雅浦海沟沉积有机碳垂向分布及其指示意义
2018-09-25刘诚刚孙承君陈建芳潘建明韩正兵
吴 彬,李 栋*,赵 军,刘诚刚,孙承君,陈建芳,潘建明,韩正兵,胡 佶
雅浦海沟沉积有机碳垂向分布及其指示意义
吴 彬1,2,李 栋1,2*,赵 军1,2,刘诚刚1,2,孙承君3,陈建芳1,2,潘建明1,2,韩正兵1,2,胡 佶1,2
(1.国家海洋局第二海洋研究所海洋生态与环境实验室,浙江 杭州 310012;2.国家海洋局海洋生态系统与生物地球化学重点实验室,浙江 杭州 310012;3.国家海洋局第一海洋研究所海洋生态中心,山东 青岛 266237)
利用蛟龙号载人深潜器采集了西太平洋雅浦海沟沉积柱样,分析了总有机碳(TOC)、δ13C、粒度和比表面积(SSA)等参数,结合端元混合模型、降解模型及主成分分析(PCA),探讨不同来源有机碳(OC)垂向分布及其影响因素,估算了TOC降解速率、累积速率及通量.结果表明,雅浦海沟TOC%均值为(0.31%±0.10%),与浅海环境相当.由于外源OC主要赋存于粉砂等大粒径颗粒物以及微生物的分解作用,TOC%与粉砂%正相关,与SSA值及粘土比例负相关.微生物来源OC是TOC的主要贡献者(52%±21%),其次为海源(37%±19%)和陆源(11%±4%)OC,3种来源OC含量均随深度增加而降低.PCA结果指出,微生物来源与外源OC相对贡献间不耦合,表明海底地下水输送的OC可能是海沟微生物的重要营养源.主导沉积物中TOC垂向分布的难降解OC降解速率约为0.0012a-1,略高于普通大洋环境,但较浅海环境更低.雅浦海沟TOC累积速率为1.8×10-5gC/(cm2·a),沉降通量为2.2×109gC/a.
雅浦海沟;沉积有机碳;垂向分布;来源;降解
大洋中水深超过6000m的海沟等超深渊带,面积仅占海洋总面积的1%,但其深度变化范围覆盖海洋总深度的45%,在地质、化学和生物等方面具有独特特征,在海洋碳循环中扮演重要角色[1-5].海沟内部的V形构造及其引起的漏斗效应,使海沟易于收集周围海底平原经海底浊流和海洋生物泵等途径输运来的有机碳(OC),成为大洋OC的捕获器[6-8].海沟沉积物中积累的有机质又为该环境中底栖生物提供了理想栖息环境和可观食物,使得海沟成为活跃的微生物反应器[7,9-12].同时,由于两侧板块的相向聚合运动使得海沟内部沉积OC可以在地质时间尺度上被裹挟进入熔融地幔,从而将地表OC输入地球深处,使得海沟成为地球岩石圈潜在终极碳汇[13-14].然而,由于超深渊带中极端高压和低温环境及其所带来的仪器操作和样品采集等技术挑战,已有的研究多限于海沟沉积OC含量空间分布及与底栖生物相互影响等方面[1,7-8,15-18],针对海沟中沉积OC生物地球化学循环的研究鲜见报道[7-8,17,19-20].
雅浦海沟紧邻雅浦群岛和帕劳群岛,北起马里亚纳海沟南端,南到帕劳海沟北端,发育有典型俯冲构造侵蚀[21].相较马里亚纳等海沟,同为世界级海沟的雅浦海沟在有机生物地球化学方面的研究未见报道.本研究利用蛟龙号载人深潜器在雅浦海沟内采集无扰动沉积柱状样,通过对沉积物中总有机碳(TOC)、总氮(TN)、碳稳定同位素(δ13C)、粒度组成和比表面积(SSA)等地球化学参数的测定,利用基于蒙特卡洛(Monte-Carlo,MC)模拟的三端元混合模型和主成分分析(PCA),探讨沉积物中海源、陆源和微生物来源OC含量的垂向变化及其可能影响因素.使用多G降解模型和质量累积模型估算海沟中沉积OC的降解速率、累积速率及通量,以获取对雅浦海沟等超深渊环境中碳的生物地球化学循环的深入认识.
1 材料与方法
于2017年6月9日,利用向阳红10号海洋科研调查船及蛟龙号载人深潜器,在雅浦海沟6501m水深处使用PushCore取样器采集了DIVE150站位的无扰动柱状沉积物(长23cm,137.597°E,8.051°N)(采样站位如图1所示).沉积物样品在船上按照1~2cm间隔进行切割,装入封口袋并在-20℃条件下保存至岸上实验室分析.
将沉积物装入具有固定体积和质量的立方体容器中,测定沉积物湿密度,随后冻干测定干密度和含水率.取未研磨冻干沉积物,加入过量双氧水除去颗粒物中OC,使用六偏磷酸钠分散剂浸泡分散,在上机测试前超声分散30s后使用马尔文Mastersizer3000激光粒度仪进行粒度测定.取未研磨冻干沉积物,于350℃条件下加热除去有机质,随后使用贝士德3H-2000PS1型比表面自动分析仪,采用静态容量法来测定SSA.取适量研磨均匀冻干沉积物,使用盐酸熏蒸除去无机碳酸盐,60℃条件下加热烘干除去水分和盐酸,使用Thermo Flash EA 1112HT氮/碳元素分析仪以及DELTA V Advantage稳定同位素比质谱仪对样品中TOC%(标准偏差小于0.08%)、TN%(标准偏差小于0.07%)和δ13C(精度优于±0.2‰)进行测定.
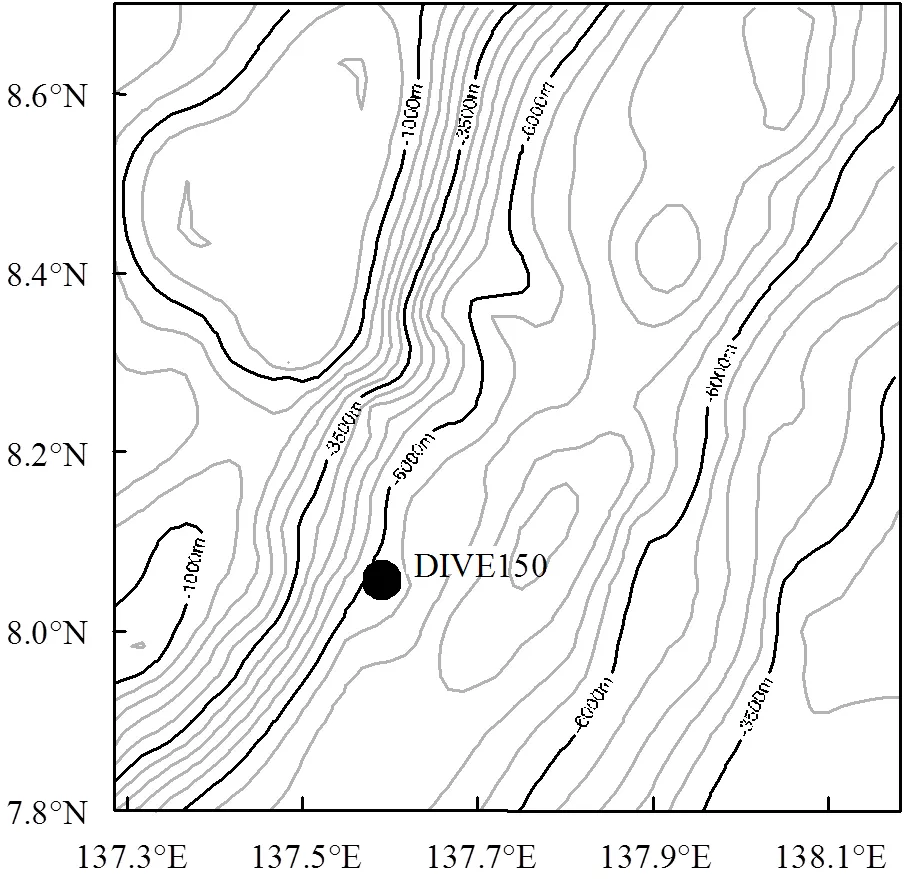
图1 柱状沉积物采样站位
