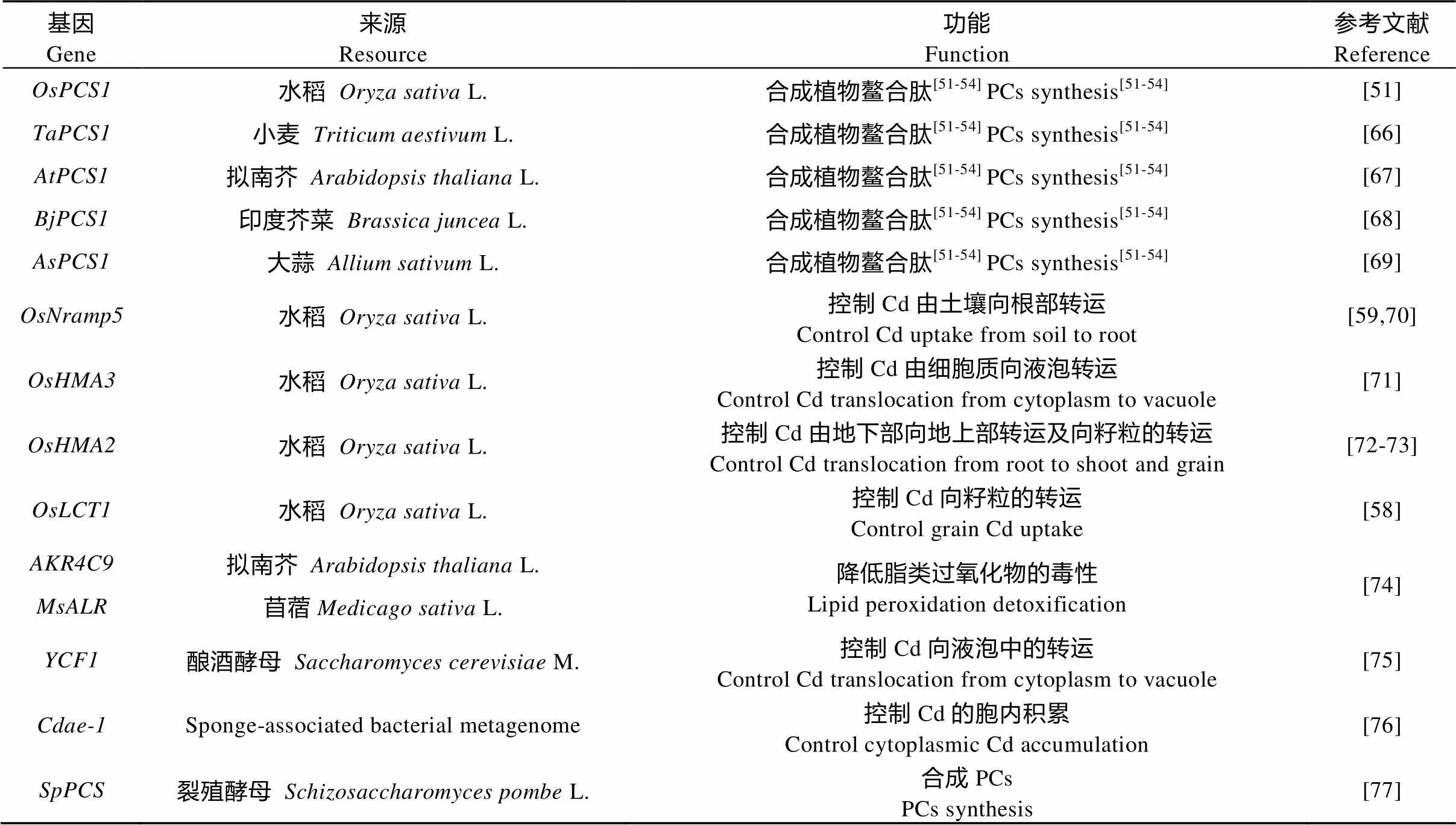
表4 作物中部分与重金属抗性相关的基因(以Cd为例)
2 土壤添加剂应用
土壤添加剂可以改变重金属在土壤中的赋存形态与生物有效性, 进而影响植物对重金属的吸收。因此, 利用土壤添加剂钝化土壤重金属是污染农田安全利用的一种重要措施。常被用于土壤重金属钝化修复的添加剂如下。
2.1 石灰性材料
大量研究表明重金属在土壤中的生物有效性与土壤pH呈负相关关系[78-81], 提高土壤pH可以钝化土壤重金属。石灰的主要成分为CaCO3, 能够显著提高土壤pH, 常被用来改善酸化土壤[82]。多种含有石灰的材料, 如煅烧的贝壳[83]、钢炉渣[84]、磷灰石[85-87]、海泡石[88]等已被用来进行土壤重金属钝化修复。但土壤pH升高也会导致营养元素的有效性和土壤酶活性的降低[89-91], 降低农作物生物量[88]。
2.2 磷酸盐材料
磷酸盐能够与重金属形成稳定的磷酸盐沉淀, 降低重金属在土壤中的迁移性[92-93]。Chen等[89]发现磷酸盐能够将土壤中的Pb、Zn、Cd由可交换态、有机结合态转化为磷氯铅矿等残渣态, 进而降低油菜(L.)中这些重金属的含量。
2.3 有机废弃物
农业有机废物如畜禽粪便、农作物秸秆等常常作为有机肥为植物提供营养元素[94]。研究表明向土壤中施加粪肥、作物秸秆等能够降低重金属的生物有效性, 减少植物对重金属的吸收[95-98]。这主要有如下几个原因: 首先, 施加粪肥、秸秆等能使土壤有机质含量增加, 这些有机物通过络合作用吸附重金属, 降低重金属的生物有效性[99-100]; 其次, 施加有机废弃物能够提高土壤pH[95,101], 进而降低重金属的生物有效性; 此外, 施加有机废弃物能够提高土壤有效磷的含量[97], 而磷能够有效钝化土壤重金属[92]。
2.4 生物炭
生物炭具有非常高的比表面积, 可高达65.85 m2∙g-1[102], 且带有负电荷, 有利于其吸附重金属离子[103-105]。此外, 生物炭表面含有大量的—OH、—COOH等官能团, 可与重金属形成稳定的络合物[106]。生物炭多呈碱性[107-109], 施用生物炭能够提高土壤pH, 强化对重金属离子的钝化[110-111]。
2.5 黏土矿物
膨润土、蒙脱石、伊利石、高岭石等黏土矿物具有较高的阳离子交换量, 能够通过离子交换作用将土壤重金属离子吸附于其表面上, 进而降低重金属的迁移性[112-113]。重金属还能与矿物晶体通过共价键形成专性吸附, 很难再从黏土矿物上解吸下来[113-114]。Sun等[115]、徐奕等[116]在Pb、Cd污染土壤中施入膨润土后, 土壤中Pb、Cd主要由可交换态转化为了残渣态, 且水稻体内的Pb、Cd浓度显著降低。
土壤添加剂在土壤重金属污染修复中也存在着一些问题。(1)过量施用添加剂会改变土壤性质。长期施用石灰等碱性物质会破坏土壤团粒结构, 造成土壤板结和养分流失, 也会对土壤微生物的群落结构产生影响[117]。(2)添加剂可能会引入新的污染物质。有机废弃物可能会携带大量重金属、有机污染物、病原菌等有害物质, 如果不经处理直接施用会对土壤造成二次污染, 并降低土壤质量[118]。(3)添加剂对重金属的钝化效果会随土壤环境的改变而改变。有机添加剂容易被降解, 被其固定的重金属会重新释放出来, 因此, 需要对其进行长期的环境风险评估[117]。(4)添加剂可能会降低农作物的产量。Sun等[88]研究发现在碱性土壤中施加0.5%~5%的海泡石会降低菠菜(L.)地上部的生物量。
3 微生物调控
微生物能够改变土壤中重金属的赋存形态, 影响其生物有效性, 也能调节植物的养分供应, 促进植物的生长发育。由于经济性与环境友好性, 微生物越来越多地被应用于土壤重金属污染的钝化修复中。当前, 多种具有重金属抗性或积累能力的微生物已经被筛选出来(表5), 这些微生物能显著降低小麦、水稻、白菜、萝卜(L.)等农作物中Cd、Pb、Cu、As、Cr等重金属的含量。

表5 部分能够抑制农作物吸收重金属的微生物
3.1 微生物调控机理
微生物抑制植物吸收重金属的机制已经有大量研究, 主要包括降低土壤重金属的有效性与影响植物吸收两个方面。
1)降低土壤重金属生物有效性。微生物可以通过分泌胞外聚合物(extracellular polymeric substances, EPS)来钝化重金属。EPS富含羟基、羧基、氨基等官能团[127-128], 可通过静电吸附、络合等作用与重金属键合并钝化重金属。Li等[129]发现白腐真菌(B.)分泌的EPS对低浓度Pb的钝化起着十分重要的作用。Joshi等[122]发现固氮菌(spp.)可以通过分泌EPS来钝化土壤中的Cd、Cr离子, 进而降低小麦体内Cd、Cr的含量。
微生物可以将重金属矿化进而钝化重金属。Li等[130]发现等细菌能够产生脲酶将尿素水解, 提高土壤pH, 使土壤溶液中的Cd、Cu、Pb、Ni等重金属在其表面沉淀形成碳酸盐结晶。Qian等[131]发现,T. CS1可以将土壤中的Pb、Cr主要转化为方解石、钒钙、碳酸钙、氧化铬等碳酸盐矿物。除了碳酸盐矿物, 微生物还能将重金属转化为磷氯铅矿、水白铅矿等[132-133]。
微生物可以通过影响土壤中有机质的转化来钝化重金属。微生物在有机质降解及腐殖质形成过程中起着重要的作用[134]。腐殖质富含羟基、羧基、氨基等官能团[135-136], 这些官能团能够与重金属形成稳定的络合物, 进而钝化重金属[137]。Zhang等[138]发现, 在堆肥过程中加入白腐真菌(B.)能够显著提高腐殖质的含量, 降低Zn、Pb、Cu和 Ni的生物有效性, 且重金属的残渣态或氧化物结合态的含量与腐殖质或其某些成分(如胡敏酸)的含量具有良好的相关性。
部分微生物对重金属具有很强的积累能力[139-140], 可以将土壤中的重金属大量吸收到体内, 进而减少可被植物吸收的重金属含量。Wu等[121]通过盆栽试验发现, 种植蘑菇(M.)显著降低了生菜(L.)体内Cu的积累量与土壤中HOAc提取态的含量, 且土壤中可交换态Cu的含量变化与蘑菇体内Cu的积累量的变化趋势一致, 这说明土壤中有效态的Cu主要被吸收从而减少了生菜对Cu的吸收。
2)影响植物对重金属的耐性和吸收。重金属会引起植物体内活性氧(ROS)的过量产生, 进而对植物产生毒害作用。通过提高体内的抗氧化酶如SOD、POD和CAT含量来清除ROS是植物应对重金属毒害的一种重要机制[141]。微生物可以影响植物体内抗氧化酶含量进而减轻重金属对作物的毒害作用。Devi等[142]发现, 向土壤中施加真菌sp. (WT2)显著提高了向日葵(L.)体内SOD、POD和CAT的含量, 增强了向日葵对Pb的耐受性。
微生物可以增强植物对营养元素的吸收, 提高植物的生物量, 对体内的重金属起到稀释作用(growth dilution effects), 进而降低植物体内的重金属含量。Wu等[143]发现接种丛枝菌根真菌(arbuscular mycorrhizal fungi, AMF)(S.)能够加强蒲公英(D.)对P的吸收, 显著提高其生物量, 降低其体内Cr的浓度。AMF外生菌丝具有很高的表面积与阳离子交换量, 能够将重金属离子结合在其表面[144-145], 在植物根系周围钝化大量重金属, 减少植物对重金属的吸收。Nayuki等[146]通过X-ray fluorescence (XRF)成像技术发现, AMF能够将Cd大量地固定在其外生菌丝的细胞壁和液泡中, 且不会转运到宿主植物的根中。即使外生菌丝能够将Cr、Zn等转运到植物根系中, 也会显著地抑制这些重金属由植物地下部向地上部的转移[146-147]。
3.2 钝化效果强化
强化微生物对重金属的钝化效果是今后的主要研究方向。添加剂可以影响微生物的活动, 进而强化其对重金属的钝化效果。Wang等[148]发现使用由畜粪制成的有机添加剂能够显著增加AMF对烟草根部的侵染率。添加硫酸盐和葡萄糖能够强化硫酸盐还原细菌(sulfate reducing bacteria)的活动, 促进其将重金属转化为硫化物沉淀[149]。与单独添加微生物相比, 微生物与生物炭、煤泥等钝化剂联合使用能显著降低Cd、Pb等重金属在土壤中的生物有效性以及在玉米、绿豆(L.)、萝卜、生菜等农作物中的含量[120,125,149-152]。微生物的活动与群落结构容易受到诸多土壤性质的影响[153], 故也可以通过调节土壤的pH[154]、C/N比[155]、有机质含量与含水率等来控制微生物对重金属的钝化活动。此外, 通过基因工程手段将与重金属抗性相关的外源基因转入微生物, 可以强化微生物对重金属的钝化修复效果。Elahian等[156]将B.的基因转入毕赤酵母(G.)后发现, 毕赤酵母能够将Ag+转化成毒性更低的纳米银并将其大量吸附在细胞表面。Li等[157]发现, 转入来自豆梨(D.)的基因能够增强大肠杆菌(E.)对Cd、Cu、Hg离子的抗性和积累量。
3.3 土著微生物利用
在需要进行安全利用的地区土壤中寻找具有重金属钝化功能的土著微生物具有重要意义。严重污染地区(如矿区)土壤的重金属含量非常高, 能够在这些土壤中存活下来的微生物往往对重金属具有很强的抗性, 这种天然筛选使人们更容易寻找到能够应用于重金属污染土壤修复的目标微生物[158-163]。但由于土壤性质存在较大的差异以及与土著微生物的竞争作用, 由污染严重的矿区分离筛选出来的微生物的活动或功能在污染程度较低的农田土壤中可能会发生改变[164-165], 进而影响这些微生物的修复效果。土著微生物能够更好地适应待修复农田的土壤环境, 进而产生更好的修复效果。Oller等[166]从当地农田土壤中筛选出的杆菌sp.、假单胞菌sp.和红球菌sp.均对As具有很强的抗性和积累能力, 且能够促进大豆的生长, 具有很大的潜力应用于当地污染土壤的粮食安全生产。Abu-Elsaoud等[119]在当地农田土壤中筛选出的AMF(N. et G.)能够增强小麦对Zn的抗性并抑制Zn向地上部的转运。此外, 随着宏基因组等技术的发展, 土壤中微生物的种质资源能够被更加充分地发掘, 这将助力人们筛选出更多的能够应用于污染土壤修复的土著微生物。
4 农艺管理
农艺措施能改变土壤的通气、水分、养分等条件, 除了能够提高农作物产量, 增加收益, 防治病虫害, 改善土壤外, 还能影响土壤中重金属的生物有效性和植物对重金属的抗性。因此, 农艺措施也是污染农田土壤安全利用的一种重要调控措施。当前, 人们主要通过施肥、水分管理、间套作等农艺措施来控制农作物对重金属的吸收。
4.1 施肥
肥料能够为农作物提供必需的营养, 增强作物对重金属的抗性, 提高生物量, 对体内的重金属起到稀释作用。此外, 肥料中的P能够与As、Cr等重金属竞争植物根系表面吸附位点, 故施加磷肥能够抑制作物对重金属的吸收[142,167-170]。肥料还能通过改变土壤pH及络合、沉淀等作用降低重金属的迁移性与生物有效性, 进而减弱植物对重金属的吸收[92,95,97,99-101]。
一些学者研究了叶面施肥对农作物吸收转运重金属的影响。叶面喷施锌肥能够降低白菜[49]、油菜[171]、黄瓜(L.)[172]、小麦[173]体内的Cd含量, 叶面喷施Si肥能够减少水稻地上部和籽粒中Pb的含量, 抑制Pb由地下部向地上部的转运[174]。这可能是因为通过叶面进入植物体内的Zn与Cd竞争植物细胞表面的吸附位点, 抑制植物对Cd的吸收[175-176]; 而Si可能将Pb等重金属固定在植物细胞壁上, 进而抑制了Pb在植物体内的运输[177-178]。此外, 叶面肥比土施肥具有更低的修复成本。在达到减少农作物重金属含量的相同效果时, 叶面肥的用量比土施肥的用量至少减少5倍[171,179]。
此外, 改变肥料的用量也能影响作物对重金属的吸收。Tang等[49]发现白菜体内Cd含量与土壤K、Zn的含量呈负相关关系, 故认为增施钾肥或锌肥能够抑制白菜对Cd的吸收。
4.2 水分管理
氧化还原条件会影响重金属在土壤中的价态和赋存形态, 而这决定了重金属的毒性与迁移性[180-182], 故通过水分条件管理控制土壤的氧化还原条件对土壤重金属污染修复具有重要意义。Hu等[183]研究了不同淹水条件对水稻吸收Cd和As的影响。结果发现, 从不淹水到定期淹水再到始终淹水, 水稻籽粒中Cd含量从0.21 mg∙kg-1减少到0.02 mg∙kg-1, 然而As的浓度却从0.14 mg∙kg-1增长到0.21 mg∙kg-1。土壤中有效态Cd与As的含量也呈现出了相反的变化趋势。还需注意的是, 减少两种重金属含量的水分管理模式下的水稻产量均较传统模式减少。因此, 需要进一步采取特定的措施来同时控制水稻Cd和As的含量, 同时保证水稻的产量。
4.3 间作
植物间作是利用不同种植物之间的互补作用达到提高产量, 控制病虫害等目的[184-185]的种植模式。将农作物与超富集植物间作, 可以在保证农产品安全的前提下, 利用超富集植物持续地减少土壤中的重金属含量, 这是污染农田安全利用的主要发展方向。Wang等[186]将Cd超富集植物龙葵(L.)与Cd低吸收品种大葱(L.)进行间作, 发现间作模式下, 大葱的Cd含量没有变化, 符合国家食品安全标准, 且大葱的产量没有降低; 另一个有利的结果是, 经过90 d的种植, 龙葵去除了表层土壤(0~20 cm)中7%的Cd。其他研究也获得了较好的结果。谭建波等[187]发现与Cd超级累植物断续菊(L.)间作能够显著减少玉米Cd的含量, 显著提高断续菊Cd的浓度, 此外, 间作均提高了两种植物的生物量。Zhan等[188]将断续菊与茄子(L.)进行间作, 也得到了相似的结果。但间作模式也存在着不理想的情况。Yang等[189]发现, 将瞿麦(L.)与白车轴草(L.)进行间作能够显著降低瞿麦体内Pb含量, 但也降低瞿麦的药用成分大黄素的含量, 这主要是由于白车轴草与瞿麦的竞争作用减少了瞿麦对营养元素的吸收。Yang等[190]发现香根草(L.)与豆科植物间作对两种植物的Cd含量与生物量均无显著影响。汤福义等[191]发现与龙葵间作甚至会提高白菜幼苗Cd的积累量。可见, 利用间作模式进行污染土壤的安全利用需要对农作物、间作植物、目标污染物进行综合考虑。
5 农业资源研究中心相关研究与展望
截止到目前, 国内外均进行过污染土壤的修复实践。国外最具代表性的污染土地的管理方法当属美国的“超级基金”, 它是为了实现污染场地的再生产利用由美国国会批准设立的管理法案, 对污染场地和责任者的确定, 修复资金的筹备与使用, 修复工程的设计和实施, 后期运营与监测等环节均进行了规范, 已经被多个国家借鉴[192]。超级基金规定由责任者或污染者对污染场地进行修复, 对找不到责任者或责任者没有修复能力的, 由超级基金支付修复费用[192]。但污染场地修复的经费投入巨大, 责任者往往难以承担, 而“超级基金”也面临着经费缺乏的问题[192]。根据“超级基金”的经验, 我国污染农田面积大, 且污染者难以确定, 无论污染企业还是地方政府, 在没有类似“超级基金”的经费支持下, 均难以进行完整的污染农田土壤的修复进而实现再生产利用。而国内农田土壤的修复实践目前多以示范为主[6], 鲜有大面积的推广应用。
