The Role of Cardiac Catheterization after Cardiac Arrest
2018-09-17AhmedHarhashMDPrashantRaoMDandKarlKernMD
Ahmed Harhash, MD, Prashant Rao, MD and Karl B. Kern, MD
1The University of Arizona Sarver Heart Center, 1501 N. Campbell Avenue, Tucson, AZ 85724, USA
Abstract Coronary angiography after cardiac arrest is important to ascertain potential treatable causes of cardiac arrest, salvage myocardium, and potentially increase long-term survival. The cause of adult out-of-hospital cardiac arrest is typically myocardial ischemia. More than 50% of such resuscitated individuals will have an acutely occluded epicardial coronary on emergency coronary angiography. This includes three in four with ST-segment elevation and one in three without ST-segment elevation. In the latter the only reliable method of detection is coronary angiography. Numerous cohort studies,now including more than 8000 patients, have shown an association between survival and early coronary angiography and/or percutaneous coronary intervention. Public reporting of percutaneous coronary intervention 30-day mortality rates has been an impediment for extending this therapy to all resuscitated individuals who experienced out-of-hospital cardiac arrest, since current databases to do fully risk-adjust rates for this subgroup. Sincere efforts are under way to correct this situation.
Keywords: cardiac arrest; coronary angiography; ST-segment elevation myocardial infarction; no ST-segment elevation myocardial infarction.
lntroduction
It is estimated that 325,000–350,000 out-of-hospital cardiac arrests (OHCAs) occur annually in the United States. An additional 210,000 in-hospital cardiac arrests (CAs) occur during the same period[1]. This incidence of OHCA has been relatively stable during the last decade [2]. The cause of sudden CA differs somewhat between individuals with OHCA and individuals with in-hospital CA and between adults and children. Approximately half of in-hospital CAs result from cardiac causes, and approximately half result from critical illnesses that cause serious hemodynamic instability, such as sepsis, pneumonia, and respiratory failure [3, 4].The vast majority of adult OHCAs are the result of sudden coronary ischemia, often an acute occlusion [5, 6]. In contrast, most children have a respiratory cause where profound hypoxia results in a bradyasystolic CA. Sadly, an increasing cause in young adults is drug overdose [7, 8].
Why Should Coronary Angiography Be Done after CA?
This high prevalence of coronary artery disease in adults experiencing out-of-hospital sudden CA has led to the concept of a potential “culprit vessel” being responsible for this catastrophic event.Numerous reports from CA registries have shown that a culprit vessel is often found when early coronary angiography is performed in resuscitated OHCA individuals [5, 9, 10]. We found that in those with postresuscitation ST-segment elevation, a culprit coronary is found 80% of the time and that the culprit vessel is occluded nine times out of ten [10].Hence in post-CA patients with ST-segment elevation, 75% will have an acutely occluded coronary.The incidence is lower in post-CA patients without ST-segment elevation, but it is not trivial. Our study found a culprit 33% of the time, and about two-thirds of such culprits were acutely occluded[10]. Therefore in 25% of all resuscitated adults experiencing an OHCA, an acutely occluded coronary was found.
The importance of such findings is obvious. If such acutely occluded coronaries are not found and reperfused in a timely fashion, significant myocardial cell death can result, leaving that resuscitated patient with decreased left ventricular function and potentially heart failure. Many registry reports have collaborated the finding that the post-CA electrocardiogram (ECG) is insufficient for identifying who has an acutely occluded culprit coronary and who does not. Currently the only dependable way to assess such coronary status is with coronary angiography. If the purpose of doing coronary angiography is to find and reperfuse such acutely occluded culprit vessels after CA, then an immediate catheterization (<2 h) is the most reasonable course.
Safety of Catheterization after CA
The safety of immediate coronary angiography after CA has been reported, but all such data are from observations during randomized trials or from nonrandomized registries. The two major issues have been whether bleeding and stent thrombosis are increased in this population.
Bleeding
Post-CA coronary angiography, typically using a femoral access, has been shown not to lead to increased bleeding even when combined with mild therapeutic hypothermia. Callaway et al.[11], using observations collected during the Resuscitation Outcomes Consortium PRI-MED trial(NCT00394706), evaluated the value and safety of early coronary angiography and therapeutic hypothermia [11]. This observational study included 3981 patients, of whom 1566 received therapeutic hypothermia, 765 underwent early coronary angiography defined as coronary angiography within the first 24 h of admission, and 705 received reperfusion [most via percutaneous coronary intervention (PCI)]. No significant increase in bleeding was seen among those undergoing angiography (3.8%;29/765) or PCI (3.1%; 22/705) compared with the group as a whole (2.7%; 106/3981).