本研究使用基于δ13C和TOC/TN摩尔比(C/N比)的三端元混合模型,分析海沟沉积物中陆源、海洋浮游植物和微生物(包括微生物活体细胞、死亡后碎屑以及改造后的外源OC)3种来源OC对沉积物TOC的相对贡献,具体方法参照文献[22].模型公式如下:
13C海源×海源+13C陆源×陆源+13C微生物×微生物=13C样品(1)
C/N海源×海源+C/N陆源×陆源+C/N微生物×微生物=C/N样品(2)
海源+陆源+微生物=1 (3)
式中:13C样品和C/N样品分别代表所测样品的13C值和C/N比,海源、陆源和微生物分别代表海洋、陆地和微生物来源OC的百分比贡献(%),其他符号代表相应来源的端元值.同时,为了尽量减少固定端元值对模型计算带来的误差,使用MC模拟方法来校正端元值的变化对不同来源OC比例计算的可能影响[23-26].该方法假定某种来源OC的13C或者C/N比端元值在给定范围内的变化符合正态分布,在此区间内按正态分布随机取值,然后选取部分或全部进行计算,计算过程所选端元值要同时满足公式(1)~(3),计算结果(海源、陆源和微生物)同样符合正态分布[27-28].使用该方法可以得到3种组分对沉积TOC相对贡献的平均值、标准偏差、中值、最大值和最小值[29-30].多次重复运行同一样品所得平均值相对标准偏差小于0.03%,表明该方法具有较高统计学稳定性.端元模型所使用端元值δ13C海源、δ13C陆源、和δ13C微生物分别设定为-20.0‰±1.0‰[29,31]、-25.6‰± 1.0‰[32]和-20.0‰±1.0‰[33-36],C/N海源、C/N陆源和C/ N微生物设定为6.5±0.3[29]、14.6±0.8[29]和3.7±0.2[37-38].其中,本研究假设沉积物柱样微生物群落以异养微生物为主,其碳源为易降解海源OC,根据微生物δ13C值取决于其碳源的原理[33-36],本研究未对微生物来源和海源OC的δ13C值进行区分,并主要依据C/N比值对海源和微生物来源OC进行区分.采样视频资料显示目标研究区域没有冷泉或者热液口发育,沉积物δ13C值高于-22.7‰,且荧光定量PCR结果表明化能合成微生物(例如氨氧化细菌和古菌)仅占总微生物丰度的极少部分(未发表数据).同时,在富氧、沉积速率较低以及有机质充足的海底表层沉积物中,异养代谢微生物在群落结构中的贡献较高[39-41].因而该方法可在一定程度上用来计算微生物来源OC的相对贡献.
本研究中使用多G降解模型对沉积物中TOC降解过程进行拟合[42],模型方程:
C=+0·(1-)·e(-k1·t)+0··e(-k2·t)(4)
式中:0表征柱状沉积物表层TOC含量,表征沉积物剖面无穷深处TOC含量的恒定值,C表征沉积物沉降时间(a)后对应深度沉积物中TOC含量,1(a-1)和2(a-1)分别代表易降解和难降解两部分TOC组分的降解速率,(0<<1)表示难降解组分所占百分比,(cm)表示沉积物深度,s(cm/a)代表沉积速率,=/s.
2 结果
2.1 TOC、TN、δ13C、SSA和粒度组成
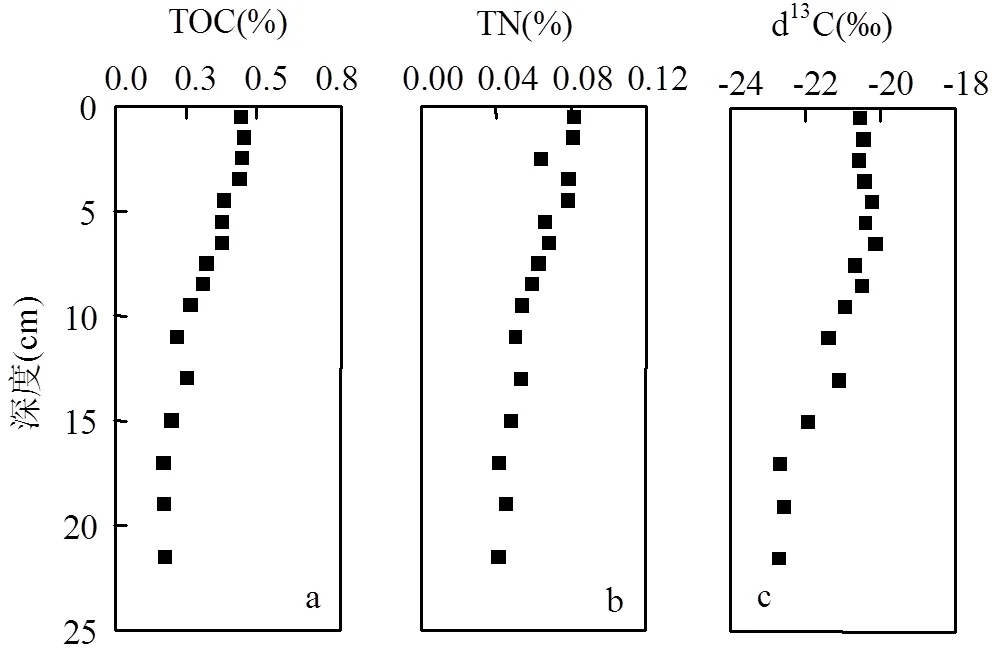
如图2所示,TOC和TN的含量变化范围分别为0.17%~0.46%(平均值0.31%±0.10%)和0.04%~ 0.08%(平均值0.06%±0.01%).δ13C为-22.70‰~ -20.13‰,平均-21.07‰±0.87‰.C/N比为4.4~8.2(平均值5.9±0.9).SSA平均值为(50.8±4.3)m2/g,在44.8~58.2m2/g范围内变化.单位SSA的TOC载荷(TOC/SSA)变化范围为0.03~0.10mgC/m2(平均值(0.06±0.03)mgC/m2).沉积物粒度组成以粉砂(4~63μm)为主(43.1%~55.2%,均值49.4%±4.0%),粘土(<4μm)次之(48.8%~28.7%,均值38.6%±5.9%),砂(>63μm)仅占6.3%~22.5%(11.9%±4.1%).各参数均呈现出由上向下的降低趋势,并最终趋于稳定.
2.2 三端元混合模型和MC模拟
利用基于MC模拟的三端元混合模型对海沟不同来源OC百分比和含量的定量计算结果如图3所示.DIVE150站位沉积物中海源、陆源和微生物来源OC在TOC中相对贡献分别为37%±19%(变化范围8%~59%)、11%±4%(变化范围6%~24%)和52%± 21%(变化范围16%~86%).沉积物中海源、陆源和微生物来源OC含量分别为0.13%±0.09% (0.01%~ 0.27%)、0.04%±0.02% (0.01%~0.11%)和0.14%± 0.03%(0.07%~0.19%).陆源垂向变化不明显,海源在7.5cm以浅波动变化,7.5cm以深显著降低至稳定值,而微生物在6.5cm以浅无明显变化,以深则显著升高到80%以上.沉积物中海源和陆源OC含量分别与其百分比例具有相似垂向分布,微生物来源OC含量呈逐渐降低的变化趋势.整体而言,8.5cm以浅沉积物以海源OC贡献为主,而8.5cm以深以微生物来源OC为主.