污染农田管控即禁止在污染农田上种植粮食也是一种保障食品安全的管理方法。但对粮食安全的坚定的要求决定了我国不能进行大面积的污染农田管控。此外, 我国农田的所有与使用制度难以保障管控措施的实施, 在被管控的农田上仍可能继续进行粮食种植, 对我国的食品安全产生潜在的威胁。维持农田生产并保障粮食与食品安全是我国当前的形势所需。因此, 在污染农田上生产出安全的农产品即污染农田的安全利用具有非常重大的意义。目前, 污染农田的安全利用已经被学界和政府普遍接受。
将多种技术手段进行整合, 实现优势互补, 是当前污染农田安全利用的重要发展趋势。就目前来看, 少数的技术集成取得了显著进展, 但可重复性与经济成本还远不能满足推广需求。农民作为实施主体对技术手段的接受程度也是影响安全利用推广的重要因素, 他们更容易接受操作简单、接近农艺措施的技术, 比如种子与肥料。这要求这些技术手段具有更佳的抑制重金属吸收转运的效果。生物技术在改变植物以及土壤微生物等对重金属的吸收转运能力方面具有极大的潜力。故发展以生物技术为核心的污染农田安全利用模式是我国重要的研究方向。
中国科学院遗传与发育生物学研究所农业资源研究中心作为中国科学院主要农业类研究所之一, 在农业环境包括农田重金属污染治理方面做了多年的布局和投入。在重金属污染农田安全利用研究方面, 相关研究组按照机理与技术并重的发展理念, 提出了以生物技术为核心的技术模式, 并在中国科学院“百人计划”和河北省杰出青年基金等项目的资助下, 系统开展了华北微生物Cd固定剂和Cd低吸收小麦品种的筛选, 并且在生物快速提取方面取得了进展。过去2年中, 研究组在微生物菌剂和修复方法方面提交并授权了多项核心专利(表6), 正在成为我国污染农田安全利用技术发展的新力量, 并提出了生物技术为核心的技术模式(图1), 可尽可能少地减少化学固定剂如石灰和磷肥的使用, 提高重金属污染农田利用的安全性。以治理理念的进步和技术积累为基础, 农业资源研究中心重金属污染修复团队已经获得了与国内相关科研单位(包括南京土壤研究所、山东师范大学、河北地质职工大学等)和相关环保企业合作, 在雄安和承德等地开展了修复示范工作。

表6 部分农业资源研究中心微生物菌剂与土壤修复方法的专利
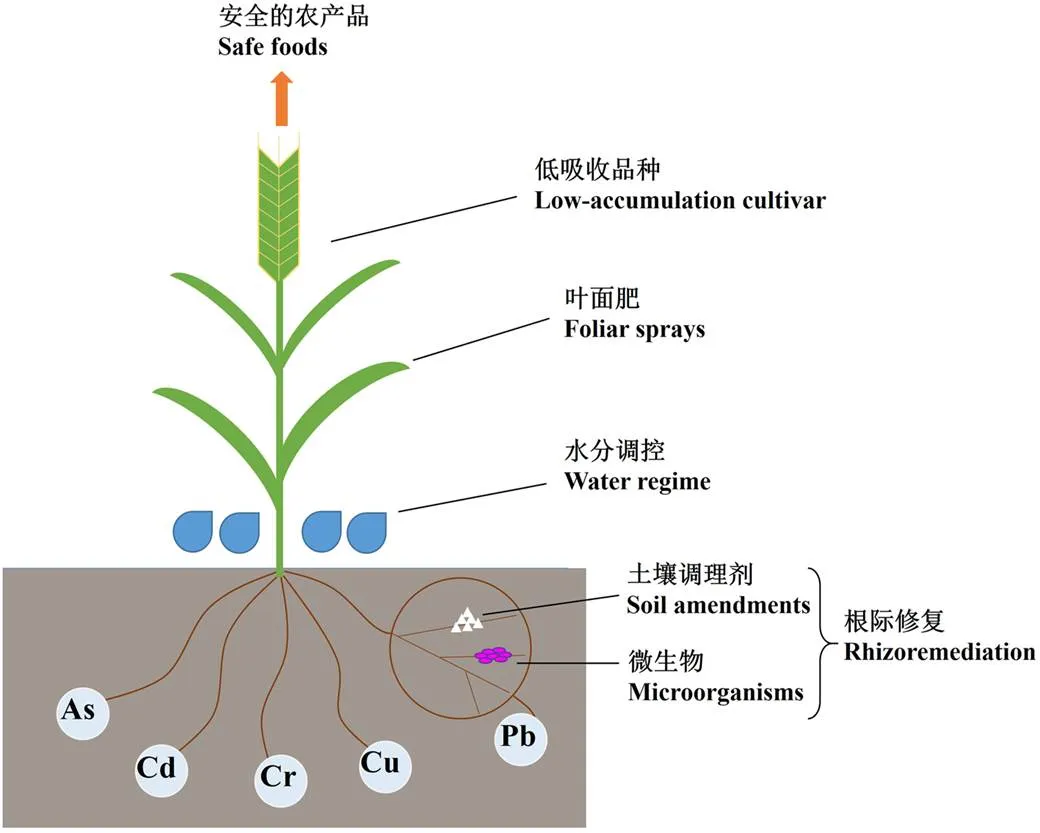
图1 当前重金属污染农田安全利用的技术模式
[1] 国土资源部. 2016中国国土资源公报[EB/OL]. http://www.mlr.gov.cn/sjpd/gtzygb/201704/P020170428532821702501.pdf Ministry of Land and Resources of China. The land resources communique of China 2016[EB/OL]. http://www.mlr.gov.cn/ sjpd/gtzygb/201704/P020170428532821702501.pdf
[2] 周健民. 浅谈我国土壤质量变化与耕地资源可持续利用[J]. 中国科学院院刊, 2015, 30(4): 459–467 ZHOU J M. Evolution of soil quality and sustainable use of soil resources in China[J]. Bulletin of Chinese Academy of Sciences, 2015, 30(4): 459–467
[3] 环境保护部, 国土资源部. 全国土壤污染调查公报[EB/OL]. (2014-04-17) http://www.mep.gov.cn/gkml/hbb/qt/201404/t20140417_ 270670.htm Ministry of Environmental Protection of China, Ministry of Land and Resources of China. Report on the national general survey of soil contamination[EB/OL]. (2014-04-17) http:// www.mep.gov.cn/gkml/hbb/qt/201404/t20140417_270670.htm
[4] 刘晓宇, 梁琼, 高如泰, 等. 长期污灌条件下农田土壤重金属污染环境风险评价[J]. 生态与农村环境学报, 2015, 31(4): 572–578 LIU X Y, LIANG Q, GAO R T, et al. Environmental risk assessment of soil heavy metal pollution of farmlands with long period of sewage irrigation[J] Journal of Ecology and Rural Environment, 2015, 31(4): 572–578
[5] 陈凤, 董泽琴, 王程程, 等. 锌冶炼区耕地土壤和农作物重金属污染状况及风险评价[J]. 环境科学, 2017, 38(10): 4360–4369 CHEN F, DONG Z Q, WANG C C, et al. Heavy metal contamination of soils and crops near a zinc smelter[J]. Environmental Science, 2017, 38(10): 4360–4369
[6] 黄益宗, 郝晓伟, 雷鸣, 等. 重金属污染土壤修复技术及其修复实践[J]. 农业环境科学学报, 2013, 32(3): 409–417HUANG Y Z, HAO X W, LEI M, et al. The remediation technology and remediation practice of heavy metals- contaminated soil[J]. Journal of Agro-Environment Science, 2013, 32(3): 409–417
[7] 吴燕玉, 陈涛, 孔庆新, 等. 张士灌区镉污染及其改良途径[J]. 环境科学学报, 1984, 4(3): 275–283 WU Y Y, CHEN T, KONG Q X, et al. Cadmium contamination of Zhangshi irrigation area and ways of improving[J]. Acta Scientiae Circumstantiae, 1984, 4(3): 275–283
[8] 侯李云, 曾希柏, 张杨珠. 客土改良技术及其在砷污染土壤修复中的应用展望[J]. 中国生态农业学报, 2015, 23(1): 20–26 HOU L Y, ZENG X B, ZHANG Y Z. Application and outlook of alien earth soil-improving technology in arsenic- contaminated soil remediation[J]. Chinese Journal of Eco-Agriculture, 2015, 23(1): 20–26
[9] 高国龙, 张望, 周连碧, 等. 重金属污染土壤化学淋洗技术进展[J]. 有色金属工程, 2013, 3(1): 49–52 GAO G L, ZHANG W, ZHOU L B, et al. Technology of chemical leaching of heavy metal-contaminated soil[J]. Nonferrous Metals Engineering, 2013, 3(1): 49–52
[10] SUN B, ZHAO F J, LOMBI E, et al. Leaching of heavy metals from contaminated soils using EDTA[J]. Environmental Pollution, 2001, 113(2): 111–120
[11] WU L H, LUO Y M, XING X R, et al. EDTA-enhanced phytoremediation of heavy metal contaminated soil with Indian mustard and associated potential leaching risk[J]. Agriculture, Ecosystems & Environment, 2004, 102(3): 307–318
[12] HE F, GAO J, PIERCE E, et al.remediation technologies for mercury-contaminated soil[J]. Environmental Science and Pollution Research, 2015, 22(11): 8124–8147
[13] YI Y M, PARK S, MUNSTER C, et al. Changes in ecological properties of petroleum oil-contaminated soil after low-temperature thermal desorption treatment[J]. Water, Air & Soil Pollution, 2016, 227: 108
[14] 王宇, 李婷婷, 魏小娜, 等. 污染土壤电动修复技术研究进展[J]. 化学研究, 2016, 27(1): 34–43 WANG Y, LI T T, WEI X N, et al. Research progress on electrokinetic remediation of contaminated soil[J]. Chemical Research, 2016, 27(1): 34–43
[15] 张涛, 陈明功, 刘宗亮. 土壤电动修复技术及其研究进展[J]. 现代农业科技, 2016, (22): 164–165 ZHANG T, CHEN M G, LIU Z L. Soil electrokinetic remediation technology and research progress[J]. Modern Agricultural Science and Technology, 2016, (22): 164–165
[16] WAN X M, LEI M, CHEN T B. Cost-benefit calculation of phytoremediation technology for heavy-metal-contaminated soil[J]. Science of the Total Environment, 2016, 563/564: 796–802
[17] SALIDO A L, HASTY K L, LIM J M, et al. Phytoremediation of arsenic and lead in contaminated soil using Chinese brake ferns () and Indian mustard ()[J]. International Journal of Phytoremediation, 2003, 5(2): 89–103
[18] JI P H, SONG Y F, JIANG Y J, et al. A two-year field study of phytoremediation usingL. in China[J]. International Journal of Phytoremediation, 2016, 18(9): 924–928
[19] LI Z, WU L H, HU P J, et al. Repeated phytoextraction of four metal-contaminated soils using the cadmium/zinc hyperaccumulator[J]. Environmental Pollution, 2014, 189: 176–183
[20] 陈亚茹, 张巧凤, 付必胜, 等. 中国小麦微核心种质籽粒铅、镉、锌积累差异性分析及低积累品种筛选[J]. 南京农业大学学报, 2017, 40(3): 393–399CHEN Y R, ZHANG Q F, FU B S, et al. Differences of lead, cadmium and zinc accumulation among Chinese wheat mini-core collections germplasms and screening for low Pb, Cd and Zn accumulative cultivars in grains[J]. Journal of Nanjing Agricultural University, 2017, 40(3): 393–399
[21] 肖亚涛. 冬小麦籽粒镉低积累品种的生产特性及其低积累机制研究[D]. 北京: 中国农业科学院, 2016 XIAO Y T. Production characteristics and mechanism of low accumulation in winter wheat with low cadmium accumulation in grain[D]. Beijing: Chinese Academy of Agricultural Sciences, 2016
[22] 邢维芹, 张红毅, SCHECKEL K G, 等. 铅冶炼污染区小麦籽粒镉含量及低积累品种筛选[J]. 农业环境科学学报, 2015, 34(10): 2039–2040XING W Q, ZHANG H Y, SCHECKEL K G, et al. Grain Cd concentrations of 100 wheat (Linn) varieties and strains grown on lead-smelting contaminated soils and screening for low Cd varieties[J]. Journal of Agro-Environment Science, 2015, 34(10): 2039–2040
[23] 孙洪欣, 薛培英, 赵全利, 等. 镉、铅积累与转运在冬小麦品种间的差异[J]. 麦类作物学报, 2015, 35(8): 1161–1167 SUN H X, XUE P Y, ZHAO Q L, et al. Differences of cadmium and lead accumulation and transportion among winter wheat varieties[J]. Journal of Triticeae Crops, 2015, 35(8): 1161–1167