Stent Thrombosis
Early observational reports raised the concern that stent thrombosis was increased in post-CA patients during early coronary angiography and PCI while they were simultaneously receiving therapeutic hypothermia. Therapeutic hypothermia could increase the risk of stent thrombosis theoretically because of the difficulty of timely administration of dual oral antiplatelet agents to comatose patients,a decreased bioavailability of such oral agents from the cooled gut, and altered pharmacokinetics at lower temperatures. The original observational reports were difficult to interpret because of the limited number of patients included [12–15]. Shah et al. [16] using the National Inpatient Sample database reported that therapeutic hypothermia given to patients admitted after resuscitation from OHCA associated with a myocardial infarction did not increase the incidence of stent thrombosis compared with that in similar patients not treated with hypothermia [3.9% (46/1193) vs. 4.7% (2271/47,916),not significant]. This much larger observation is reassuring compared with the earlier reports(Table 1). Clearly the rate is higher among acutely ill patients compared with stable PCI patients, as reported in non-CA ST-segment elevation myocardial infarction (STEMI) patients [16].
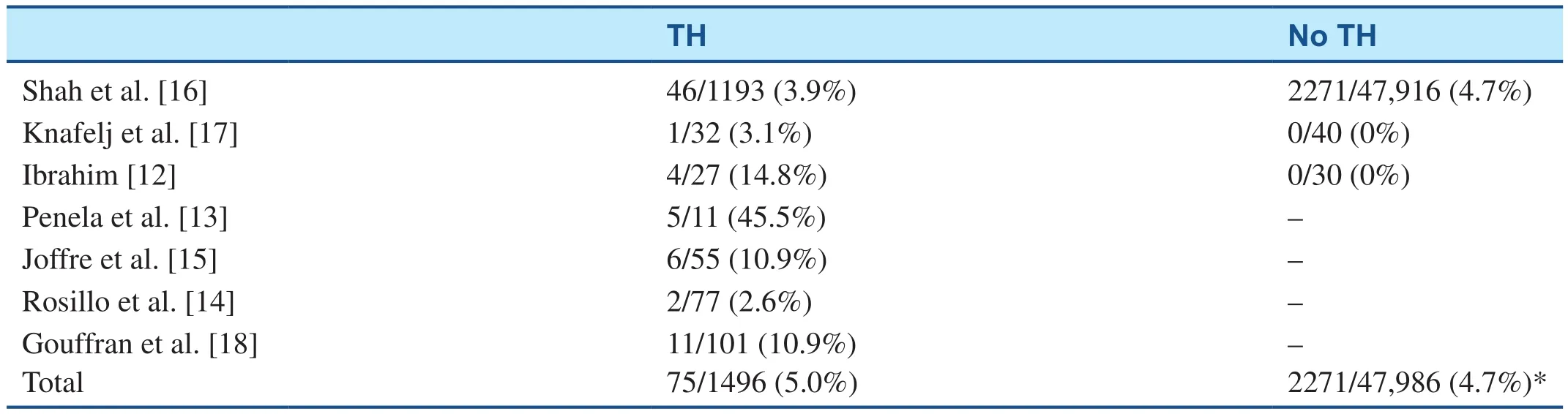
Table 1 Stent Thrombosis After Cardiac Arrest After Primary Percutaneous Coronary Intervention; with and without Therapeutic Hypothermia (TH) Treatment.
For Whom Should Coronary Angiography Be Done after CA?