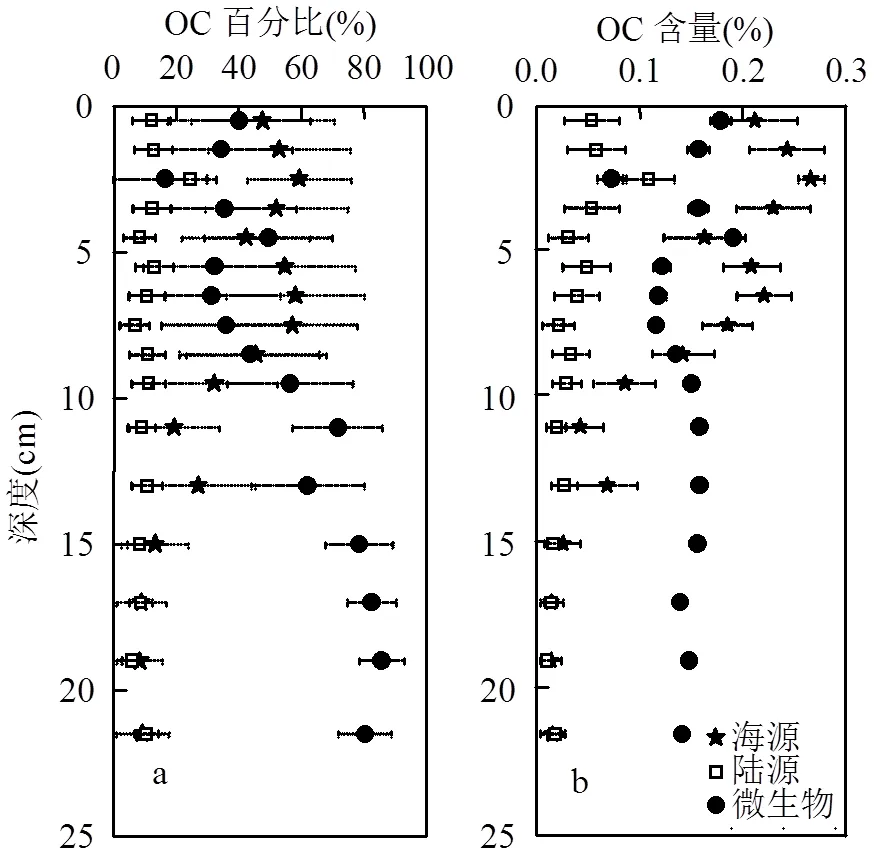
图3 基于MC模拟计算所得柱状沉积物中海源、陆源和微生物来源OC的百分比(a)以及含量(b)的垂向分布
2.3 PCA分析
对DIVE150站位沉积物中19个参数进行PCA分析,结果表明前2个主成分的累积贡献率为87%.其中,主成分1和2分别解释了数据集总变异的75%和12%(图4).主成分1在颗粒物粒级及其相关参数上具有显著区分度,而主成分2则主要在OC来源上存在不同负荷,正负荷表征陆源和海源等外源物质输入,而负值则表征沉积物中微生物来源OC贡献.
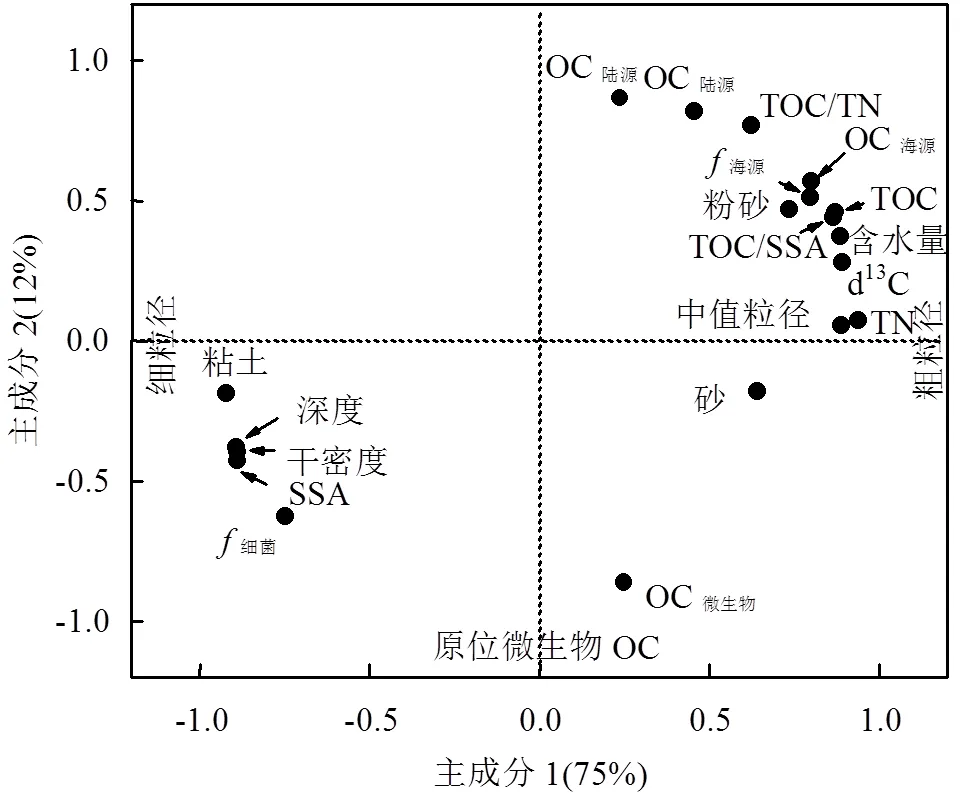
图4 基于主成分分析所得沉积柱状样中不同参数的负荷分布
2.4 降解模型
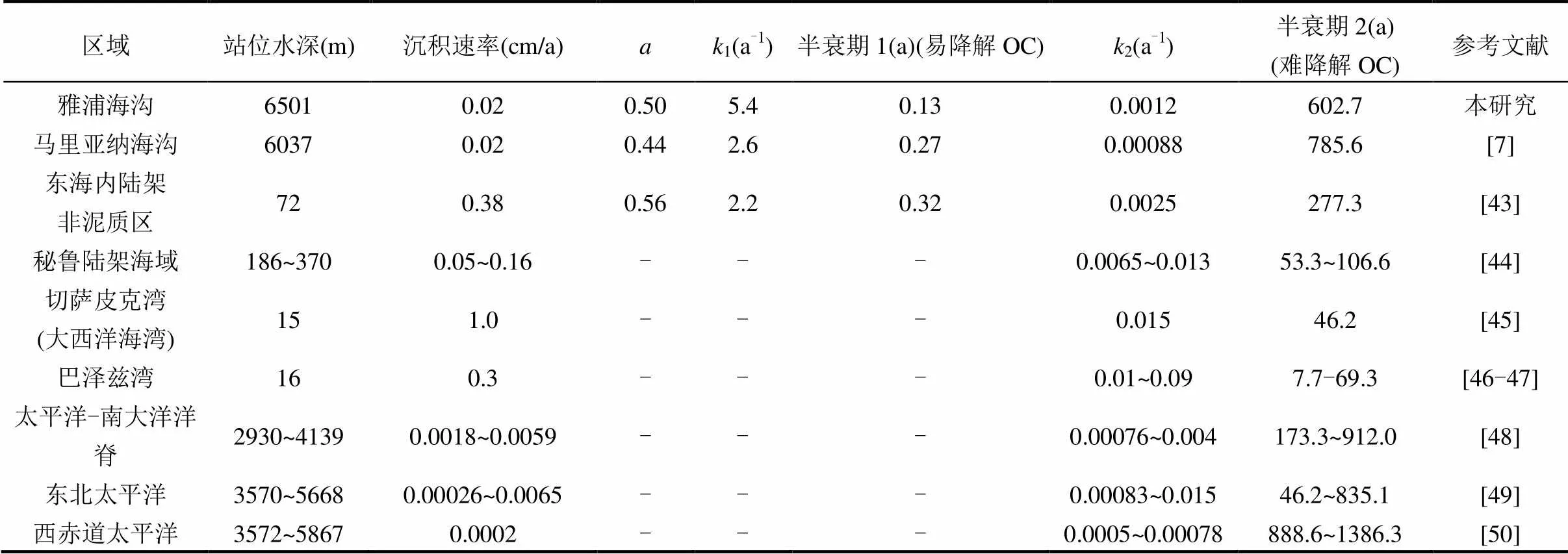
表1 世界不同海域沉积柱状样中TOC的降解参数比较
注:为难降解OC所占比例,1(a-1)为易降解OC的降解速率,2(a-1)为难降解OC降解速率,-表示未计算.
由于雅浦海沟紧邻马里亚纳海沟南端,两者均位于西太平洋暖池和热带环流海区,具有相似的初级生产力和沉降通量,因此,本研究使用与马里亚纳海沟南部区域相似水深处(6037m)的沉积速率(约为0.02cm/a)[7,17],对雅浦海沟沉积TOC降解速率进行估算.计算结果如表1所示.沉积物中TOC剖面符合指数型降解分布,与多G降解模型方程拟合度较高(2=0.93),沉积物中易降解OC降解速率1为5.4a-1,难降解OC降解速率2为0.0012a-1,且难降解OC的降解过程对TOC的垂向分布影响显著.