[24] 李平远, 娄运生, 王琦, 等. 6个小麦品种对铜镉吸收积累差异的比较[J]. 安徽农学通报, 2007, 13(12): 102–104 LI P Y, LOU Y S, WANG Q, et al. Comparison of Cu and Cd accumulation among six wheat cultivars[J]. Anhui Agricultural Science Bulletin, 2007, 13(12): 102–104
[25] 杨素勤, 程海宽, 张彪, 等. 不同品种小麦Pb积累差异性研究[J]. 生态与农村环境学报, 2014, 30(5): 646–651 YANG S Q, CHENG H K, ZHANG B, et al. Differences in Pb accumulation between wheat varieties[J]. Journal of Ecology and Rural Environment, 2014, 30(5): 646–651
[26] 李正文, 张艳玲, 潘根兴, 等. 不同水稻品种籽粒Cd、Cu和Se的含量差异及其人类膳食摄取风险[J]. 环境科学, 2003, 24(3): 112–115 LI Z W, ZHANG Y L, PAN G X, et al. Grain contents of Cd, Cu and Se by 57 rice cultivars and the risk significance for human dietary uptake[J]. Environmental Science, 2003, 24(3): 112–115
[27] 张路, 张锡洲, 李廷轩, 等. 水稻镉安全亲本材料对镉的吸收分配特性[J]. 中国农业科学, 2015, 48(1): 174–184ZHANG L, ZHANG X Z, LI T X, et al. Cd uptake and distribution characteristics of Cd pollution-safe rice materials[J]. Scientia Agricultura Sinica, 2015, 48(1): 174–184
[28] 张儒德, 李军, 秦利, 等. 辽宁省5种不同基因型水稻对镉吸收差异的研究[J]. 农业环境科学学报, 2016, 35(5): 842–849 ZHANG R D, LI J, QIN L, et al. Study on the difference of cadmium absorption in five rice genotypes of Liaoning Province[J]. Journal of Agro-Environment Science, 2016, 35(5): 842–849
[29] 何玉龙. 镉、铅复合污染条件下不同基因型水稻镉、铅积累特性及低积累品种筛选的研究[D]. 沈阳: 沈阳农业大学, 2016 HE Y L. Screening of Cd and Pb accumulation characteristics and low accumulation varieties of different genotypes of rice under Cd and Pb combined pollution[D]. Shenyang: Shenyang Agricultural University, 2016
[30] 段桂兰, 张红梅, 刘云霞, 等. 水稻基因类型与生长环境对精米中砷积累的影响[J]. 生态毒理学报, 2013, 8(2): 156–162 DUAN G L, ZHANG H M, LIU Y X, et al. Impact of rice genotype and growing environment on arsenic accumulation in rice polished grains[J]. Asian Journal of Ecotoxicology, 2013, 8(2): 156–162
[31] 伍钧, 吴传星, 孟晓霞, 等. 重金属低积累玉米品种的稳定性和环境适应性分析[J]. 农业环境科学学报, 2011, 30(11): 2160–2167WU J, WU C X, MENG X X, et al. The analysis of stability and adaptability on low accumulation of heavy metals in various cultivars of[J]. Journal of Agro-Environment Science, 2011, 30(11): 2160–2167
[32] 于蔚, 李元, 陈建军, 等. 铅低累积玉米品种的筛选研究[J]. 环境科学导刊, 2014, 33(5): 4–9 YU W, LI Y, CHEN J J, et al. Selection ofcultivars with low accumulation of heavy metals of lead[J]. Environmental Science Survey, 2014, 33(5): 4–9
[33] 辛艳卫. 不同玉米品种对镉的吸收和转运差异性研究[D]. 沈阳: 沈阳农业大学, 2017 XIN Y W. Uptake and translocation of cadmium in different maize cultivars[D]. Shenyang: Shenyang Agricultural University, 2017
[34] 吴传星, 伍钧, 杨刚, 等. 重金属低积累玉米品种的筛选[C]//第三届全国农业环境科学学术研讨会论文集. 天津: 中国农业生态环境保护协会, 2009: 363–370WU C X, WU J, YANG G, et al. Screeningcultivars with high tolerance and low grain accumulation of heavy metals[C]//National Agricultural Environmental Science Academic Seminar. Tianjin: China Agricultural Ecological Environment Protection Association, 2009: 363–370
[35] 杜彩艳, 张乃明, 雷宝坤, 等. 砷、铅、镉低积累玉米品种筛选研究[J]. 西南农业学报, 2017, 30(1): 5–10 DU C Y, ZHANG N M, LEI B K, et al. Selection of varieties ofwith low accumulation of heavy metals of arsenic, lead and cadmium[J]. Southwest China Journal of Agricultural Sciences, 2017, 30(1): 5–10
[36] 孙洪欣. 府河污灌区农田镉铅含量调查及其低积累作物品种筛选[D]. 保定: 河北农业大学, 2015 SUN H X. Investigation of Cd, Pb concentrations in soil-crops and screen of cultivars of wheat/maize with low Cd and Pb accumulation in the sewage irrigation farmlands along Fuhe river[D]. Baoding: Agricultural University of HeBei, 2015
[37] HE B Y, LING L, ZHANG L Y, et al. Cultivar-specific differences in heavy metal (Cd, Cr, Cu, Pb, and Zn) concentrations in water spinach (‘Forsk’) grown on metal-contaminated soil[J]. Plant and Soil, 2015, 386(1/2): 251–262
[38] XIAO Q Q, WONG M H, HUANG L, et al. Effects of cultivars and water management on cadmium accumulation in water spinach (Forsk.)[J]. Plant and Soil, 2015, 391(1/2): 33–49
[39] ZENG W A, LI F, TAN L, et al. Comparison of absorption capacity of different flue-cured tobacco cultivars for six kinds of heavy metals[J]. Agricultural Science & Technology, 2016, 17(9): 2081–2084
[40] CHEN F, DONG J, WANG F, et al. Identification of barley genotypes with low grain Cd accumulation and its interaction with four microelements[J]. Chemosphere, 2007, 67(10): 2082–2088
[41] ZHANG K, WANG J B, YANG Z Y, et al. Genotype variations in accumulation of cadmium and lead in celery (L.) and screening for low Cd and Pb accumulative cultivars[J]. Frontiers of Environmental Science & Engineering, 2013, 7(1): 85–96
[42] DAI H W, YANG Z Y. Variation in Cd accumulation among radish cultivars and identification of low-Cd cultivars[J]. Environmental Science and Pollution Research, 2017, 24(17): 15116–15124
[43] WANG S Q, WEI S H, CHEN Y Q, et al. Comparison of soybean cultivars enriching Cd and the application foreground of the low-accumulating cultivar in production[J]. Polish Journal of Environmental Studies, 2017, 26(3): 1299–1304
[44] 智杨, 孙挺, 周启星, 等. 铅低积累大豆的筛选及铅对其豆中矿物营养元素的影响[J]. 环境科学学报, 2015, 35(6): 1939–1945 ZHI Y, SUN T, ZHOU Q X, et al. Identification of Chinese soybean cultivars with low lead accumulation and the effect of lead on their mineral ion complement[J]. Acta Scientiae Circumstantiae, 2015, 35(6): 1939–1945
[45] WANG L, XU Y M, SUN Y B, et al. Identification of pakchoi cultivars with low cadmium accumulation and soil factors that affect their cadmium uptake and translocation[J]. Frontiers of Environmental Science & Engineering, 2014, 8(6): 877–887
[46] XIN J L, HUANG B F, LIU A Q, et al. Identification of hot pepper cultivars containing low Cd levels after growing on contaminated soil: Uptake and redistribution to the edible plant parts[J]. Plant and Soil, 2013, 373(1/2): 415–425
[47] QIU Q, WANG Y T, YANG Z Y, et al. Responses of different Chinese flowering cabbage (L.) cultivars to cadmium and lead exposure: Screening for Cd+Pb pollution-safe cultivars[J]. CLEAN-Soil Air Water, 2011, 39(11): 925–932
[48] HE B Y, YU D P, CHEN Y, et al. Use of low-calcium cultivars to reduce cadmium uptake and accumulation in edible amaranth (L.)[J]. Chemosphere, 2017, 171: 588–594
[49] TANG L, LUO W J, TIAN S K, et al. Genotypic differences in cadmium and nitrate co-accumulation among the Chinese cabbage genotypes under field conditions[J]. Scientia Horticulturae, 2016, 201: 92–100
[50] HUANG B F, XIN J L, DAI H W, et al. Identification of low-Cd cultivars of sweet potato ((L.) Lam.) after growing on Cd-contaminated soil: Uptake and partitioning to the edible roots[J]. Environmental Science and Pollution Research, 2015, 22(15): 11813–11821