Post-CA Patients with ST-Segment Elevation
For the lucky minority who survive CA with no apparent extracardiac cause, the American College of Cardiology/American Heart Association (AHA)resuscitation guidelines updated in 2015 [19] recommend emergency coronary angiography with an attempt to revascularize the culprit vessel (class I recommendation if there is evidence of STEMI on surface ECG after return of spontaneous circulation).The 2015 update deferred to the 2013 STEMI guidelines for defining the ECG criteria for STEMI and how to discern a culprit lesion on coronary angiography [20]. Although the ECG criteria for STEMI were originally defined as “ST segment elevation in two or more leads, a new LBBB [left bundle branch block], or ST elevation in lead aVR with multi-lead ST depression” [20], some studies have included new LBBB, while others included “any lead with ST elevation” as an inclusion criterion for their designated studies. Although it is established that ST-segment elevation in two or more consecutive leads is generally associated with acute coronary occlusion, it is less certain whether LBBB or ST-segment elevation in one lead (including lead aVR) has the same association. Indeed, one of the studies reported only 24% of CA patients with presumably new LBBB to have an acute coronary occlusion [21]. Defining“culprit lesions” on coronary angiography is another potential for variability among studies. Although all studies included acutely thrombotic lesions with distal TIMI flow of 1 or 0, some considered severe more than 90% of lesions despite intact TIMI 3 flow, and others used rates of PCI as a surrogate for the presence of a culprit lesion. Overall, the culprit occlusion rate ranged from 55% to 82% in OHCA patients with STEMI, which is comparable with the rate in the non-CA STEMI population.
Studies of Post-CA Patients with STEMl
Originally noted more than two decades ago by Spaulding et al. [5], coronary occlusion after CA is frequent (48%), and coronary angiography seemed to improve clinical outcome in those patients regardless of their ECG findings. In a retrospective study,Gorjup et al. [22] reported a 5.7% incidence of CA in a series of 2393 STEMI patients. In their study,post-CA patients who regained consciousness and were awake at hospital arrival experienced the same outcome as those who presented with no CA (the rate of survival to hospital discharge was 100% vs.94.8%, respectively). However, patients who did not regain consciousness and remained comatose had significantly worse outcome (the survival rate was only 51%, and only 29% for those with cerebral performance category 1 or 2) than the non-CA cohort[22]. Another study, by Garot et al. [23], investigating longer-term survival in patients with CA complicating STEMI, found a rate of survival to hospital discharge of 55% and a 6-month survival rate of 54%. Of note, most of the patients in both studies underwent invasive coronary angiography and PCI,which was felt to be the main attribute contributing to their improvement in clinical outcome [22, 23].More recently, we reported the incidence of STEMI in post-CA patients, where we found no difference in outcome between CA patients with STEMI and CA patients without STEMI on their postresuscitation ECG [10]. However, we found a strong correlation between coronary angiography and survival to hospital discharge. STEMI patients who underwent coronary angiography had better survival (54.7%) than those who did not (33.3%) [10]. Redfors et al. [24]reported from a large national database in Sweden that 63% of resuscitated OHCA patients (144 of 230) with STEMI had an acute culprit occlusion.
The most recent report, from Zeyons et al. [25],found the highest incidence of acute culprit occlusion yet (82% of their CA STEMI patients), while Stær-Jensen et al. [21] reported in 2015 the lowest rate of acute culprit lesion occlusion (55%) in patients with ST-segment elevation on a post-CA ECG.
Other studies using large datasets/registries to identify OHCA patients have focused on outcomes with less emphasis on detailed ECG or angiographic criteria. Vyas et al. [26] reported a large multicenter retrospective analysis on patients presenting with ventricular fibrillation or pulseless electrical activity (data from the CARES registry).Although data regarding ST-segment elevation or angiography details were not available for a large portion of patients, Vyas et al. clearly demonstrated that STEMI patients undergoing coronary angiography and PCI – if indicated – had higher survival than those who did not [odds ratio (OR) of survival to discharge 1.52, 95% confidence interval (CI)1.28–1.80]. Geri et al. [27] reported a retrospective analysis demonstrating not only short-term but also long-term survival benefit for patient undergoing coronary angiography with PCI (OR for 30-day and long-term mortality 0.71, 95% CI 0.54–0.92). In an observational study extending for nearly 15 years,Mylotte et al. [28] showed that a full revascularization approach might even be better than the usual culprit artery approach in those with STEMI after resuscitation from OHCA.
Although most studies advocated early coronary angiography after CA, Kim et al. [29] recently reported that a more cautious approach might be better. In their retrospective analysis of an Asian OHCA cohort, they found a correlation between subarachnoid hemorrhage and ST-segment changes on surface ECG, however mostly related to ST-segment depression and prolonged QT interval rather than classic STEMI [29].