3 讨论
3.1 雅浦海沟沉积OC的组成及其影响因素
海沟等超深渊环境是深海沉积OC的潜在捕获器.DIVE150站位柱样0~4cm沉积物中TOC含量稳定在0.44%以上,与邻近的马里亚纳海沟相当(~0.45%)[7],但明显低于日本海沟(~1.0%)[9]、中美洲海沟(2.0%~4.0%)[51]和卡里亚科海沟(~3.0%)[52].与陆地距离的远近和真光层初级生产力的差异是造成该趋势的主要原因.雅浦海沟紧邻马里亚纳海沟,同处于西太平洋暖池和热带环流海区,具有相似的初级生产力(分别为82gC/(m2·a)和59gC/(m2·a))和颗粒OC沉降通量(分别为(0.56±0.29)gC/(m2·a)和(0.55±0.20)gC/ (m2·a))[53-54],且均位于西太平洋深水环流通道中[40],因而具有相似水动力环境并导致了相似TOC含量.而日本海沟、中美洲海沟以及卡里亚科海沟由于邻近陆地并处于上升流区域,真光层水体初级生产力较高(例如日本海沟193gC/(m2·a)和卡里亚科海沟190gC/(m2·a)),导致较高的颗粒OC沉降通量(日本海沟(3.05±0.91)gC/(m2·a)、卡里亚科海沟(0.77± 0.46)gC/(m2·a))和沉积物TOC含量[40,51-52].此外,与具有较高初级生产力的我国东海内陆架海域(200m以浅,0.2%~0.6%)[30]、黄海(140m以浅,0.1%~1.1%)[55]和南极半岛东北海域(2000~3000m,0.4%~0.6%)[56]等浅海海洋环境同样具有可比性,并显著高于普通开阔大洋海底沉积物(5000m,0.1%~0.3%)[11,57],表明海沟V型构造对海底沉积OC的物理汇聚作用显著.
沉积物粒度组成和微生物活动对海沟沉积物中TOC垂向分布具有显著影响.由PCA分析结果可知(图4),沉积物粒径大小及相关参数是影响沉积有机质垂向分布的主要因素.沉积物TOC含量与中值粒径大小,特别是粉砂粒级%(4~63μm)正相关,而与SSA大小以及粘土粒级%(<4μm)负相关(图5).这与在陆架边缘海等海洋环境中所得中值粒径越低、粘土粒级越多、SSA越高会导致TOC含量越高的规律相反[30,58].该现象可能是由海沟沉积物含水率、水动力环境、外源OC输入以及微生物活动等多因素共同造成的.如图4所示,含水率、外源OC百分比例和含量、TOC和TN含量、中值粒径以及粉砂比例均分布在负荷分布图的第一象限,表明受沉积物压实效应影响,沉积物含水率自上而下逐渐降低(从80%降低至60%),虽然海沟沉积物上覆水水体流速整体较低(cm/s量级),但依然会对表层沉积物造成一定程度的冲刷,导致沉积物粒径自上向下的降低(图2f).相比粒径更小的微生物来源颗粒物,富含OC的新鲜外源有机质可能更多的赋存于粒径更大的粉砂粒级颗粒物上(4~63μm).同时,海沟沉积物上覆水来源于南极绕极深层水[40],溶解氧浓度较高(~5.1mg/L)且溶氧渗透深度较大[7],使得海沟等深海沉积物中好氧矿化成为有机质分解的唯一重要路径[7,40,59-60],因而在活跃微生物新陈代谢作用影响下,外源OC含量迅速降低,而微生物则显著升高.同时,微生物和SSA、粘土比例等参数均位于负荷分布图的第3象限,表明微生物在SSA高的粘土粒级颗粒物表面更活跃,这与已有的研究结果相同,即SSA越大越利于OC的附着以及被微生物吸收利用[61-63].因而形成了海沟环境中粒径与TOC含量等参数间独特的关系和分布特征.
原位微生物来源OC相对贡献与外源OC输入相对贡献间的去耦合(图4),以及8.5cm以下微生物来源OC的显著贡献(>50%),在一定程度上表明除了经颗粒物自然沉降或海底浊流等过程输运来的陆地和海洋初级生产来源OC外,还有经其他途径输运而来的OC供给来维持海沟沉积物小生境中微生物的生命活动.作为连通地球内部和表层的窗口和通道,超深渊带中碳库可能受到深部生物圈和海底地下水物质输送的调控[64-73].例如,洋壳上层沉积物及岩层是地球上容量最大的连续含水层系统,其中存在着受潮汐驱动的可贯穿几百米厚沉积物层的海底地下水流动(流速达到10cm/d),这些发生于沉积物间隙中的水体流动,能够促进大洋底层水体与海底地下水之间利用沉积物内部的裂隙和其他可渗透性的通道,进行溶解态和颗粒态的物质交换(如O2、NO3-、溶解态和颗粒态OC等),从而更新海沟沉积物中碳等营养物质,并对沉积物中微生物生物量以及有机质的降解产生影响[39,73-76].图4中微生物与粘土比例的显著正相关(=0.82)即表明小粒径颗粒物及其附着的OC对微生物生命活动的重要作用.雅浦海沟沉积物较高的含水率和松软质地,更为其中物质交换提供了基础.另外,作为地球上最大的微生物碳储库,地球总生物量的50%~ 66%存在于深部生物圈中,其中海底沉积物又是地球上最为广袤的微生物孵化器和栖息地[39].本研究沉积物中显著微生物来源OC相对贡献与该结论相一致.