[51] GASIC K, KORBAN S S. Transgenic Indian mustard () plants expressing anphytochelatin synthase () exhibit enhanced As and Cd tolerance[J]. Plant Molecular Biology, 2007, 64(4): 361–369
[52] GUO J B, DAI X J, XU W Z, et al. Overexpressingandsimultaneously increases the tolerance and accumulation of cadmium and arsenic in[J]. Chemosphere, 2008, 72(7): 1020–1026
[53] LI Y J, DHANKHER O P, CARREIRA L, et al. Overexpression of phytochelatin synthase inleads to enhanced arsenic tolerance and cadmium hypersensitivity[J]. Plant & Cell Physiology, 2004, 45(12): 1787–1797
[54] PAL R, RAI J P N. Phytochelatins: Peptides involved in heavy metal detoxification[J]. Applied Biochemistry and Biotechnology, 2010, 160(3): 945–963
[55] ÉVA C, SOLTI Á, OSZVALD M, et al. Improved reactive aldehyde, salt and cadmium tolerance of transgenic barley due to the expression of aldo-keto reductase genes[J]. Acta Physiologiae Plantarum, 2016, 38: 99
[56] WANG Y G, LIU H H, WANG S P, et al. Overexpression of a common wheat geneenhances tolerance to zinc inand rice through the modulation of reactive oxygen species production[J]. Plant Molecular Biology Reporter, 2016, 34(4): 794–806
[57] NAHAR N, RAHMAN A, NAWANI N N, et al. Phytoremediation of arsenic from the contaminated soil using transgenic tobacco plants expressinggene of[J]. Journal of Plant Physiology, 2017, 218: 121–126
[58] URAGUCHI S, KAMIYA T, SAKAMOTO T, et al. Low-affinity cation transporter () regulates cadmium transport into rice grains[J]. Proceedings of the National Academy of Sciences of the United States of America, 2011, 108(52): 20959–20964
[59] SASAKI A, YAMAJI N, YOKOSHO K, et al. Nramp5 is a major transporter responsible for manganese and cadmium uptake in rice[J]. The Plant Cell, 2012, 24(5): 2155–2167
[60] YAMAJI N, XIA J X, MITANI-UENO N, et al. Preferential delivery of zinc to developing tissues in rice is mediated by P-type heavy metal ATPase OsHMA2[J]. Plant Physiology, 2013, 162(2): 927–939
[61] SASAKI A, YAMAJI N, MA J F. Overexpression ofenhances Cd tolerance and expression of Zn transporter genes in rice[J]. Journal of Experimental Botany, 2014, 65(20): 6013–6021
[62] WU G, KANG H B, ZHANG X Y, et al. A critical review on the bio-removal of hazardous heavy metals from contaminated soils: Issues, progress, eco-environmental concerns and opportunities[J]. Journal of Hazardous Materials, 2010, 174(1/3): 1–8
[63] HU Y N, CHENG H F, TAO S. The challenges and solutions for cadmium-contaminated rice in China: A critical review[J]. Environment International, 2016, 92/93: 515–532
[64] AHANGER M A, AKRAM N A, ASHRAF M, et al. Plant responses to environmental stresses—from gene to biotechnology[J]. AoB Plants, 2017, 9(4): plx025
[65] LUO J S, HUANG J, ZENG D L, et al. A defensin-like protein drives cadmium efflux and allocation in rice[J]. Nature Communications, 2018, 9(1): 645
[66] CLEMENS S, KIM E J, NEUMANN D, et al. Tolerance to toxic metals by a gene family of phytochelatin synthases from plants and yeast[J]. The EMBO Journal, 1999, 18(12): 3325–3333
[67] VATAMANIUK O K, MARI S, LU Y P, et al. AtPCS1, a phytochelatin synthase from: Isolation andreconstitution[J]. Proceedings of the National Academy of Sciences of the United States of America, 1999, 96(12): 7110–7115
[68] HEISS S, WACHTER A, BOGS J, et al. Phytochelatin synthase (PCS) protein is induced inleaves after prolonged Cd exposure[J]. Journal of Experimental Botany, 2003, 54(389): 1833–1839
[69] ZHANG H Y, XU W Z, GUO J B, et al. Coordinated responses of phytochelatins and metallothioneins to heavy metals in garlic seedlings[J]. Plant Science, 2005, 169(6): 1059–1065
[70] ISHIMARU Y, TAKAHASHI R, BASHIR K, et al. Characterizing the role of rice NRAMP5 in manganese, iron and cadmium transport[J]. Scientific Reports, 2012, 2: 286
[71] UENO D, YAMAJI N, KONO I, et al. Gene limiting cadmium accumulation in rice[J]. Proceedings of the National Academy of Sciences of the United States of America, 2010, 107(38): 16500–16505
[72] SATOH-NAGASAWA N, MORI M, NAKAZAWA N, et al. Mutations in rice () heavy metal ATPase 2 () restrict the translocation of zinc and cadmium[J]. Plant and Cell Physiology, 2012, 53(1): 213–224
[73] TAKAHASHI R, ISHIMARU Y, SHIMO H, et al. The OsHMA2 transporter is involved in root-to-shoot translocation of Zn and Cd in rice[J]. Plant Cell & Environment, 2012, 35(11): 1948–1957
[74] ÉVA C, SOLTIÁ, OSZVALD M, et al. Improved reactive aldehyde, salt and cadmium tolerance of transgenic barley due to the expression of aldo-keto reductase genes[J]. Acta Physiologiae Plantarum, 2016, 38: 99
[75] LI Z S, LU Y P, ZHEN R G, et al. A new pathway for vacuolar cadmium sequestration in: YCF1-catalyzed transport of bis (glutathionato) cadmium[J]. Proceedings of the National Academy of Sciences of the United States of America, 1997, 94(1): 42–47
[76] MORI T, IWAMOTO K, WAKAOJI S, et al. Characterization of a novel gene involved in cadmium accumulation screened from sponge-associated bacterial metagenome[J]. Gene, 2016, 576(2): 618–625
[77] KANG S H, SINGH S, KIM J Y, et al. Bacteria metabolically engineered for enhanced phytochelatin production and cadmium accumulation[J]. Applied and Environmental Microbiology, 2007, 73(19): 6317–6320
[78] SPEIR T W, VAN SCHAIK A P, PERCIVAL H J, et al. Heavy metals in soil, plants and groundwater following high-rate sewage sludge application to land[J]. Water, Air, and Soil Pollution, 2003, 150(1/4): 319–358
[79] BANG J, HESTERBERG D. Dissolution of trace element contaminants from two coastal plain soils as affected by pH[J]. Journal of Environmental Quality, 2004, 33(3): 891–901
[80] WANG A S, ANGLE J S, CHANEY R L, et al. Soil pH effects on uptake of Cd and Zn by[J]. Plant and Soil, 2006, 281(1/2): 325–337
[81] DU LAING G, VANTHUYNE D R J, VANDECASTEELE B, et al. Influence of hydrological regime on pore water metal concentrations in a contaminated sediment-derived soil[J]. Environmental Pollution, 2007, 147(3): 615–625
[82] YANG J E, SKOUSEN J G, OK Y S, et al. Reclamation of abandoned coal mine waste in Korea using lime cake by-products[J]. Mine Water and the Environment, 2006, 25(4): 227–232
[83] ISLAM M N, TAKI G, NGUYEN X P, et al. Heavy metal stabilization in contaminated soil by treatment with calcined cockle shell[J]. Environmental Science and Pollution Research, 2017, 24(8): 7177–7183
