Tail Waving Speed Affects Territorial Response in the Toad-headed Agama Phrynocephalus vlangalii
2018-09-27XiaQIUJinzhongFUandYinQI
Xia QIU, Jinzhong FU and Yin QI
Chengdu Institute of Biology, Chinese Academy of Sciences, Chengdu 610041, China
Abstract Territorial response affects a resident’s access to resources and mates, but the response level is likely flexible due to the trade-off between benefits and costs. Residents have to decide the response based on their own quality,resource benefits and intruder threat. Here we examined the association between territorial response and intruder threat using a newly developed 3D animation in male Phrynocephalus vlangalii. Three intruder stimuli (fast tail waving plus tail coil, slow tail waving plus tail coil and tail coil only) were animated based on display characters of real male P. vlangalii, and played to the resident in the field using a within subject design. We found that residents who faced fast tail waving plus tail coil displays more often emerged from their burrows compared with those faced slow tail waving plus tail coil displays and tail coil only displays, suggesting high speed tail waving display likely encodes high threat and triggers high territorial response. We also found that residents who faced slow tail waving plus tail coil displays opted to stay in the burrow compared with those faced tail coil only displays, suggesting slow tail waving display likely encodes low threat and functions in social conflict alleviation. Taken together, our study casts important insight on the association between intruder tail waving speed and resident’s territorial response, and provides some evidence that intruder tail waving speed is positively associated with its threat level.
Keywords tail display, territorial response, 3D animation, playback, Phrynocephalus vlangalii
1. Introduction
Territorial defense is expected to affect residents’ access to resources and mates, but the level of territorial response is likely flexible due to the trade-off between benefits and costs (Anderssonet al., 2002; Duckworth, 2006; Mareset al., 2012). Residents have to decide the territorial response according to their own physical condition,resource quality, and intruder threat. While dominant residents likely defend large areas to ensure access to high quality resources, inferior residents likely take flexible territorial tactics, and may or may not defend depending on resource quality and intruder threat (de Kortet al.,2009; Mowleset al., 2010; Reedyet al., 2017; Sinervo and Lively, 1999). Nevertheless, the effect of intruder threat on territorial defense is generally unknown, because it is difficult to decouple intruder threat from resource quality and resident condition (Behret al., 2009; Illes and Yunes-Jimenez, 2009; Reedyet al., 2017). To test for intruder threat effects, it is essential to manipulate the intruder threat while controlling for resident morphology and resource quality.
Using display behavior as an indicator of intruder threat can facilitate this research. To avoid the risk of injury associated with physical combat during territorial defense, many species rely on displays that allow individuals to assess one another (e.g. claw waving in crabPagurus bernhardus, Mowleset al., 2010; push up in Jacky dragonAmphibolurus muricatus, Peters and Ord,2003). Display measurements, such as speed, function as important indicators of individual bite force (Andersonet al., 2008), fight ability (Briffa and Fortescue, 2017;Lailvauxet al., 2004; Mowleset al., 2010), endurance,and social status (Perryet al., 2004). With the advancement of computer technology, display behavior during territorial defense can be re-created and animated using 3D animation. Using the playback approach, the effect of intruder threat on territorial response can be tested while keeping other factors constant (Nelsonet al.,2010; Peters and Evans, 2003; Watanabe and Troje, 2006).A similar method has been used in the Jacky dragonA.muricatus, in which aggressive and submission displays of intruders were animated and played to the resident, and territorial response was found to vary with the intruder displays (Van Dyk and Evans, 2008). Similarly, the drum display of intruders in downy woodpecker (Dryobates pubescens) was engineered and played to the resident,and the residents were found to adjust their territorial response according to the drum speed of intruders, with high speed drums evoking high territorial response from residents (Schuppeet al., 2017).
Phrynocephalus vlangaliiis an excellent model for testing the association between territorial response and intruder threat. Male and femaleP. vlangaliidig burrows,which they defend using complex tail displays (Qiet al., 2011). There are clear patterns in the use of tail displays. For example, the display of male and female lizards generally begins with low tail coil, followed by high tail coil, and ends with low tail coil. Nevertheless,the display patterns vary with social contexts. During aggressive male-male competition and male courtship,males always showcase fast tail waving after high tail coil. Occasionally, juveniles and females show slow tail waving during social interaction (Peterset al., 2016). The speed of tail displays evidently signals male social status inP. vlangalii(Qiet al., 2011), and reflects important information on individual sprint speed, bite force, and burrow quality (Xia Qiu, unpublished data). Hence tail displays can be used as indicators of individual threat inP. vlangalii.