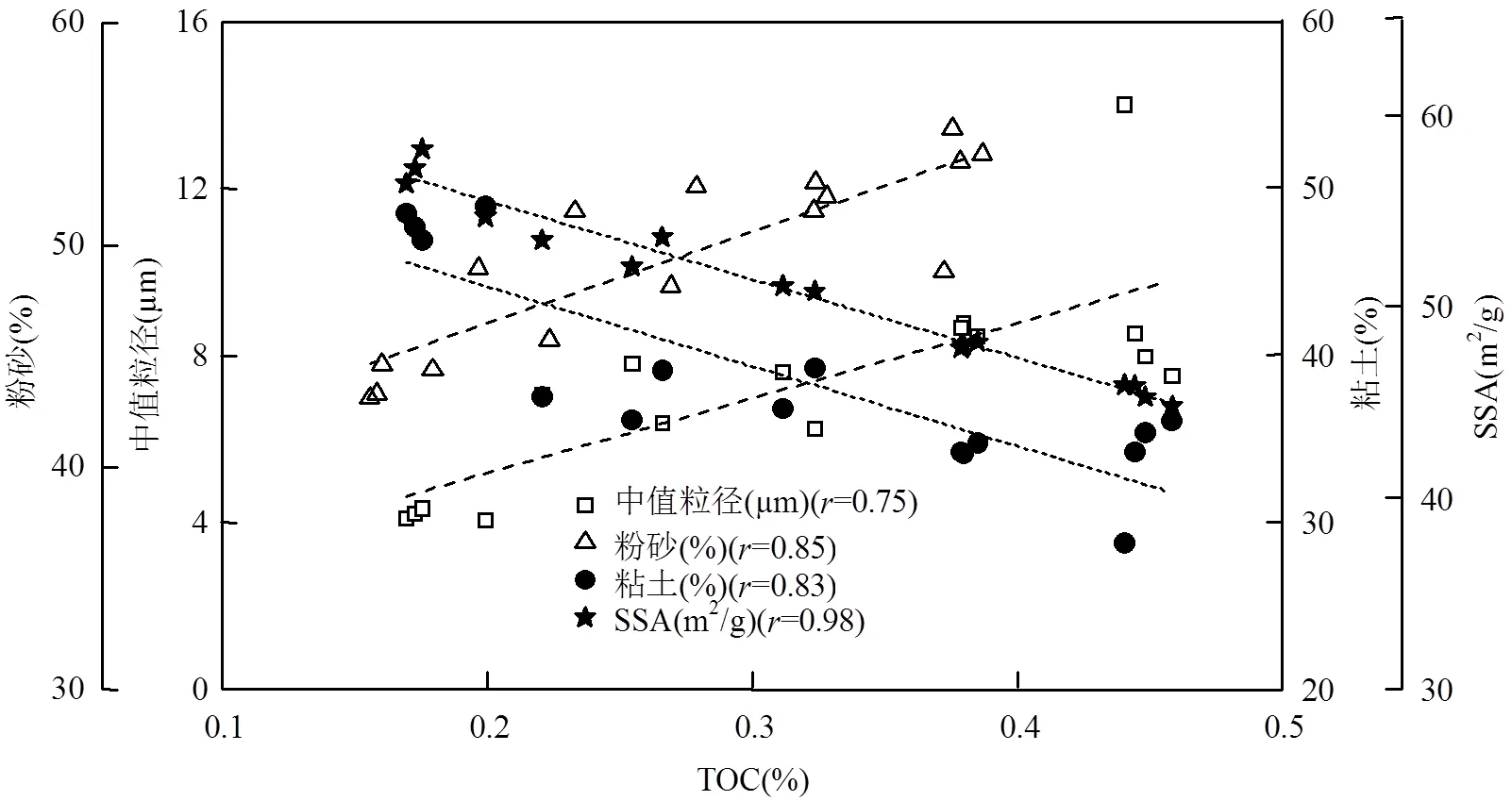
图5 沉积柱状样中值粒径、粉砂、粘土比例与TOC间的相关性
3.2 雅浦海沟沉积TOC的降解与埋藏
沉积OC的组成特征会决定沉积物中再矿化过程损失掉的OC的来源.本研究对沉积物中TOC/ SSA比值与δ13C·TOC/SSA比值进行线性拟合(图6),根据拟合方程的斜率判断再矿化过程损失的OC的来源特征[77-78].从图6可以看出,随着沉积OC再矿化过程(TOC/SSA比值由表层到底层逐渐降低)的进行,沉积物中被微生物降解和利用的OC的δ13C值约为-19.5‰,表明海沟沉积物中海源OC被优先降解.可能原因是沉积物总碳库中易降解的小分子海源OC贡献(37%±19%)较高,而难降解大分子陆源OC百分比例相对较低(陆源=11%±4%),具有低C/N比的微生物会更倾向于分解利用含氮量高的海源有机质.虽然有研究表明,在距离陆地较近的海沟中,陆源有机质能够作为一种特殊食物来源促进海沟中高效、专性分解陆源高等植物来源有机质生物的发育,例如马里亚纳海沟中专门摄食陆源木质碎屑的可可螺科腹足类和片脚类等动物[17,79-83].同时,发生于海洋环境中的激发效应同样也会促进海底沉积物中陆源有机质的快速降解[84-86].但本研究中,可能由于沉积物中陆源OC贡献以及微生物多样性较低(未发表数据),且缺少能够专性降解陆源OC的微生物,因而导致陆源OC被较好的保存(图3).
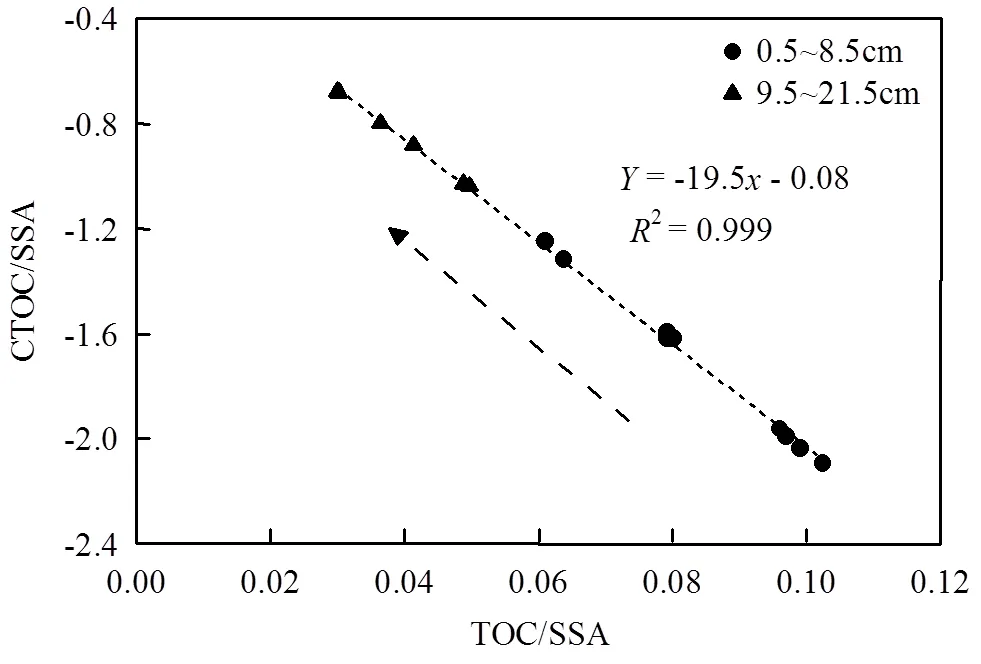
图6 沉积柱状样中δ13C·TOC/SSA与TOC/SSA之间的相关性
沉积速率以及沉降颗粒物的物质组成对海沟沉积OC的降解和埋藏具有重要影响.多G降解模型结果表明,雅浦海沟与马里亚纳海沟同等深度沉积物中难降解OC所占比例相似,降解速率较为一致(表1).雅浦海沟和马里亚纳海沟沉积OC的降解速率显著高于太平洋-南大洋洋脊、东北太平洋、西赤道太平洋等6000m以浅的普通开阔大洋的降解速率,同时,又较陆架边缘海以及近岸海湾等浅海环境更低(表1).沉积速率可以通过对沉积物中孔隙度、溶解氧渗透深度、微生物群落组成和沉积OC矿化路径等方式的调控,影响沉积OC降解速率[41,49].虽然一般情况下海底沉积物沉积速率与水深成反比[41,87],但由于海沟地形的汇聚作用,使得海沟中颗粒物沉积速率显著的高于周边海底平原(表1).与海底平原相比,较高的沉积速率和随水深降低的水体流速导致了海沟沉积物中高含水率(~72%)和高溶解氧渗透深度(马里亚纳海沟6037m水深处沉积物5cm深度溶解氧浓度依然高达~170μmol/L)[7].同时,海沟中较海底平原更高的TOC含量和微生物丰度[7],更为沉积物中以好氧呼吸为主要代谢途径的微生物活动提供了有力保证[39,60,88],因而导致了海沟沉积OC具有较普通开阔大洋更高的降解速率.与陆架边缘海等浅海环境相比,虽然两者沉积物中海源OC均有重要贡献,但边缘海环境中更高的初级生产力(例如东海161gC/(m2·a))导致其沉积物中易降解海源OC绝对含量显著高于海沟(东海内陆架约为0.4%,而雅浦海沟约为0.13%±0.09%)[30,89-91],因而可降解的潜力更高.同时,陆架浅水区中强烈的水动力条件和沉积物再悬浮[78,92],以及更高的微生物丰度(比海沟高3个数量级)[7,93-95],更使得陆架边缘海成为沉积OC的焚化炉[78,96],因而其沉积OC降解速率显著高于海沟.
利用雅浦海沟沉积物干、湿密度、TOC含量以及沉积物沉积速率等参数,并结合质量累积模型[17,41],估算了雅浦海沟中沉积OC的累积速率、埋藏效率和埋藏通量.结果表明,雅浦海沟表层沉积物中OC的累积速率约为1.8×10-5gC/(cm2·a),与Jamieson[40]、Lutz等[97]模型计算的雅浦海沟颗粒OC沉降速率处于同一量级(约为5.6×10-5gC/ (cm2·a)),并与邻近马里亚纳海沟南部区域沉积OC累积速率(0.35×10-5gC/(cm2·a)~1.5×10-5gC/(cm2·a))基本相当[17],约为全球陆架边缘海颗粒OC累积速率平均值的4.3%(4.2×10-4gC/(cm2·a))[98]以及大洋(>1000m)颗粒OC累积速率平均值的18%(1.0× 10-4gC/(cm2·a))[99].结合雅浦海沟海域面积(1.21× 104km2)[40]计算可得雅浦海沟颗粒OC沉降通量约为2.2×109gC/a.同时,由于雅浦海沟初级生产力和面积均显著低于全球其他海沟[40],因而其他海沟将具有更可观的颗粒OC沉降速率和通量,表明超深渊带对深海大洋颗粒OC具有显著的汇聚作用.