These data confirm the clinical benefit from coronary angiography and PCI in patients with STEMI on postresuscitation ECG (Table 2). Given the clear short-term and long-term survival benefit, we recommend emergency coronary angiography in CA patients with STEMI, and we screen them for an intracranial cause only if the history or physical examination is suggestive of such, rather than on a routine basis.
Post-CA Patients without ST-Segment Elevation
The role of coronary angiography in postresuscitation patients with STEMI is widely acknowledged.In such patients the early restoration of coronary blood flow after resuscitation is essential to prevent repeated CA and circulatory shock and to preserve myocardial function [31]. However, the role of coronary angiography in post-CA patients with non–ST-segment elevation is less well established.
Guidelines for Coronary Angiography in CA Survivors without ST-Segment Elevation
According to the AHA 2015 statement, emergency coronary angiography is a class II recommendation in select CA survivors without ST-segment elevation [19]. The statement emphasizes that appropriate patient selection is vital to focus on those patients who will benefit most from emergency coronary angiography, while avoiding high-risk invasive procedures in those who do not have significant coronary lesions. Major criteria for appropriate patient selection include hemodynamic or electrical instability, evidence of ongoing ischemia,comorbidities, and other patient-specific factors[32]. Furthermore, initial CA rhythm also influences outcomes. Ventricular fibrillation or ventricular tachycardia is associated with more favorable outcome, although nonshockable rhythms may also be caused by coronary artery occlusion [33].A detailed list of unfavorable factors for post-CA coronary angiography is given in Figure 1 [34]. A major challenge of appropriate patient selection is ascertaining some of these risk factors, particularly in a comatose survivor of an OHCA.
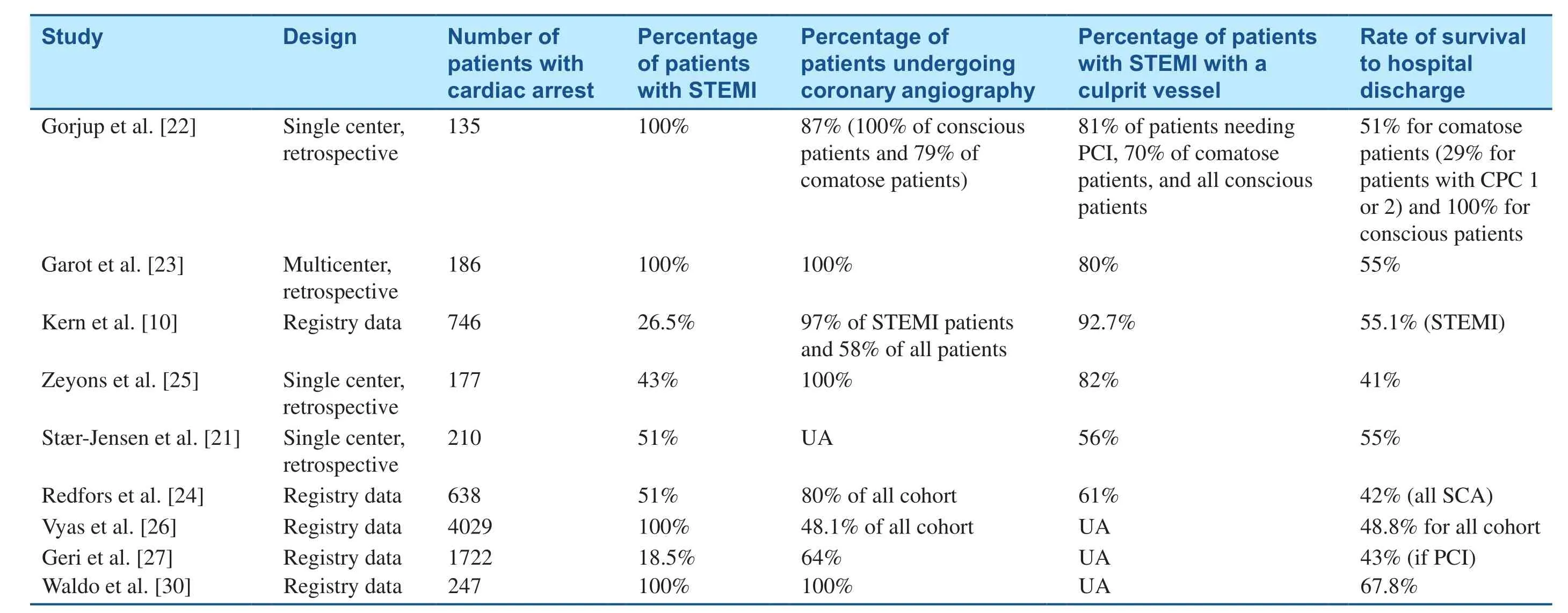
Table 2 Studies of ST-Segment Elevation Myocardial Infarction (STEMI) and Out-of-Hospital Cardiac Arrest.