[84] MOON D H, WAZNE M, CHEONG K H, et al. Stabilization of As-, Pb-, and Cu-contaminated soil using calcined oyster shells and steel slag[J]. Environmental Science and Pollution Research, 2015, 22(14): 11162–11169
[85] MAURIC A, LOTTERMOSER B G. Phosphate amendment of metalliferous waste rocks, Century Pb-Zn mine, Australia: Laboratory and field trials[J]. Applied Geochemistry, 2011, 26(1): 45–56
[86] OLIVA J, CAMA J, CORTINA J L, et al. Biogenic hydroxyapatite (Apatite Ⅱ™) dissolution kinetics and metal removal from acid mine drainage[J]. Journal of Hazardous Materials, 2012, 213/214: 7–18
[87] SELIM H M. Phosphate in Soils: Interaction with Micronutrients, Radionuclides and Heavy Metals[M]. London: CRC Press, 2015
[88] SUN Y B, ZHAO D, XU Y M, et al. Effects of sepiolite on stabilization remediation of heavy metal-contaminated soil and its ecological evaluation[J]. Frontiers of Environmental Science & Engineering, 2016, 10(1): 85–92
[89] CHEN S B, XU M G, MA Y B, et al. Evaluation of different phosphate amendments on availability of metals in contaminated soil[J]. Ecotoxicology and Environmental Safety, 2007, 67(2): 278–285
[90] GARAU G, CASTALDI P, SANTONA L, et al. Influence of red mud, zeolite and lime on heavy metal immobilization, culturable heterotrophic microbial populations and enzyme activities in a contaminated soil[J]. Geoderma, 2007, 142(1/2): 47–57
[91] ACIEGO PIETRI J C, BROOKES P C. Relationships between soil pH and microbial properties in a UK arable soil[J]. Soil Biology and Biochemistry, 2008, 40(7): 1856–1861
[92] CAO X D, MA L Q, SINGH S P, et al. Phosphate-induced lead immobilization from different lead minerals in soils under varying pH conditions[J]. Environmental Pollution, 2008, 152(1): 184–192
[93] ZENG G M, WAN J, HUANG D L, et al. Precipitation, adsorption and rhizosphere effect: The mechanisms for phosphate-induced Pb immobilization in soils — A review[J]. Journal of Hazardous Materials, 2017, 339: 354–367
[94] ROSEN C J, ALLAN D L. Exploring the benefits of organic nutrient sources for crop production and soil quality[J]. Horttechnology, 2007, 17(4): 422–430
[95] TANG X J, LI X, LIU X M, et al. Effects of inorganic and organic amendments on the uptake of lead and trace elements bygrown in an acidic red soil[J]. Chemosphere, 2015, 119: 177–183
[96] MORI M, KOTAKI K, GUNJI F, et al. Suppression of cadmium uptake in rice using fermented bark as a soil amendment[J]. Chemosphere, 2016, 148: 487–494
[97] YANG Z B, LIU L X, LYU Y F, et al. Metal availability, soil nutrient, and enzyme activity in response to application of organic amendments in Cd-contaminated soil[J]. Environmental Science and Pollution Research, 2018, 25(3): 2425–2435
[98] YAO Y, SUN Q, WANG C, et al. Evaluation of organic amendment on the effect of cadmium bioavailability in contaminated soils using the DGT technique and traditional methods[J]. Environmental Science and Pollution Research, 2017, 24(9): 7959–7968
[99] GRIMES S M, TAYLOR G H, COOPER J. The availability and binding of heavy metals in compost derived from household waste[J]. Journal of Chemical Technology and Biotechnology, 1999, 74(12): 1125–1130
[100] SONG Q J, GREENWAY G M. A study of the elemental leachability and retention capability of compost[J]. Journal of Environmental Monitoring, 2004, 6(1): 31–37
[101] LIU L, CHEN H S, CAI P, et al. Immobilization and phytotoxicity of Cd in contaminated soil amended with chicken manure compost[J]. Journal of Hazardous Materials, 2009, 163(2/3): 563–567
[102] YASMIN K K, ALI B, CUI X Q, et al. Impact of different feedstocks derived biochar amendment with cadmium low uptake affinity cultivar of pak choi (L.) on phytoavoidation of Cd to reduce potential dietary toxicity[J]. Ecotoxicology and Environmental Safety, 2017, 141: 129–138
[103] AMONETTE J E, JOSEPH S. Characteristics of biochar: Microchemical properties[M]//LEHMANN J, JOSEPHS. Biochar for Environmental Management, Science and Technology. London: Earthscan, 2009: 33–52
[104] BEESLEY L, MARMIROLI M. The immobilisation and retention of soluble arsenic, cadmium and zinc by biochar[J]. Environmental Pollution, 2011, 159(2): 474–480
[105] PARK J H, CHOPPALA G K, BOLAN N S, et al. Biochar reduces the bioavailability and phytotoxicity of heavy metals[J]. Plant and Soil, 2011, 348(1/2): 439–451
[106] HUANG J H, HSU S H, WANG S L. Effects of rice straw ash amendment on Cu solubility and distribution in flooded rice paddy soils[J]. Journal of Hazardous Materials, 2011, 186(2/3): 1801–1807
[107] LU K P, YANG X, SHEN J J, et al. Effect of bamboo and rice straw biochars on the bioavailability of Cd, Cu, Pb and Zn to[J]. Agriculture, Ecosystems & Environment, 2014, 191: 124–132
[108] PUGA A P, ABREU C A, MELO L C A, et al. Biochar application to a contaminated soil reduces the availability and plant uptake of zinc, lead and cadmium[J]. Journal of Environmental Management, 2015, 159: 86–93
[109] KHAN K Y, ALI B, CUI X Q, et al. Effect of biochar amendment on bioavailability and accumulation of cadmium and trace elements inL. (Chinese cabbage)[J]. Journal of Agricultural Science, 2016, 8(9): 23–36
[110] MA L, XU R K, JIANG J. Adsorption and desorption of Cu(Ⅱ) and Pb(Ⅱ) in paddy soils cultivated for various years in the subtropical China[J]. Journal of Environmental Sciences, 2010, 22(5): 689–695
[111] SUN J K, LIAN F, LIU Z Q, et al. Biochars derived from various crop straws: Characterization and Cd(Ⅱ) removal potential[J]. Ecotoxicology and Environmental Safety, 2014, 106: 226–231
[112] BUSINELLI M, CASCIARI F, BUSINELLI D, et al. Mechanisms of Pb (Ⅱ) sorption and desorption at some claysand goethite-water interfaces[J]. Agronomie, 2003, 23(3): 219–225
[113] HAMIDPOUR M, KALBASI M, AFYUNI M, et al. Sorption hysteresis of Cd(Ⅱ) and Pb(Ⅱ) on natural zeolite and bentonite[J]. Journal of Hazardous Materials, 2010, 181(1/3): 686–691
[114] MOZGAWA W. The influence of some heavy metals cations on the FTIR spectra of zeolites[J]. Journal of Molecular Structure, 2000, 555(1/3): 299–304
[115] SUN Y B, LI Y, XU Y M, et al.stabilization remediation of cadmium (Cd) and lead (Pb) co-contaminated paddy soil using bentonite[J]. Applied Clay Science, 2015, 105/106: 200–206
[116] 徐奕, 赵丹, 徐应明, 等. 膨润土对轻度镉污染土壤钝化修复效应研究[J]. 农业资源与环境学报, 2017, 34(1): 38–46XU Y, ZHAO D, XU Y M, et al. Immobilization and remediation of low-level Cd contaminated soil using bentonite[J]. Journal of Agricultural Resources and Environment, 2017, 34(1): 38–46