4 结论
4.1 雅浦海沟TOC含量与邻近马里亚纳海沟相当,但显著低于日本海沟和中美洲海沟等海沟.漏斗效应导致雅浦海沟表层沉积OC含量显著高于开阔大洋,并与陆架边缘海等浅海环境具有可比性.
4.2 沉积物粒度组成和微生物活动对海沟沉积OC垂向分布影响显著.海沟沉积物TOC%与中值粒径大小,特别是粉砂%具有较强正相关关系,而与SSA大小以及粘土粒级%呈现显著负相关.
4.3 微生物来源OC是海沟沉积OC主要来源,其次为海洋浮游植物来源和陆地来源OC.原位微生物来源OC相对贡献与外源OC输入相对贡献间的去耦合,以及8.5cm以下微生物来源OC的显著贡献(>50%),在一定程度上表明海底地下水的流动及其输运而来的OC对维持海沟沉积物小生境中微生物的生命活动具有重要影响.
4.4 海源OC是雅浦海沟沉积物中微生物优先利用的营养来源.雅浦海沟沉积TOC降解速率高于普通开阔大洋,又较陆架边缘海以及近岸海湾等浅海环境更低.
[1] Jamieson A J, Fujii T, Solan M, et al. First findings of decapod crustacea in the hadal zone [J]. Deep Sea Research Part I Oceanographic Research Papers, 2009,56(4):641-647.
[2] Jamieson A J, Fujii T. Trench connection [J]. Biology Letters, 2011,7(5):641-643.
[3] Canganella F, Kato C. Deep-Ocean Ecosystems [M]. United States: John Wiley and Sons, 2014,doi:10.1002/9780470015902.a0003.
[4] Gallo N D, Cameron J, Hardy K, et al. Submersible- and lander- observed community patterns in the Mariana and New Britain trenches: Influence of productivity and depth on epibenthic and scavenging communities [J]. Deep Sea Research Part I Oceanographic Research Papers, 2015,99:119-133.
[5] Leduc D, Rowden A A, Glud R N, et al. Comparison between infaunal communities of the deep floor and edge of the Tonga Trench: Possible effects of differences in organic matter supply [J]. Deep Sea Research Part I Oceanographic Research Papers, 2015,116:264-275.
[6] Danovaro R, Croce N D, Dell’Anno A, et al. A depocenter of organic matter at 7800m depth in the SE Pacific Ocean [J]. Deep Sea Research Part I Oceanographic Research Papers, 2003,50(12):1411-1420.
[7] Glud R N, Wenzhöfer F, Middelboe M, et al. High rates of microbial carbon turnover in sediments in the deepest oceanic trench on Earth [J]. Nature Geoscience, 2013,6(4):284-288.
[8] Oguri K, Kawamura K, Sakaguchi A, et al. Hadal disturbance in the Japan Trench induced by the 2011 Tohoku–Oki Earthquake [J]. Scientific Reports, 2013,3(1915):1915.
[9] Brassell S C, Comet P A, Eglinton G, et al. The origin and fate of lipids in the Japan Trench [J]. Physics and Chemistry of the Earth, 1980, 12:375-392.
[10] Brassell S C E G, Maxwell J R. Preliminary lipid analyses of two quaternary sediments from the middle America trench, southern mexico transect [J]. Initial Reposts Deep Sea Drilling Project, 1981, 66:557-580.
[11] 张海生,于培松,倪建宇,等.赤道太平洋沉积有机质物性、源性的地球化学及其沉积环境对比研究[J]. 海洋学报, 2008,30(6):60-68.
[12] Nunoura T, Takaki Y, Hirai M, et al. Hadal biosphere: Insight into the microbial ecosystem in the deepest ocean on Earth [J]. Proceedings of the National Academy of Sciences of the United States of America, 2015,112(11):E1230.
[13] Jamieson A J, Fujii T, Mayor D J, et al. Hadal trenches: the ecology of the deepest places on Earth [J]. Trends in Ecology and Evolution, 2010,25(3):190-197.
[14] Čížková H, Bina C R. Geodynamics of trench advance: Insights from a Philippine-Sea-style geometry [J]. Earth and Planetary Science Letters, 2015,430:408-415.
[15] Gage J D. Food inputs, utilization, carbon flow and energetics. In: Tyler P A (Ed), Ecosystems of the Deep Oceans, Ecosystems of the World [M]. Amsterdam: Elsevier, 2003:313-380.
[16] Itou M, Matsumura I, Noriki S. A large flux of particulate matter in the deep Japan Trench observed just after the 1994Sanriku-Oki earthquake [J]. Deep Sea Research Part I Oceanographic Research Papers, 2000,47(10):1987-1998.
[17] Luo M, Gieskes J, Chen L, et al. Provenances, distribution, and accumulation of organic matter in the southern Mariana Trench rim and slope: Implication for carbon cycle and burial in hadal trenches [J]. Marine Geology, 2017,386(2):486-498.
[18] Jamieson, Alan J. Ecology of Deep Oceans: Hadal Trenches [M]. United States: John Wiley and Sons, 2011,doi:10.1002/9780470015902. a0023606.
[19] Hulme S M, Wheat C G, Fryer P, et al. Pore water chemistry of the Mariana serpentinite mud volcanoes: A window to the seismogenic zone [J]. Geochemistry Geophysics Geosystems, 2010,11(1):414-431.
[20] 李 栋,赵 军,刘诚刚,等.超深渊生境特征及生物地球化学过程研究进展[J]. 地球科学, 2018,doi:10.3799/dqkx.2018.196.
[21] Kobayashi K. Origin of the Palau and Yap trench-arc systems [J]. Geophysical Journal International, 2004,157(3):1303-1315.
[22] 潘慧慧,姚 鹏,赵 彬,等.基于水淘选分级的长江口最大浑浊带附近颗粒有机碳的来源、分布和保存[J]. 海洋学报, 2015,37(4):1-15.
[23] Andersson A. A systematic examination of a random sampling strategy for source apportionment calculations [J]. Science of the Total Environment, 2011,s(412-413):232-238.
[24] Carreira R S, Wagener A L R, Readman J W, et al. Changes in the sedimentary organic carbon pool of a fertilized tropical estuary, Guanabara Bay, Brazil: an elemental, isotopic and molecular marker approach [J]. Marine Chemistry, 2002,79(3/4):207-227.
[25] Hu L, Guo Z, Feng J, et al. Distributions and sources of bulk organic matter and aliphatic hydrocarbons in surface sediments of the Bohai Sea, China [J]. Marine Chemistry, 2009,113(3/4):197-211.
[26] Hu B, Li J, Zhao J, et al. Late Holocene elemental and isotopic carbon and nitrogen records from the East China Sea inner shelf: Implications for monsoon and upwelling [J]. Marine Chemistry, 2014,162(6): 60-70.
[27] Vonk J E, S Nchezgarc A L, Semiletov I, et al. Molecular and radiocarbon constraints on sources and degradation of terrestrial organic carbon along the Kolyma paleoriver transect, East Siberian Sea [J]. Biogeosciences Discussions, 2010,7(4):3153-3166.
[28] Karlsson E S, Charkin A, Dudarev O, et al. Carbon isotopes and lipid biomarker investigation of sources, transport and degradation of terrestrial organic matter in the Buor-Khaya Bay, SE Laptev Sea [J]. Biogeosciences Discussions, 2011,8(8):3463-3496.
[29] Li X, Bianchi T S, Allison M A, et al. Composition, abundance and age of total organic carbon in surface sediments from the inner shelf of the East China Sea [J]. Marine Chemistry, 2012,s(145-147):37-52.
[30] Li D, Yao P, Bianchi T S, et al. Organic carbon cycling in sediments of the Changjiang Estuary and adjacent shelf: Implication for the influence of Three Gorges Dam [J]. Journal of Marine Systems, 2014, 139(139):409-419.
[31] Tesi T, Miserocchi S, Go I M A, et al. Source, transport and fate of terrestrial organic carbon on the western Mediterranean Sea, Gulf of Lions, France [J]. Marine Chemistry, 2007,105(1/2):101-117.
[32] Xing L, Zhang H, Yuan Z, et al. Terrestrial and marine biomarker estimates of organic matter sources and distributions in surface sediments from the East China Sea shelf [J]. Continental Shelf Research, 2011,31(10):1106-1115.