The objective of the present study was to examine whether male residents ofP. vlangaliiadjust their territorial response according to intruder threat. In order to manipulate intruder threat, we used a video playback approach (Ord and Evans, 2002), utilizing 3D animations of displaying lizards (Van Dyk and Evans, 2008). Three stimuli, fast tail waving plus tail coil, slow tail waving plus tail coil, and tail coil only, were designed and animated to reflect varying intruder threat according to the social scenarios they are used (Figure 1A, B). Stimuli were played back to the resident male lizards (Figure 1C).We predicted that intruders with fast tail waving plus tail coil displays encode high threat and would provoke high territorial response in residents. We also predicted that intruders with slow tail waving plus tail coil encodes low threat and thereby provoke low territorial response.
2. Materials and methods
2.1. Study siteThe study was conducted in Xiaman Conservation Station of Zoige Wetland Nature Reserve in Sichuan province, China (33°43'25.0" N, 102°29'04.0"E, elevation: 3 475 m above sea level). In Zoige,P.vlangaliimainly occurs in sand dunes nested within wetland and grassland, with a high population density of approximately 3 000 lizards/ha (Wuet al., 2002). The vegetation around sand dunes is predominantly composed ofDracocephalum heterophyllumandCarex aridula,occasionally alongsideAstagalus sutchenensis,Anaphalis lacteal,Vicia cracca,Morina kokonorica,Oxytropsis glabra,Linum stelleroidesandClematis tangutica. The climate in this area is characterized by a short spring and summer (four months, from April to July) and a long autumn and winter (eight months, from August to March of the following year).
2.2. Animation designWe animated the tail displays using the methods described by Bianet al. (2017).Briefly, we constructed a wireframe model ofP. vlangaliibased on morphological data, and used photographs of lizard skin as the texture. To re-create the dynamics of the display, we used position data from 3D reconstructions ofP. vlangaliidisplay movements (Peterset al., 2016).Three different display bouts were created that exhibited fast tail waving (15.99 cm/s) plus tail coiling (2.77 cm/s,FTW hereafter), slow tail waving (2.20 cm/s) plus tail coiling (2.77 cm/s, STW hereafter), and tail coiling only(2.77 cm/s, TCO hereafter, Figure 1A) . Tail waving at fast speed was nearly eight times faster than at slow speed, but within the normal range (Peterset al., 2016).We predicted these three stimuli (FTW, TCO and STW)represent three levels of intruder threat, from high to low, according to different social scenarios they are used(Peterset al., 2016). Each bout, regardless of display type, was 20 s duration and was repeated five times with inter-bout intervals of 2-8 s, resulting in final sequences of 11 m duration (Figure 1A). All animated displays were saved as MP4 files and presented using a tablet personal computer (brand Teclast, tablet PC).
2.3. Experiment procedureWe examined the territorial response of adult male resident (>50mm snout-vent length, SVL) lizard to three different animation playback stimuli using a within subject design in July and August 2015. Each resident experienced all three animated stimuli(FTW, STW, or TCO), and the order of presentation was randomized between trials (Caselliet al., 2015).Before trials, we searched the study area for resident males and captured them by noose. The body mass and snout-vent length (SVL) were measured respectively using an electronic scale (MAXN, precision 0.01g) and a clear plastic ruler as soon as they were caught. We then released the resident to its burrow and marked the burrow using a chopstick.