The European consensus statement (2014) recommended a similar selective approach for emergency coronary angiography following non–ST-segment elevation CA [31]. In the absence of any obvious noncoronary cause of CA and if the CA situation is favorable, it recommends that coronary angiography be performed within 2 h. In addition, the authors of the statement advocate a strategy of a dedicated short “stop” area in the emergency department or intensive care unit for comatose patients after resuscitation without ST-segment elevation. The purpose of this period is to establish details surrounding the event and perform further investigations to exclude an obvious noncoronary cause of CA.
The ambiguity of the emergency “stop” and the class II recommendation for early coronary angiography in select CA survivors without ST-segment elevation is a reflection of a lack of consensus regarding the value of early reperfusion in this population. It is imperative that future research focus on a more precise way of identifying patients who will gain most benefit from early invasive therapies after resuscitation [34].
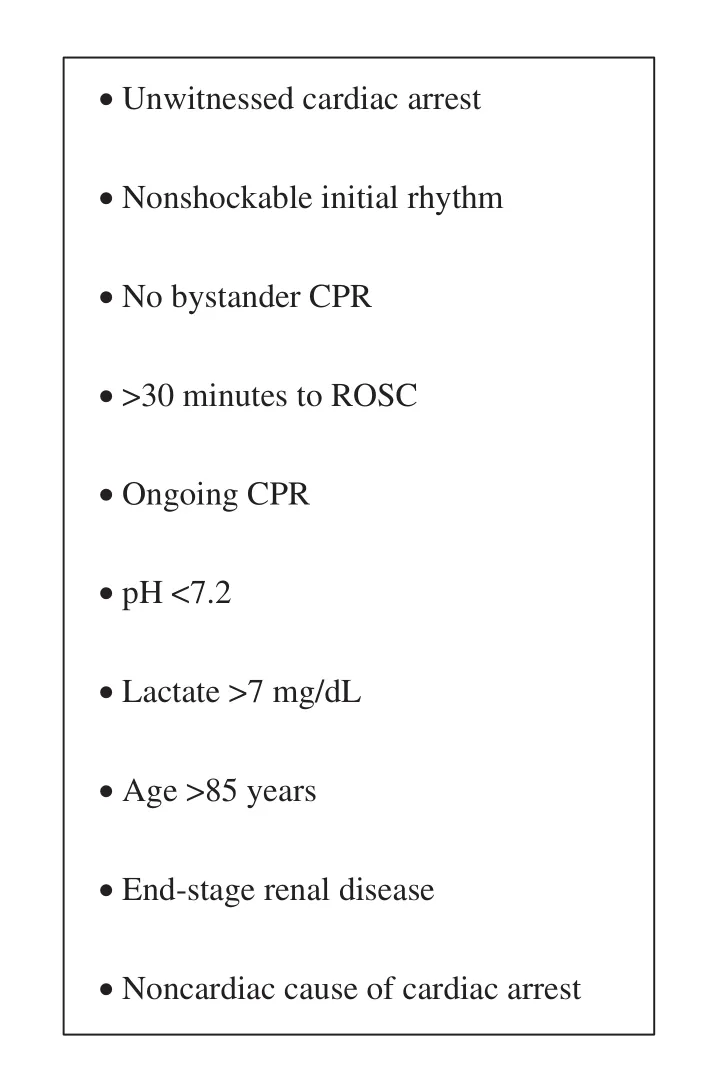
Figure 1 Unfavorable Features for Coronary Angiography after Cardiac Arrest.ROSC, return of spontaneous circulation. Adapted from Rab et al. [34] with permission.