[117] 赵乾程, 杨欣, 曹田, 等. 土壤重金属污染原位钝化修复及效果评价进展研究[J]. 环境科学与管理, 2016, 41(12): 98–102 ZHAO Q C, YANG X, CAO T, et al. Advances in research on in situ immobilization of heavy metal in contaminated soil and its effect evaluation[J]. Environmental Science and Management, 2016, 41(12): 98–102
[118] SHARMA A, NAGPAL A K. Soil amendments: A tool to reduce heavy metal uptake in crops for production of safe food[J]. Reviews in Environmental Science and Bio/Technology, 2018, 17(1): 187–203
[119] ABU-ELSAOUD A M, NAFADY N A, ABDEL-AZEEM A M. Arbuscular mycorrhizal strategy for zinc mycoremediation and diminished translocation to shoots and grains in wheat[J]. PLoS One, 2017, 12(11): e0188220
[120] LIU L, LI J W, YUE F X, et al. Effects of arbuscular mycorrhizal inoculation and biochar amendment on maize growth, cadmium uptake and soil cadmium speciation in Cd-contaminated soil[J]. Chemosphere, 2018, 194: 495–503
[121] WU B, CHEN R, YAO Y, et al. Mycoremediation potential ofin soil co-contaminated with copper and naphthalene[J]. RSC Advances, 2015, 5(83): 67524–67531
[122] JOSHI P M, JUWARKAR A A. In vivo studies to elucidate the role of extracellular polymeric substances fromin immobilization of heavy metals[J]. Environmental Science & Technology, 2009, 43(15): 5884–5889
[123] LI Y, PANG H D, HE L Y, et al. Cd immobilization and reduced tissue Cd accumulation of rice (wuyun-23) in the presence of heavy metal-resistant bacteria[J]. Ecotoxicology and Environmental Safety, 2017, 138: 56–63
[124] ZHOU G T, XIA X, WANG H, et al. Immobilization of lead bysp. WH16–1 in pot experiments of Pb-contaminated paddy soil[J]. Water, Air, and Soil Pollution, 2016, 227: 339
[125] WANG Q, CHEN L, HE L Y, et al. Increased biomass and reduced heavy metal accumulation of edible tissues of vegetable crops in the presence of plant growth-promoting NeorhizobiumT1–17 and biochar[J]. Agriculture, Ecosystems & Environment, 2016, 228: 9–18
[126] WANG X H, NIE Z W, HE L Y, et al. Isolation of As-tolerant bacteria and their potentials of reducing As and Cd accumulation of edible tissues of vegetables in metal(loid)- contaminated soils[J]. Science of the Total Environment, 2017, 579: 179–189
[127] YIN Y R, HU Y Y, XIONG F. Sorption of Cu(Ⅱ) and Cd(Ⅱ) by extracellular polymeric substances (EPS) from[J]. International Biodeterioration & Biodegradation, 2011, 65(7): 1012–1018
[128] CHEN Y P, ZHANG P, GUO J S, et al. Functional groups characteristics of EPS in biofilm growing on different carriers[J]. Chemosphere, 2013, 92(6): 633–638
[129] LI N J, ZHANG X H, WANG D Q, et al. Contribution characteristics of the in situ extracellular polymeric substances (EPS) into Pb immobilization[J]. Bioprocess and Biosystems Engineering, 2017, 40(10): 1447–1452
[130] LI M, CHENG X H, GUO H X. Heavy metal removal by biomineralization of urease producing bacteria isolated from soil[J]. International Biodeterioration & Biodegradation, 2013, 76: 81–85
[131] QIAN X Y, FANG C L, HUANG M S, et al. Characterization of fungal-mediated carbonate precipitation in the biomineralization of chromate and lead from an aqueous solution and soil[J]. Journal of Cleaner Production, 2017, 164: 198–208
[132] RHEE Y J, HILLIER S, PENDLOWSKI H, et al. Pyromorphite formation in a fungal biofilm community growing on lead metal[J]. Environmental Microbiology, 2014, 16(5): 1441–1451
[133] RHEE Y J, HILLIER S, GADD G M. A new lead hydroxycarbonate produced during transformation of lead metal by the soil fungus[J]. Geomicrobiology Journal, 2016, 33(3/4): 250–260
[134] TANG L, ZENG G M, SHEN G L, et al. Simultaneous amperometric determination of lignin peroxidase and manganese peroxidase activities in compost bioremediation using artificial neural networks[J]. Analytica Chimica Acta, 2006, 579(1): 109–116
[135] COLES C A, YONG R N. Humic acid preparation, properties and interactions with metals lead and cadmium[J]. Engineering Geology, 2006, 85(1/2): 26–32
[136] CAMPITELLI P, CEPPI S. Effects of composting technologies on the chemical and physicochemical properties of humic acids[J]. Geoderma, 2008, 144(1/2): 325–333
[137] SOLER-ROVIRA P, MADEJÓN E, MADEJÓN P, et al.remediation of metal-contaminated soils with organic amendments: Role of humic acids in copper bioavailability[J]. Chemosphere, 2010, 79(8): 844–849
[138] ZHANG C S, XU Y, ZHAO M H, et al. Influence of inoculating white-rot fungi on organic matter transformations and mobility of heavy metals in sewage sludge based composting[J]. Journal of Hazardous Materials, 2018, 344: 163–168
[139] DAMODARAN D, SHETTY K V, MOHAN B R. Effect of chelaters on bioaccumulation of Cd(Ⅱ), Cu(Ⅱ), Cr(Ⅵ), Pb(Ⅱ) and Zn(Ⅱ) infrom soil[J]. International Biodeterioration & Biodegradation, 2013, 85: 182–188
[140] ZOTTI M, DI PIAZZA S, ROCCOTIELLO E, et al. Microfungi in highly copper-contaminated soils from an abandoned Fe-Cu sulphide mine: Growth responses, tolerance and bioaccumulation[J]. Chemosphere, 2014, 117: 471–476
[141] SHAHID M, POURRUT B, DUMAT C, et al. Heavy-metal- induced reactive oxygen species: Phytotoxicity and physicochemical changes in plants[M]//WHITACRE D M. Reviews of Environmental Contamination and Toxicology. Cham: Springer, 2014: 1–44
[142] DEVI S S, SREENIVASULU Y, RAO K V B. Protective role oflogibrachiatum (WT2) on lead induced oxidative stress inL.[J]. Indian Journal of Experimental Biology, 2017, 55(4): 235–241
[143] WU S L, CHEN B D, SUN Y Q, et al. Chromium resistance of dandelion (Diels.) and bermudagrass ([Linn.] Pers.) is enhanced by arbuscular mycorrhiza in Cr(Ⅵ)-contaminated soils[J]. Environmental Toxicology and Chemistry, 2014, 33(9): 2105–2113
[144] JONER E J, BRIONES R, LEYVAL C. Metal-binding capacity of arbuscular mycorrhizal mycelium[J]. Plant and Soil, 2000, 226(2): 227–234
[145] CHEN B D, CHRISTIE P, LI X L. A modified glass bead compartment cultivation system for studies on nutrient and trace metal uptake by arbuscular mycorrhiza[J]. Chemosphere, 2001, 42(2): 185–192
[146] NAYUKI K, CHEN B, OHTOMO R, et al. Cellular imaging of cadmium in resin sections of arbuscular mycorrhizas using synchrotron micro X-ray fluorescence[J]. Microbes & Environments, 2014, 29(1): 60–66
[147] WU S L, ZHANG X, CHEN B D, et al. Chromium immobilization by extraradical mycelium of arbuscular mycorrhiza contributes to plant chromium tolerance[J]. Environmental and Experimental Botany, 2016, 122: 10–18
[148] WANG F Y, WANG L, SHI Z Y, et al. Effects of AM inoculation and organic amendment, alone or in combination, on growth, P nutrition, and heavy-metal uptake of tobacco in Pb-Cd-contaminated soil[J]. Journal of Plant Growth Regulation, 2012, 31(4): 549–559