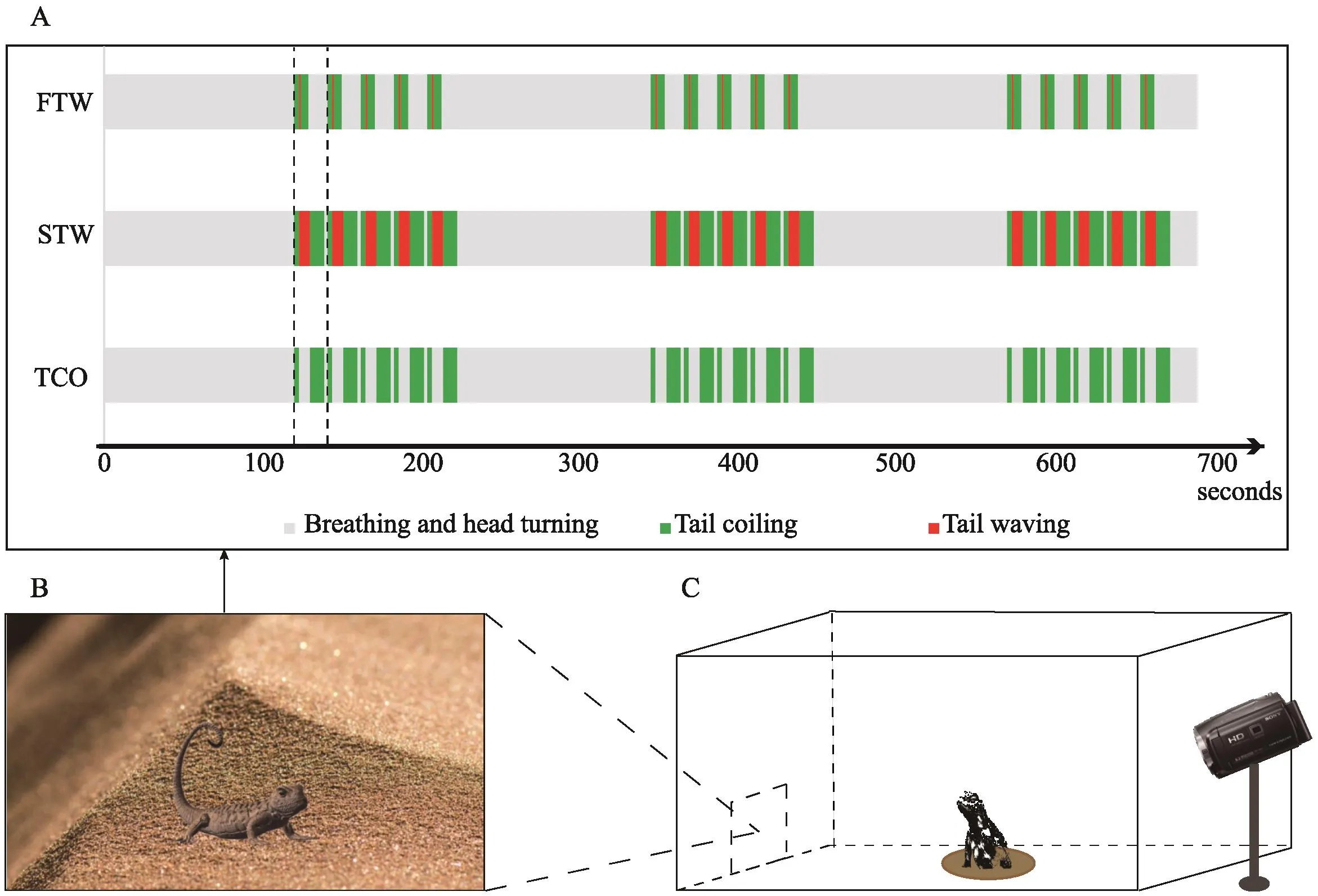
Figure 1 Schematic of A: animation design; B: screenshot of animation; and C: animation playback.
A minimum of 24 h later, we returned to the marked residents and tested their responses to each of the stimuli.Before presenting the animation, we ensured the presence of the resident with binoculars, before placing a portable wooden box (1.2×0.4×0.8 m3) around the burrow (Figure 1C). The top of the box is open to allow enough light for visual communication. The purpose of the box is to provide a consistent light environment for the display device and minimize the effect of light reflected from the screen. Two rectangle windows were open at different sides of the box for animation presentation and footage recording.