Angiographic Findings in CA Survivors without ST-Segment Elevation
A seminal study by Spaulding et al. [5] showed that ST-segment elevation is a poor predictor of acute coronary occlusion in the post-CA patient and has a high false negative rate for detection of acute coronary occlusion. Consistent with these findings, Hollenbeck et al. [35] identified acute coronary occlusion in nearly 30% of comatose patients without ST-segment elevation following CA. The Parisian Region Out-of-Hospital Cardiac Arrest(PROCAT) registry showed that 58% of postresuscitation patients without ST-segment elevation had at least one significant coronary artery lesion [36].The same authors later showed that one-third of OHCA patients without ST-segment elevation had a culprit coronary lesion requiring PCI [37]. These observations were corroborated by the Resuscitation Outcomes Consortium [38].
Using the International Cardiac Arrest Registry(INTCAR), Kern et al. [10] compared coronary angiographic findings of post-CA patients with and without ST-segment elevation. The analysis included 746 comatose post-CA patients (198 with and 548 without ST-segment elevation). In these patients, Kern et al. identified a culprit coronary vessel in one-third of patients without ST-segment elevation after CA. Of particular interest, they found that 69% of such culprit vessels were acutely occluded. Similar findings have been observed in smaller studies [9, 39].
Outcomes after Catheterization among Post-CA Patients without ST-Segment Elevation
INTCAR has also provided valuable insight into the impact of coronary angiography on clinical outcomes in CA survivors without ST-segment elevation. Survival to hospital discharge and functional outcome at discharge were significantly greater in the group that underwent coronary angiography versus the group that did not undergo coronary angiography [12]. Improved clinical outcomes associated with coronary angiography in this post-CA group without ST-segment elevation have been corroborated by Patel et al. [40] showed that coronary angiography in post-CA patients without ST-segment elevation was significantly more likely to survive to discharge than that of their ST-segment elevation counterparts (OR 7.02, 95% CI 6.60–7.46 vs. OR 3.71, 95% CI 3.27–4.22; P < 0.001). This study also showed that coronary angiography itself was associated with high rates of survival to discharge, even when no subsequent PCI was performed. Although these results appear to suggest that even patients without an acutely obstructed coronary artery benefit from early intervention, a selection bias where patients with a more favorable prognosis are selected for coronary angiography cannot be ruled out.
When Should Patients after CA Undergo Coronary Angiography?
The timing of early coronary angiography plays an important role in outcomes following CA. If most CAs are precipitated by an acute ischemic event,then an early invasive strategy with potential for PCI is imperative. The importance of early reperfusion therapy after CA has been highlighted in animal studies where delayed coronary angiography,despite early targeted temperature management(TTM), results in the same myocardial infarct size as in those receiving neither hypothermia nor early reperfusion [41].
Emergency catheterization was first shown to be feasible in CA survivors by Kahn et al. [42]. Bendz et al. [43] showed that the post-CA group are a high-risk population, and those with ST-segment elevation who undergo early reperfusion have a higher 2-year mortality rate compared with matched STEMI controls without CA (27.5% vs. 7.1%).
Hollenbeck et al. [35] showed that in CA survivors without ST-segment elevation, there was significantly increased survival to hospital discharge in patients who underwent early coronary angiography compared with late coronary angiography(defined as at least 24 h after admission) (66% vs.49%; P = 0.017).
Within the initial 24 h following CA, the benefit ofemergentcoronary angiography (<2 h) versusurgentcoronary angiography (<6 h) versusearlycoronary angiography (<24 h) on clinical outcomes is unclear. Data from a 7-year retrospective French study showed the survival rate of patients who underwent coronary angiography within 2 h of arrival in the emergency department was 23%(30/133) versus only 9.6% (30/312) in those who did not undergo coronary angiography [44]. However,if the purpose of coronary angiography after CA is to identify acute coronary occlusions and then reperfuse such vessels in a timely fashion,emergentcoronary angiography would seem a better strategy than eitherurgentorearlycoronary angiography.In this regard, Garcia et al. [33] performed coronary angiography in 231 OHCA survivors within 6 h of arrival in the emergency department; 74% of patients survived to hospital discharge and 65% survived to hospital discharge with good neurological outcome. A notable feature was that most of these patients (90%) gained access to the catheterization laboratory within 2 h of presentation.
Strote et al. [45] compared the role ofurgentcoronary angiography (≤6 h) versusdelayedcoronary angiography (>6 h) versus no coronary angiography in OHCA survivors. In a series of 240 individuals,coronary angiography within 6 h was associated with increased survival compared with delayed coronary angiography or no coronary angiography.Seventy-two percent (44/61) of those who underwent urgent coronary angiography survived to hospital discharge compared with only 49% (87/179)in the delayed/no coronary angiography groups.Of those who survived to hospital discharge, similar rates of good neurological outcome (either full recovery or mild impairment) were observed in both groups.