[149] KO M S, PARK H S, LEE J U. Influence of indigenous bacteria stimulation on arsenic immobilization in field study[J]. CATENA, 2017, 148: 46–51
[150] WANG T, SUN H W, MAO H J, et al. The immobilization of heavy metals in soil by bioaugmentation of a UV-mutant38 assisted by NovoGro biostimulation and changes of soil microbial community[J]. Journal of Hazardous Materials, 2014, 278: 483–490
[151] CHEN L, HE L Y, WANG Q, et al. Synergistic effects of plant growth-promotingT1-17 and immobilizers on the growth and heavy metal accumulation of edible tissues of hot pepper[J]. Journal of Hazardous Materials, 2016, 312: 123–131
[152] SENEVIRATNE M, WEERASUNDARA L, OK Y S, et al. Phytotoxicity attenuation inunder heavy metal stress at the presence of biochar and N fixing bacteria[J]. Journal of Environmental Management, 2017, 186: 293–300
[153] FIERER N, JACKSON R B. The diversity and biogeography of soil bacterial communities[J]. Proceedings of the National Academy of Sciences of the United States of America, 2006, 103(3): 626–631
[154] ROUSK J, BÅÅTH E, BROOKES P C, et al. Soil bacterial and fungal communities across a pH gradient in an arable soil[J]. The ISME Journal, 2010, 4(10): 1340–1351
[155] XU H J, WANG X H, LI H, et al. Biochar impacts soil microbial community composition and nitrogen cycling in an acidic soil planted with rape[J]. Environmental Science & Technology, 2014, 48(16): 9391–9399
[156] ELAHIAN F, REIISI S, SHAHIDI A, et al. High-throughput bioaccumulation, biotransformation, and production of silver and selenium nanoparticles using genetically engineered[J]. Nanomedicine: Nanotechnology, Biology and Medicine, 2017, 13(3): 853–861
[157] LI H, CONG Y, LIN J, et al. Enhanced tolerance and accumulation of heavy metal ions by engineeredexpressingphytochelatin synthase[J]. Journal of Basic Microbiology, 2015, 55(3): 398–405
[158] OLADIPO O G, AWOTOYE O O, OLAYINKA A, et al. Heavy metal tolerance potential ofstrains isolated from mining sites[J]. Bioremediation Journal, 2016, 20(4): 287–297
[159] BAN Y H, XU Z Y, YANG Y R, et al. Effect of dark septate endophytic funguson plant growth, photosynthesis and Pb tolerance of maize (L.)[J]. Pedosphere, 2017, 27(2): 283–292
[160] CECCHI G, ROCCOTIELLO E, DI PIAZZA S, et al. Assessment of Ni accumulation capability by fungi for a possible approach to remove metals from soils and waters[J]. Journal of Environmental Science and Health, Part B: Pesticides, Food Contaminants, and Agricultural Wastes, 2017, 52(3): 166–170
[161] MOHAMMADIAN E, AHARI A B, ARZANLOU M, et al. Tolerance to heavy metals in filamentous fungi isolated from contaminated mining soils in the Zanjan Province, Iran[J]. Chemosphere, 2017, 185: 290–296
[162] MOHANTY S, GHOSH S, NAYAK S, et al. Isolation, identification and screening of manganese solubilizing fungi from low-grade manganese ore deposits[J]. Geomicrobiology Journal, 2017, 34(4): 309–316
[163] MOHANTY S, GHOSH S, NAYAK S, et al. Bioleaching of manganese bysp. isolated from mining deposits[J]. Chemosphere, 2017, 172: 302–309
[164] KUMAR B L, GOPAL D V R S. Effective role of indigenous microorganisms for sustainable environment[J]. 3 Biotech, 2015, 5(6): 867–876
[165] JEONG S, MOON H S, SHIN D, et al. Survival of introduced phosphate-solubilizing bacteria (PSB) and their impact on microbial community structure during the phytoextraction of Cd-contaminated soil[J]. Journal of Hazardous Materials, 2013, 263: 441–449
[166] OLLER A L W, TALANO M A, AGOSTINI E. Screening of plant growth-promoting traits in arsenic-resistant bacteria isolated from the rhizosphere of soybean plants from Argentinean agricultural soil[J]. Plant and Soil, 2013, 369(1/2): 93–102
[167] MEHARG A A, HARTLEY-WHITAKER J. Arsenic uptake and metabolism in arsenic resistant and nonresistant plant species[J]. New Phytologist, 2002, 154(1): 29–43
[168] TU S, MA L Q. Interactive effects of pH, arsenic and phosphorus on uptake of As and P and growth of the arsenic hyperaccumulatorL. under hydroponic conditions[J]. Environmental and Experimental Botany, 2003, 50(3): 243–251
[169] CHATTERJEE J, CHATTERJEE C. Phytotoxicity of cobalt, chromium and copper in cauliflower[J]. Environmental Pollution, 2000, 109(1): 69–74
[170] QIAN H F, SUN Z Q, SUN L W, et al. Phosphorus availability changes chromium toxicity in the freshwater alga[J]. Chemosphere, 2013, 93(6): 885–891
[171] 董如茵, 徐应明, 王林, 等. 土施和喷施锌肥对镉低积累油菜吸收镉的影响[J]. 环境科学学报, 2015, 35(8): 2589–2596 DONG R Y, XU Y M, WANG L, et al. Effects of soil application and foliar spray of zinc fertilizer on cadmium uptake in a pakchoi cultivar with low cadmium accumulation[J]. Acta Scientiae Circumstantiae, 2015, 35(8): 2589–2596
[172] YANG J X, WANG L Q, WEI D P, et al. Foliar spraying and seed soaking of zinc fertilizers decreased cadmium accumulation in cucumbers grown in Cd-contaminated soils[J]. Soil and Sediment Contamination: An International Journal, 2011, 20(4): 400–410
[173] GUO G H, LEI M, CHEN T B, et al. Evaluation of different amendments and foliar fertilizer for immobilization of heavy metals in contaminated soils[J]. Journal of Soils and Sediments, 2018, 18(1): 239–247
[174] LIU J G, CAI H, MEI C C, et al. Effects of nano-silicon and common silicon on lead uptake and translocation in two rice cultivars[J]. Frontiers of Environmental Science & Engineering, 2015, 9(5): 905–911
[175] HART J J, WELCH R M, NORVELL W A, et al. Transport interactions between cadmium and zinc in roots of bread and durum wheat seedlings[J]. Physiologia Plantarum, 2002, 116(1): 73–78
[176] KUO S, HUANG B, BEMBENEK R. The availability to lettuce of zinc and cadmium in a zinc fertilizer[J]. Soil Science, 2004, 169(5): 363–373
[177] MA J F, TAMAI K, YAMAJI N, et al. A silicon transporter in rice[J]. Nature, 2006, 440(7084): 688–691
[178] Liu J G, ZHANG W, QU P, et al. Cadmium tolerance and accumulation in fifteen wetland plant species from cadmium-polluted water in constructed wetlands[J]. Frontiers of Environmental Science & Engineering, 2016, 10(2): 262–269
[179] CAKMAK I, KUTMAN U B. Agronomic biofortification of cereals with zinc: A review[J]. European Journal of Soil Science, 2018, 69(1): 172–180
[180] CHENG X D, GOLEMOVIC M, GILES F, et al. Organic arsenic lipid derivatives are more potent and less toxic than inorganic arsenic trioxide in preclinical testing[J]. Blood, 2004, 104(11): 1803