During the trial, we positioned the tablet in the box through one of the windows that allow the residents to view it at a distance of approximately 0.2 m to the burrow entrance. This can avoid variation in responses due to different receiver distances. After a 10 minutes’acclimation period (with tablet being off), we started the animation via remote control. The resident territorial response was monitored and recorded using a Sony HDV PJ670 camcorder. Each trial was ended after the resident emerged and stood towards the animation, or lasting ten minutes if the resident did not emerge. We waited at least 15 minutes before presenting the next animation, which is consistent with natural display rates of one every 20 minutes inP. vlangalii(Qiet al., 2011). We measured the latency to emerge from burrow as territorial response. A maximum of 10 minutes’ latency was given if the resident did not emerge. All trials were conducted between 10:00 am to 17:00 pm when lizards were active, to reduce the potential physiological effect.
2.4. Statistical analysisIn total, the latency to emerge was measured in 22 resident males and 66 trials were carried out. Because lizards did not emerge in high proportion of trials (36/66), we simultaneously consider the emerging probability as binary territorial response,with “1” representing emerging, while “0” representing not emerging. We examined the association between territorial response and the intruder stimuli using a generalized linear mixed-effect model in “lme4” package(Bateset al., 2005) in R version 3.12 (R Core Team,2018). For the latency to emerge, we considered the intruder stimuli and resident SVL as fixed effects, with lizard identity as random effect and a Poisson error distribution. For the binary response, we considered the intruder stimuli and resident SVL as fixed effects,with lizard identity as random effect and a binomial error distribution. The resident SVL was considered as a covariate, because the body size likely affects the response of resident in lizards (Aubretet al., 2014; de Barroset al., 2010; Delaney and Warner, 2017). The significance of fixed effects was obtained from the model and when significant we examined pairwise contrasts from the model by changing the reference value using relevel function. Using our model, we predicted the territorial response to test how model predictions were related to our intruder stimuli using predict function.