Of note, the INTCAR study findings in this regard highlight the need for caution in interpreting such data from nonrandomized populations [10]. In the INTCAR study, post-CA outcomes were compared between those who underwent immediate coronary angiography and those who underwent delayed coronary angiography. The rate of survival to hospital discharge was 56% (205/364) in the patients who underwent immediate coronary angiography compared with 89% (67/75) in the patients who underwent delayed coronary angiography. This result is likely secondary to a selection bias, as delayed coronary angiography is likely to be performed in the stabler and more neurologically intact patient population, after the more critically ill have often died.
Despite the limitations of retrospective analyses and the selection bias inherent in a physician’s decision to perform coronary angiography following CA, early invasive reperfusion therapy for this population appears reasonable.
Ongoing lssues for Coronary Angiography after CA
Therapeutic Hypothermia and Coronary Angiography
Therapeutic hypothermia efficacy after CA was established more than a decade ago with the publication of two landmark studies with active external cooling to reach a core temperature of 32–34°C for 12–24 h in comatose patients after OHCA [46,47]. These two randomized trials achieved a survival benefit and a significant improvement in neurological recovery in comparison with patients not treated with hypothermia. The benefits from therapeutic hypothermia were attributed to retardation of destructive enzymatic reactions, suppression of free-radical production, and reduction of the oxygen demand in low-flow regions. More recently,another landmark study (TTM), investigating the optimal degree of hypothermia for OHCA, randomized patients to two groups, either to a 33°C group or to a 36°C group – using active cooling –and found no differences in outcomes between both approaches. However, this study has been misinterpreted by some practitioners, who have suggested that the lack of difference between the 33°C group and the 36°C group suggests cooling is not necessary since 36°C and 37°C (normothermia) are not very different. Of note, in the TTM trial, all patients received active TTM via transcutaneous (76%) or intravascular (24%) devices, and they were carefully monitored for 3 days after the randomization period to actively prevent hyperthermia [48].
Acknowledging the dispute around the benefit of combining PCI and therapeutic hypothermia in OHCA patients, we investigated the synergistic effect of combining these two approaches in a large animal (porcine) translational study simulating patients with CA complicating left anterior descending artery occlusion [41]. After occluding the left anterior descending artery with an intracoronary balloon and inducing ventricular fibrillation, we randomly assigned 32 swine to one of the following treatment groups: group A, hypothermia and reperfusion; group B, hypothermia and no reperfusion;group C, no hypothermia but reperfusion; and group D, no hypothermia and no reperfusion. Resuscitated animals randomized to receive hypothermia were rapidly cooled to 34°C, whereas those randomized to receive reperfusion received this after 45 min of left anterior descending artery occlusion. At 4 h,myocardial infarct size was calculated. Group A had the smallest infarct size [16% of the area at risk(AAR)], whereas groups B and D (no reperfusion)had the largest infarct sizes (both 40% of AAR),and group C was in between (30% of AAR) [41].Several clinical trials examined the same concept in patients with STEMI but without associated CA.These trials have proven the feasibility of delivering cold saline via large-bore intravenous catheters and achieving core temperatures as low as 33°C while performing coronary intervention. This approach successfully limited the infarct size and region of myocardium at risk in comparison with those who received PCI alone, confirming the feasibility and potential advantages of combining reperfusion and therapeutic hypothermia for both brain and heart in the treatment of CA patients with suspected cardiac origin [49, 50].