[33] Taipale S J, Peltomaa E, Hiltunen M, et al. Inferring phytoplankton, terrestrial plant and bacteria bulk δ¹³C values from compound specific analyses of lipids and fatty acids [J]. Plos One, 2015,10(7):e0133974.
[34] Abraham W R, Hesse C, Pelz O. Ratios of Carbon Isotopes in Microbial Lipids as, an Indicator of Substrate Usage [J]. Applied and Environmental Microbiology, 1998,64(11):4202-4209.
[35] Blair N, Leu A, Muñoz E, et al. Carbon isotopic fractionation in heterotrophic microbial metabolism [J]. Applied and Environmental Microbiology, 1985,50(4):996-1001.
[36] Coffin R B, Velinsky D J, Devereux R, et al. Stable carbon isotope analysis of nucleic acids to trace sources of dissolved substrates used by estuarine bacteria [J]. Applied and Environmental Microbiology, 1990,56(7):2012-2020.
[37] Lee S, Fuhrman J A. Relationships between Biovolume and Biomass of Naturally Derived Marine Bacterioplankton [J]. Applied and Environmental Microbiology, 1987,53(6):1298-1303.
[38] Goñi A, Hedges J I. Sources and reactivities of marine-derived organic matter in coastal sediments as determined by alkaline CuO oxidation [J]. Geochimica Et Cosmochimica Acta, 1995,59(14):2965-2981.
[39] 方家松,张 利.探索深部生物圈[J]. 中国科学:地球科学, 2011, 6:9-18.
[40] Jamieson A. The hadal zone: life in the deepest oceans [M]. England: Cambridge University Press, 2015.
[41] Tromp J. Normal-mode splitting observations from the great 1994Bolivia and Kuril Islands earthquakes: constraints on the structure of the mantle and inner core [J]. GSA Today, 1995,5:137.
[42] Li D, Yao P, Bianchi T S, et al. Historical reconstruction of organic carbon inputs to the East China Sea inner-shelf: Implications for anthropogenic activities and regional climate variability [J]. Holocene, 2015,25(12):1869-1881.
[43] 李 栋.长江口—东海内陆架沉积有机碳的生物地球化学过程及生态环境演变历史的重建[D]. 青岛:中国海洋大学, 2015.
[44] Reimers C E, Suess E. Late Quaternary Fluctuations in the Cycling of Organic matter off Central Peru: A Proto-Kerogen Record [M]. United States:Springer, 1983.
[45] Zimmerman A R, Canuel E A. A geochemical record of eutrophication and anoxia in Chesapeake Bay sediments: anthropogenic influence on organic matter composition [J]. Marine Chemistry, 2000,69(1):117- 137.
[46] Andersen B L, Broffitt B. Carbon cycling in coastal sediments: 1. A quantitative estimate of the remineralization of organic carbon in the sediments of Buzzards Bay, MA [J]. Geochimica Et Cosmochimica Acta, 1988,52(6):1531-1543.
[47] Henrichs S M, Farrington J W. Peru upwelling region sediments near 15°S. 1. Remineralization and accumulation of organic matter [J]. Limnology and Oceanography, 1984,29(1):1-19.
[48] Reimers C E, Suess E. The partitioning of organic carbon fluxes and sedimentary organic matter decomposition rates in the ocean [J]. Marine Chemistry, 1983,13(2):141-168.
[49] Murray J W, Kuivila K M. Organic matter diagenesis in the northeast Pacific: transition from aerobic red clay to suboxic hemipelagic sediments [J]. Deep Sea Research Part I Oceanographic Research Papers, 1990,37(1):59-80.
[50] Grundmanis V, Murray J W. Aerobic respiration in pelagic marine sediments [J]. Geochimica Et Cosmochimica Acta, 1982,46(6):1101- 1120.
[51] Paull C K, Dillon W P. Gas Hydrates Along the Peru and Middle America Trench Systems [M]. American Geophysical Union, 2013, 124:257-271.
[52] Wakeham S G, Ertel J R. Diagenesis of organic matter in suspended particles and sediments in the Cariaco Trench [J]. Organic Geochemistry, 1988,13(4-6):815-822.
[53] Longhurst A, Sathyendranath S, Platt T, et al. An estimate of global primary production in the ocean from satellite radiometer data [J]. Population Environment and Development, 1995,17(6):1245-1271.
[54] Lutz M J, Ken C, Dunbar R B, et al. Seasonal rhythms of net primary production and particulate organic carbon flux to depth describe the efficiency of biological pump in the global ocean [J]. Journal of Geophysical Research Oceans, 2007,112:C10011.
[55] Wu B, Liu S M, Ren J L. Dissolution kinetics of biogenic silica and tentative silicon balance in the Yellow Sea [J]. Limnology and Oceanography, 2017,62,doi:10.1002/lno.10514.
[56] 韩喜彬,赵 军,初凤友,等.南极半岛东北海域表层沉积有机质来源及其沉积环境[J]. 海洋学报, 2015,37(8):26-38.
[57] 曾 湘.深海沉积物中细菌对有机碳源的反应:细菌群落和酶活的变化 [D].厦门:国家海洋局第三海洋研究所, 2005.
[58] 蓝先洪,蜜蓓蓓,李日辉,等.渤海东部和黄海北部沉积物中重金属分布特征[J]. 中国环境科学, 2014,34(10):2660-2668.
[59] Reeburgh W S. Rates of biogeochemical processes in anoxic sediments [J]. Annual Review of Earth and Planetary Sciences, 1983, 11(1):269-298.
[60] 朱茂旭,史晓宁,杨桂朋,等.海洋沉积物中有机质早期成岩矿化路径及其相对贡献[J]. 地球科学进展, 2011,26(4):355-364.
[61] Beazley M J. The significance of organic carbon and sediment surface area to the benthic biogeochemistry of the slope and deep water environments of the northern Gulf of Mexico [D]. United States:Texas A and M University, 2004.
[62] Hargrave, Barry T. Aerobic decomposition of and detritus as a function of particle surface area and organic sediment content [J]. Limnology and Oceanography, 1972,17(4):583-586.
[63] Deflaun M F, Mayer L M. Relationships between bacteria and grain surfaces in intertidal sediments [J]. Limnology and Oceanography, 1983,28(5):873-881.
[64] Blankenship-Williams L E, Levin L A. Living Deep: A Synopsis of Hadal Trench Ecology [J]. Marine Technology Society Journal, 2009, 43(5):137-143.
[65] Ichino M C, Clark M R, Drazen J C, et al. The distribution of benthic biomass in hadal trenches: A modelling approach to investigate the effect of vertical and lateral organic matter transport to the seafloor [J]. Deep Sea Research Part I Oceanographic Research Papers, 2015, 100:21-33.
[66] 党宏月,宋林生,李铁刚,等.海底深部生物圈微生物的研究进展[J]. 地球科学进展, 2005,20(12):1306-1313.
[67] Jiao N, Herndl G J, Hansell D A, et al. Microbial production of recalcitrant dissolved organic matter: long-term carbon storage in the global ocean [J]. Nature Reviews Microbiology, 2010,8(8):593-599.
[68] 焦念志.海洋固碳与储碳——并论微型生物在其中的重要作用[J]. 中国科学:地球科学, 2012,42(10):1473-1486.
[69] Fang J, Barcelona M J, Nogi Y, et al. Biochemical implications and geochemical significance of novel phospholipids of the extremely barophilic bacteria from the Marianas Trench at 11,000m [J]. Deep-Sea Research Part I, 2015,47(6):1173-1182.
[70] D’Hondt S, Inagaki F, Ferdelman T, et al. Exploring Subseafloor Life with the Integrated Ocean Drilling Program [J]. Scientific Drilling, 2007,5(5):4-12.
[71] 谢树成,殷鸿福.地球生物学前沿:进展与问题[J]. 中国科学:地球科学, 2014,6:1072-1086.
[72] 焦念志,骆庭伟,张 瑶,等.海洋微型生物碳泵—从微型生物生态过程到碳循环机制效应[J]. 厦门大学学报(自然版), 2011,50(2): 387-401.
[73] 汪品先.从海洋内部研究海洋[J]. 地球科学进展, 2013,28(5):517- 520.
[74] Rathburn A E, Levin L A, Tryon M, et al. Geological and biological heterogeneity of the Aleutian margin (1965-4822m) [J]. Progress in Oceanography, 2009,80(1/2):22-50.