3. Results
The predicted latency to emerge and emerging probability as a function of intruder stimuli are presented in Figure 2A and B. We found that resident males were quicker to emerge from burrows when faced FTW stimulus compared with those faced STW stimulus and TCO stimulus (Figure 2A, Table 1). In addition, resident males were quicker to emerge from burrows when faced TCO stimulus compared with those faced STW stimulus(Table 1). Similarly, we found that resident males more often emerge from burrows when faced FTW stimulus compared with those faced STW stimulus (Figure 2B,Table 2). We also found a trend that resident males were less likely to emerge from burrows when faced STW stimulus compared with those faced TCO stimulus(Table 2).
4. Discussion
Our results are consistent with our prediction that tail waving speed reflects varying intruder threat and residents’ territorial response is flexible according to intruder tail waving speed. This is inferred from the fact that resident males who faced intruders with high speed tail waving are quicker and more likely to emerge from their burrows. We also find some evidence that slow tail waving display likely encodes low threat, because resident males who faced slow tail waving intruders opt to stay in the burrow compared with those faced tail coil only displays.
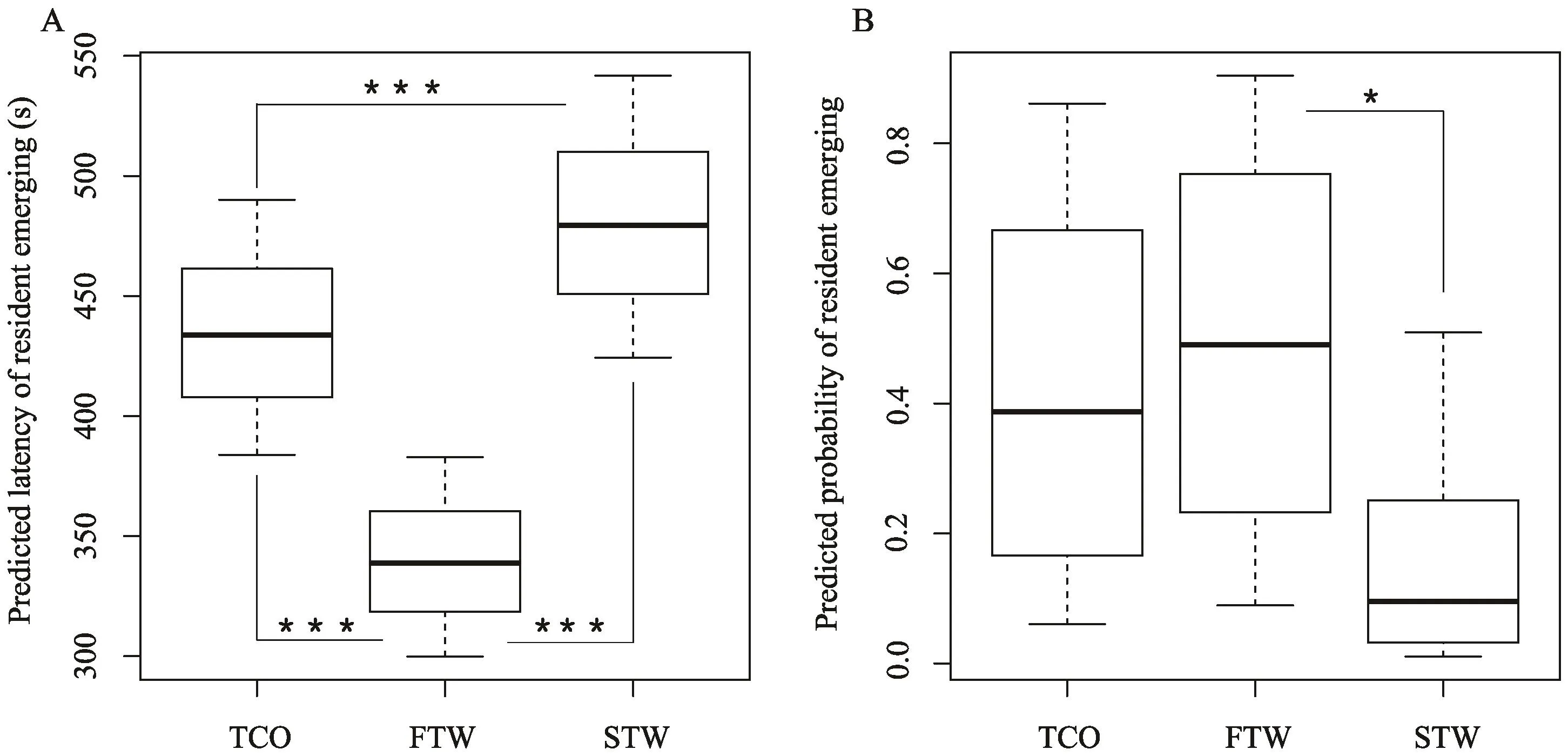
Figure 2 Comparison of A: predicted latency of resident emerging; B: predicted probability of resident emerging among different intruder stimuli. TCO: tail coil only; FTW: fast tail waving plus tail coil; STW: slow tail waving plus tail coil. Data are plotted as median, 10th, 25th,75th, and 90th percentiles within each treatment. The asterisk represents significant effects, * means P < 0.05, *** means P < 0.001.
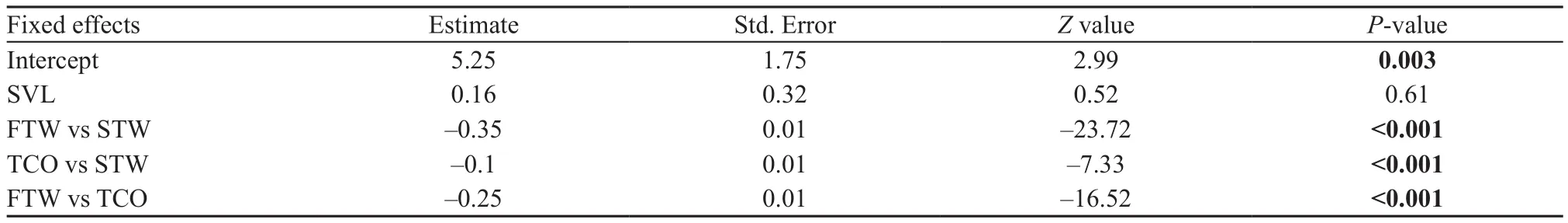
Table 1 Summary of generalized linear mixed-effect model testing for the effect of intruder threat on resident emerging latency in Phrynocephalus vlangalii.