Public Reporting of Coronary Angiography and PCl Outcomes
Public reporting of hospital mortality rates for selective conditions has been proposed for several decades. The original intent was to allow patients the opportunity to educate themselves regarding the quality of care provided by different medical institutions. The hope was that such transparency would result in more informed and educated patient choices concerning where patients would like to receive their medical care. Unfortunately, the experience during the last decade has been less fulfilling.Concerns quickly surfaced that the challenge of public reporting of outcomes would be to ensure that comparative populations were equivalent. Ellis et al. [51] noted in 2011 that the common databases used to track PCI mortality lack important data on noncardiac causes of death after the procedure, even though more than 50% of the 30-day post-PCI deaths were from noncardiac causes. In 2013 the AHA published a scientific statement on the impact of PCI performance reporting on AHA-recommended cardiac resuscitation centers (CRCs)[52]. It calculated that if a CRC did 11 post-CA STEMI coronary angiograms/PCIs annually, the observed PCI mortality rate would double from 5.0 to 10%. This is a reflection of the different expected post-PCI mortality rates for non-CA STEMI (5%)and post-CA STEMI (50%). The obvious point is that such cases should not be combined in public reports regarding center or individual operator PCI mortality statistics. It was concluded that OHCA cases should be monitored but not publically reported or used in PCI performance ranking [52].This would stop the current “penalizing of highvolume CRCs for following the AHA 2010 CPR guidelines” recommending early coronary angiography for such patients. A second report appeared in 2013, suggesting that death within 30 days of PCI was just as likely to be non-PCI related as attributed to the procedure [53]. The two most common conditions associated with non-PCI-related deaths were CA and cardiogenic shock. Data comparing public reporting states (New York, Massachusetts,Pennsylvania) have shown that since public reporting was mandated less PCI is being done for patients after CA or those with cardiogenic shock[30, 54]. This “risk avoidance” patient selection is most noticeable in comparisons of total mortality for those with STEMI. Initial PCI deaths appear to be lower, but overall mortality for STEMI patients is actually higher since some whose best longterm chance was reperfusion never received such treatment because of their known higher risk. This results in the sickest patients with the most to gain,albeit with the most risk, not being considered for aggressive therapy because of the fear they will increase the hospital or 30-day mortality rates. One editorial citing this issue noted that “many patients die in spite of PCI rather than from PCI” [55].
The American College of Cardiology Interventional Council made a similar statement in 2015 that states with mandatory public reporting of PCI mortality now rank 42nd, 48th, and 50thfor the use of PCI for acute myocardial infarction in the nation,while total mortality for STEMI in those states is 35% higher than in states without mandated public reporting. This strongly suggests a risk-averse selection of patients considered for primary PCI or for whom primary PCI is declined in public reporting states. To provide optimal care for all patients,the American College of Cardiology Interventional Council endorsed the 2013 statement of the AHA calling for “out-of-hospital STEMI-PCI cases to be separately categorized from other STEMI-PCI cases” and that they “should not be included in public reporting” [34].
Significant progress with this issue is still hard to document. In 2010, New York changed its reporting requirement, allowing selected OHCA STEMI patients to be excluded. However, the requirements for exclusion were narrow, resulting in most cases continuing to be included in the mortality reports.This change seemed insufficient to increase the rate of PCI after CA in the state of New York [56].
If optimal care is the goal for all post-CA patients,we must finally deal with this issue. The fair solution is simply to track PCI mortality for STEMI patients with and without associated OHCA separately,finally acknowledging that a 10-fold increase in expected mortality between two subsets of patients is enough to require apples be reported with apples and oranges be reported with oranges.
Conclusions
Most adults who experience OHCA have an acute coronary issue, often with an acute coronary occlusion. The 12-lead ECG findings of ST-segment elevation support the likelihood that a major coronary is acutely occluded. However, the absence of ST-segment elevation is not evidence that all coronaries are patent and without need for emergent reperfusion. At least one-third of resuscitated adults without ST-segment elevation have an acutely occluded coronary and need emergent coronary intervention. Such patients have no more bleeding or stent thrombosis than other acute coronary syndrome patients without CA. Increasing evidence suggests that procedural success for CA patients undergoing emergent coronary angiography and PCI is similar to that for non-CA patients, but they have a higher risk of death because of the sequelae of their CA. Such patients should not be included in current public reported mortality statistics, but rather should be reported separately for more accurate comparisons.
杂志排行
Cardiovascular Innovations and Applications的其它文章
- Speckle Tracking Echocardiography ldentifies lmpaired Longitudinal Strain as a Common Deficit in Various Cardiac Diseases
- Current Status of Coronary Atherectomy
- The Use of Direct Oral Anticoagulants for Prevention of Stroke and Systemic Embolic Events in East Asian Patients with Nonvalvular Atrial Fibrillation
- Bioresorbable Vascular Scaffold in the Midportion of the Left Anterior Descending Artery for Cardiac Allograft Vasculopathy in a Cardiac Transplant Patient
- The Contemporary Role of Femoral Artery Access
- Cardiovascular Innovations and Applications