[181] TAKAHASHI Y, MINAMIKAWA R, HATTORI K H, et al. Arsenic behavior in paddy fields during the cycle of flooded and non-flooded periods[J]. Environmental Science & Technology, 2004, 38(4): 1038–1044
[182] XU X Y, MCGRATH S P, MEHARG A A, et al. Growing rice aerobically markedly decreases arsenic accumulation[J]. Environmental Science & Technology, 2008, 42(15): 5574–5579
[183] HU P J, OUYANG Y N, WU L H, et al. Effects of water management on arsenic and cadmium speciation and accumulation in an upland rice cultivar[J]. Journal of Environmental Sciences, 2015, 27: 225–231
[184] OUMA G, JERUTO P. Sustainable horticultural crop production through intercropping: The case of fruits and vegetable crops: A review[J]. Agriculture and Biology Journal of North America, 2010, 1(5): 1098–1105
[185] ALTIERI M A, NICHOLLS C. Biodiversity and Pest Management in Agroecosystems[M]. 2nd ed. New York: Food Products Press, 2004
[186] WANG S Q, WEI S H, JI D D, et al. Co-planting Cd contaminated field using hyperaccumulatorL. through interplant with low accumulation Welsh onion[J]. International Journal of Phytoremediation, 2015, 17(9): 879–884
[187] 谭建波, 陈兴, 郭先华, 等. 续断菊与玉米间作系统不同植物部位Cd、Pb分配特征[J]. 生态环境学报, 2015, 24(4): 700–707 TAN J B, CHEN X, GUO X H, et al. Distribution characteristics of Pb and Cd in different parts ofandin an intercropping system[J]. Ecology and Environmental Sciences, 2015, 24(4): 700–707
[188] ZHAN F D, QIN L, GUO X H, et al. Cadmium and lead accumulation and low-molecular-weight organic acids secreted by roots in an intercropping of a cadmium accumulatorL. withL.[J]. RSC Advances, 2016, 6(40): 33240–33248
[189] YANG X Y, MA S Y, LI J H. Effects of different soil remediation methods on inhibition of lead absorption and growth and quality ofL.[J]. Environmental Science and Pollution Research, 2017, 24(36): 28190–28196
[190] YANG B, SHU W S, YE Z H, et al. Growth and metal accumulation in vetiver and twospecies on lead/zinc mine tailings[J]. Chemosphere, 2003, 52(9): 1593–1600
[191] 汤福义, 林立金, 杨代宇, 等. 少花龙葵种间嫁接后代对小白菜生长及镉积累的影响[J]. 土壤通报, 2016, 47(1): 207–212TANG F Y, LIN L J, YANG D Y, et al. Effects of inter-species post-grafting generation ofon growth and cadmium accumulation of[J]. Chinese Journal of Soil Science, 2016, 47(1): 207–212
[192] 谷庆宝, 颜增光, 周友亚, 等. 美国超级基金制度及其污染场地环境管理[J]. 环境科学研究, 2007, 20(5): 84–88 GU Q B, YAN Z G, ZHOU Y Y, et al. Critical review of superfund act and environmental management of superfund sites[J]. Research of Environmental Science, 2007, 20(5): 84–88
Safe utilization of farmland contaminated with heavy metals in China: Progress and outlook*
YANG Shushen1,2†, SUN Yanqin3†, ZHENG Xin1, LI Xiaofang1**
(1. Center for Agricultural Resources Research, Institute of Genetics and Developmental Biology, Chinese Academy of Sciences / Key Laboratory of Agricultural Water Resources, Chinese Academy of Science, Shijiazhuang 050022, China; 2. University of Chinese Academy of Sciences, Beijing 100049, China; 3. Hebei Vocational College of Geology, Shijiazhuang 050081, China )
Farmland heavy metal pollution is now a serious problem in China. Food heavy metal contents in some agricultural regions exceed the national limits and threaten human health and the development of economy and society. Meanwhile, farmland resources are very limited in China. Therefore, farmland heavy metal pollution needs to be resolved urgently. Among available remediation tools, the physical or chemical ones are costly and are not suitable for the slightly / moderately contaminated farmland at a large scale. The phytoextraction method is cost-effective and environmentally friendly, but requires a long time. Overall, techniques aiming at heavy metal removal have limitations in solving heavy metal pollution in farmland. Safe utilization is to produce safe agricultural products, without removing the heavy metal content in soil purposefully. Safe utilization of heavy metal contaminated farmland is preferable for China at this stage. Cultivation of low-accumulation crops is an important option for the safe utilization scheme. A number of low-accumulation cultivars of grain crops and vegetables have been screened out in China. Genetic engineering has potential in breeding of low-metal-accumulation crops. Soil additives such as clay minerals, organic wastes and biochar can inhibit heavy metal uptake of crops by immobilization of heavy metals in soil, via ion-exchange, precipitation, etc. However, use of soil additives may impact soil qualities by changing soil structure, loss of nutrition and secondary pollution. Some microorganisms showing strongly resistant to heavy metals have already been used to inhibit the heavy metal uptake of crops. Genetically modified microbes possessing stronger immobilization ability should be paid more attention. Furthermore, agronomic strategies including fertilization, water management, and intercropping can transform the heavy metal forms in soil and influence the heavy metal uptake by crops. However, the crop yield and quality like nutrient content can also be influenced by the safe utilization measures. Efforts should be made to get a balance between low accumulation of heavy metals and crop yield and qualities. In the future, researches may focus on the integration of various remediation techniques for the large-scale field implementation.
Heavy metal contamination; Farmland; Safe utilization; Crops; Soil amendments; Microorganism; Agronomic strategy; Heavy metal immobilization
Supported by theNational Key Research and Development Program of China (2018YFD0800306), the ‘100 Talents Project’ of Chinese Academy of Sciences (Y726012203) and Hebei Science Fund for Distinguished Young Scholars (D2018503005)
Corresponding author, E-mail: xfli@sjziam.ac.cn
Jun. 19, 2018;
Jul. 4, 2018
10.13930/j.cnki.cjea.180573
K53
A
1671-3990(2018)10-1555-18
通信作者:李小方, 主要从事重金属污染环境修复的生态可持续、廉价、易于实施的现代生物技术研究。E-mail: xfli@sjziam.ac.cn
†同等贡献者: 杨树深, 主要从事重金属污染农田土壤修复与安全利用研究, E-mail: ssyang@sjziam.ac.cn; 孙衍芹, 主要从事生态环境修复技术研究, E-mail:dzzgdx@163.com
2018-06-19
2018-07-04
†Equal contributors
*国家重点研发计划项目(2018YFD0800306)、中国科学院率先行动“百人计划”(Y726012203)和河北杰出青年基金(D2018503005)资助
杨树深, 孙衍芹, 郑鑫, 李小方. 重金属污染农田安全利用: 进展与展望[J]. 中国生态农业学报, 2018, 26(10): 1555-1572
YANG S S, SUN Y Q, ZHENG X, LI X F. Safe utilization of farmland contaminated with heavy metals in China: Progress and outlook[J]. Chinese Journal of Eco-Agriculture, 2018, 26(10): 1555-1572