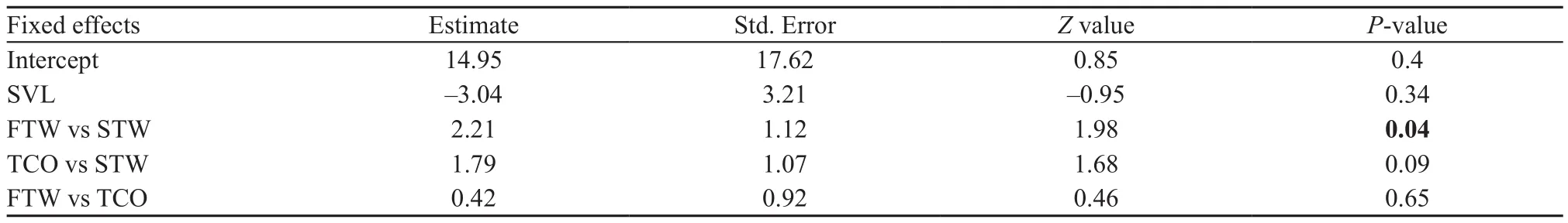
Table 2 Summary of generalized linear mixed-effect model testing for the effect of intruder threat on resident emerging probability in Phrynocephalus vlangalii.
Resident males respond differently to different displays, with high speed waving evoking high territorial response inP. vlangalii. This is conformed to the theory that territorial response is a trade-off process, during which residents would assess the rivals and choose a proper response. This is particular true when the physical condition and social dominance differ markedly between residents and intruders (Anderssonet al., 2002;Duckworth, 2006; Mareset al., 2012). This would either increase the efficiency of territorial defense or avoid unecessary costs (Stevens, 2013). Similar signal based territorial trade-offs have been found in several other species. For example, a female bat (Vespertilio sinensis)assesses the competitive ability of rivals via call rate during agonistic interactions. If the residents defend food resource using high call rate, the intruder female would choose to retreat (Luoet al., 2017). In banded wren (Thryophilus pleurostictus), the intensity of acoustic signals represents individual performance, the residents are less likely to approach the high intensity stimulus compared with the low-and medium-intensity stimuli (de Kortet al., 2009).
Slow tail waving likely encodes low threat, because residents exhibit low response to slow waving tail display compared with tail coil only display. This is corroborated by the result from another stage-encounter study usingP. vlangalii, which shows that low tail waving display associates (12/25 aggressive interactions) with social reconciliation and represents low probability of conflict escalation (Yin Qi, unpublished data). Similar display has been found in Jacky dragonA. muricatus, in which subordinate males produce high rates of slow armwaves during intra-sexual interactions than dominant males (Van Dyk and Evans, 2008). In male mandrillsMandrillus sphinx, males use facial and gestural signals to communicate dominance and sub-ordinance, avoiding escalated conflict (Setchell and Wickings, 2005).
As a conclusion, we find the links between residents’territorial response and intruder tail display. The high speed tail waving likely encodes high threat and triggers high territorial response in residentP. vlangalii, while the low speed tail waving likely encodes low threat and functions in alleviating social conflict. In the future, a specific experiment should be carried out to ascertain why different displays provoke different territorial response and whether different territorial response can ensure different fitness to residents.
AcknowledgementThanks to Yayong WU for field assistance in data collection. Thanks to Richard PETERS for display digitizing and manuscript comments. This project is supported by grants from the National Natural Science Foundation of China (grant numbers 31572273,31872233 to Yin QI).
杂志排行
Asian Herpetological Research的其它文章
- Massive Molecular Parallel Evolution of the HSP90AA1 Gene between High-elevation Anurans
- Predatory Cues Influence the Behavioral Responses and Metamorphic Traits of Polypedates maculatus (Anura:Rhacophoridae)
- Perch Associated Expression of Phenotypic Plasticity in Limb Development and Sprint Speed in Agamid Lizard Calotes versicolor: A Laboratory Study
- A Review for Life-history Traits Variation in Frogs Especially for Anurans in China
- Population Size, Genetic Diversity and Molecular Evidence of a Recent Population Bottleneck in Hynobius chinensis, an Endangered Salamander Species
- A New Species of Genus Microhyla (Amphibia: Anura:Microhylidae) from Zhejiang Province, China