[75] Krause D C, White W C, Piper D J W, et al. Turbidity currents and cable breaks in the western New Britain Trench [J]. Geological Society of America Bulletin, 1970,81(7):2153-2160.
[76] D'hondt S.大洋钻探对洋底以下生命的探索[J]. 地球科学进展, 2003,18(5):759-763.
[77] Blair N E, Aller R C. The fate of terrestrial organic carbon in the marine environment [J]. Annual Review of Marine Science, 2012,4(1): 401.
[78] Yao P, Zhao B, Bianchi T S, et al. Remineralization of sedimentary organic carbon in mud deposits of the Changjiang Estuary and adjacent shelf: Implications for carbon preservation and authigenic mineral formation [J]. Continental Shelf Research, 2014,91:1-11.
[79] Lemche H, Hansen B, Madsen F, et al. Hadal life as analysed from photographs [J]. Videnskabelige Meddelelser Fra Dansk Naturhistorik Forening, 1976,139:263-336.
[80] George R Y, Higgins R P. Eutrophic hadal benthic community in the Puerto Rico Trench [J]. Ambio Special Report, 1979,6:51-58.
[81] Baird B H, White D C. Biomass and community structure of the abyssal microbiota determined from the ester-linked phospholipids recovered from Venezuela Basin and Puerto Rico Trench sediments [J]. Marine geology, 1985,68(1-4):217.
[82] Smith C R, Demopoulos A W J. The deep Pacific ocean floor [J]. Ecosystems of the World, 2003,28:179-218.
[83] Kobayashi H, Hatada Y, Tsubouchi T, et al. The hadal amphipod hirondellea gigas possessing a unique cellulase for digesting wooden debris buried in the deepest seafloor [J]. Plos One, 2012,7(8):e42727.
[84] Bianchi T S. The role of terrestrially derived organic carbon in the coastal ocean: A changing paradigm and the priming effect [J]. Proceedings of the National Academy of Sciences of the United States of America, 2011,108(49):19473-19481.
[85] Bianchi T S, Thornton D C O, Yvon-Lewis S A, et al. Positive priming of terrestrially derived dissolved organic matter in a freshwater microcosm system [J]. Geophysical Research Letters, 2015,42(13): 5460-5467.
[86] Ward N D, Bianchi T S, Sawakuchi H O, et al. The reactivity of plant-derived organic matter and the potential importance of priming effects along the lower Amazon River [J]. Journal of Geophysical Research Biogeosciences, 2016,121(6):doi.10.1002/2016JG003342.
[87] Burwicz E B, Rüpke L, Wallmann. Estimation of the global amount of submarine gas hydrates formed via microbial methane formation based on numerical reaction-transport modeling and a novel parameterization of Holocene sedimentation [J]. Geochimica et Cosmochimica Acta 75(16):4562-4576.
[88] Wenzh Fer F, Oguri K, Middelboe M, et al. Benthic carbon mineralization in hadal trenches: assessment by in situ O 2microprofile measurements [J]. Deep Sea Research Part I Oceanographic Research Papers, 2016,116:276-286.
[89] 林 晶,吴 莹,张 经,等.长江有机碳通量的季节变化及三峡工程对其影响[J]. 中国环境科学, 2007,27(2):246-249.
[90] 赵晨英,藏家业,刘 军,等.黄渤海氮磷营养盐的分布、收支与生态环境效应[J]. 中国环境科学, 2016,36(7):2115-2127.
[91] 高 嵩,韩秀荣,王 婷.浒苔绿潮与南黄海近岸海域水质的关系[J]. 中国环境科学, 2014,34(1):213-218.
[92] Xu B, Bianchi T S, Allison M A, et al. Using multi-radiotracer techniques to better understand sedimentary dynamics of reworked muds in the Changjiang River estuary and inner shelf of East China Sea [J]. Marine Geology, 2015,370(2):76-86.
[93] Zhang Y, Wang X, Zhen Y, et al. Microbial Diversity and Community Structure of Sulfate-Reducing and Sulfur-Oxidizing Bacteria in Sediment Cores from the East China Sea [J]. Frontiers in Microbiology, 2017,8:2133, doi:10.3389/fmico.2017.02133.
[94] 张 玉,贺 惠,米铁柱,等.东海海域表层沉积物中硫酸盐还原菌分布特征研究[J]. 中国环境科学, 2016,36(12):3750-3758.
[95] 李佳霖,白 洁,高会旺,等.长江口海域夏季沉积物反硝化细菌数量及反硝化作用[J]. 中国环境科学, 2009,29(7):756-761.
[96] Aller R C, Blair N E. Carbon remineralization in the Amazon–Guianas tropical mobile mudbelt: A sedimentary incinerator [J]. Continental Shelf Research, 2006,26(17):2241-2259.
[97] Lutz M J, Caldeira K, Dunbar R B, et al. Seasonal rhythms of net primary production and particulate organic carbon flux to depth describe the efficiency of biological pump in the global ocean [J]. Journal of Geophysical Research Oceans, 2007,112:C10011.2007.
[98] Berner R A. Burial of organic carbon and pyrite sulfur in the modern ocean: Its geochemical and environmental significance [J]. American Journal of Science, 1982,282(4):451-473.
[99] Sarmiento J L, Gruber N. Ocean Biogeochemical Dynamics-Calcium Carbonate Cycle [M]. United States:Princeton University Press, 2006.
致谢:感谢向阳红10号海洋科研调查船全体船员、国家海洋局第二海洋研究所鹿博、于培松、朱秋红及郭晓泽在调查采样和样品分析上给予的帮助!
Vertical distribution of sedimentary organic carbon in the Yap Trench and its implications.
WU Bin1,2, LI Dong1,2*, ZHAO Jun1,2, LIU Cheng-gang1,2, SUN Cheng-jun3, CHEN Jian-fang1,2, PAN Jian-ming1,2, HAN Zheng-Bing1,2, HU Ji1,2
(1.Laboratory of Marine Ecosystem and Environment, Second Institute of Oceanography, State Oceanic Administration, Hangzhou 310012,China;2.Key laboratory of Marine Ecosystem and Biogeochemistry, State Oceanic Administration, Hangzhou 310012, China;3.Marine Ecology Center, First Institute of Oceanography, State Oceanic Administration, Qingdao 266237, China)., 2018,38(9):3502~3511
Sediment core from the Yap Trench located in the west Pacific Ocean was collected by manned deep-sea research submersible. Geochemical parameter analyses (e.g. total organic carbon (TOC), δ13C, grain size composition and specific surface area (SSA)), combined with an end-member mixing model, a degradation model and principal component analysis (PCA) were conducted in order to 1) study the vertical distribution of the organic carbon (OC) and corresponding affecting factors, 2) assess the degradation and accumulation rate and the flux of the sedimentary OC in the Yap Trench. The mean TOC content of the Yap Trench was (0.31%±0.10%) and was comparable with those of the neritic sediments. Probably due to enrichment of the allochthonous organic matter in the large grain-sized particles (e.g. silt) and intense decomposition of OC by microorganisms, the TOC content was positively related with silt% and negatively related with SSA and clay%. The microorganism-derived OC was the dominant proportion of the sedimentary TOC (52%±21%), followed by the marine (37%±19%) and terrestrial (11%±4%) OC. The OC contents of those three sources all decreased downward. Based on PCA, decoupling between the microorganism-derived and the allochthonous OC indicated that the OC delivered by the submarine groundwater was probably an important nutritional supply for the microorganisms in the trench. The degradation rate of the refractory OC which dominated the vertical profile of TOC was around 0.0012a-1, slightly higher than that of the normal deep ocean but lower than the neritic environment. The accumulation rate and settling flux of TOC in the Yap Trench were around 1.8×10-5gC/(cm2·a) and2.2×109gC/a.
Yap Trench;sedimentary organic carbon;vertical distribution;source;degradation
X55
A
1000-6923(2018)09-3502-10
吴 彬(1986-),女,河北省秦皇岛人,博士后,主要从事海洋沉积物中生源要素的生物地球化学过程研究.发表论文4篇.
2018-02-07
国家自然科学基金青年科学基金项目(41606090);国家重点基础研究发展计划(973计划)项目(2015CB755904);国家海洋局第二海洋研究所基本科研业务费专项(JG1516)
* 责任作者, 助理研究员, lidong@sio.org.cn
