Synthesis and Neuroprotective Activity of Neolamellarin A Analogues
2018-08-24ZHANGMengYINRuijuanZHANGYiranHAOCuiZHANGLijuanandJIANGTao2
ZHANG Meng, YIN Ruijuan, ZHANG Yiran, HAO Cui, ZHANG Lijuan,, and JIANG Tao2),
Synthesis and Neuroprotective Activity of Neolamellarin A Analogues
ZHANG Meng1), 2), #, YIN Ruijuan2), 3), #, ZHANG Yiran1), 2), HAO Cui1), ZHANG Lijuan1), 2),*, and JIANG Tao2), *
1) Systems Biology and Medicine Center for Complex Diseases, Affiliated Hospital of Qingdao University, Qingdao 266003, China 2)School of Medicine and Pharmacy, Ocean University of China, Qingdao 266003, China 3) Marine Biomedical Research Institute of Qingdao, Qingdao 266003, China
Alkaloids are a class of natural products with a wide range of biological activities. Due to the special living environment, the alkaloids from marine sponges have exhibited different biological activities and promising medical application potential. Neolamellarin A is a marine alkaloid possessing bisaryl-pyrrole structural features. Here, the synthesis of 12 different 3,4-bisaryl-N-alkylated permethylated analogues of neolamellarin A and their outstanding neuroprotective activity in PC12 cells are presented and discussed.
alkaloids;neolamellarin A; synthesis; PC12 cells; neuroprotective activity
1 Introduction
The pyrrole-derived alkaloids lamellarins are a related rapidly growing class of marine natural products (Bailly, 2014; Fan., 2008; Jia, 2011). Investigations of several lamellarins analogues have revealed their multi- drug resistance (MDR) reversal properties by inhibiting the P-glycoprotein (P-gp)-mediated drug efflux (Bharate., 2015; Plisson., 2012; Quesada., 1996). These compounds possess 3,4-bisaryl-pyrrole structural features and high lipotropy. The bioassay of these alkaloids showed relatively low cytotoxicity (Zhang., 2010), which limits the potential applications of these compounds. Neolamellarin A was isolated from spongeby Liu(2007).Our group has reported the synthesis of permethylated neolamellarin A and its analogues (Yin., 2015). In current study, we used the newly synthesized 3,4-bisaryl- N-alkylated permethylated neolamellarin A analogues not only to test their cytotoxicity but also their neuroprotective activity by using both cancer and neuronal PC12 cell lines. The synthetic route and detailed characterization of each neolamellarin A analogue will be described and published elsewhere.

Fig.1 The structures of neolamellarin A and permethylated neolamellarin A.
2 Results and Discussion
The 3,4-bisaryl-N-alkylated permethylated neolamellarin A possess the structural feature that 3- and 4-positions of pyrrole ring have an identical aryl group, and N position locates a benzyl group. So, we adopted the One- pot AgOAc-mediated synthetic way to synthesize this kind of alkaloids, which was reported by Li and co- workers (Li., 2010).
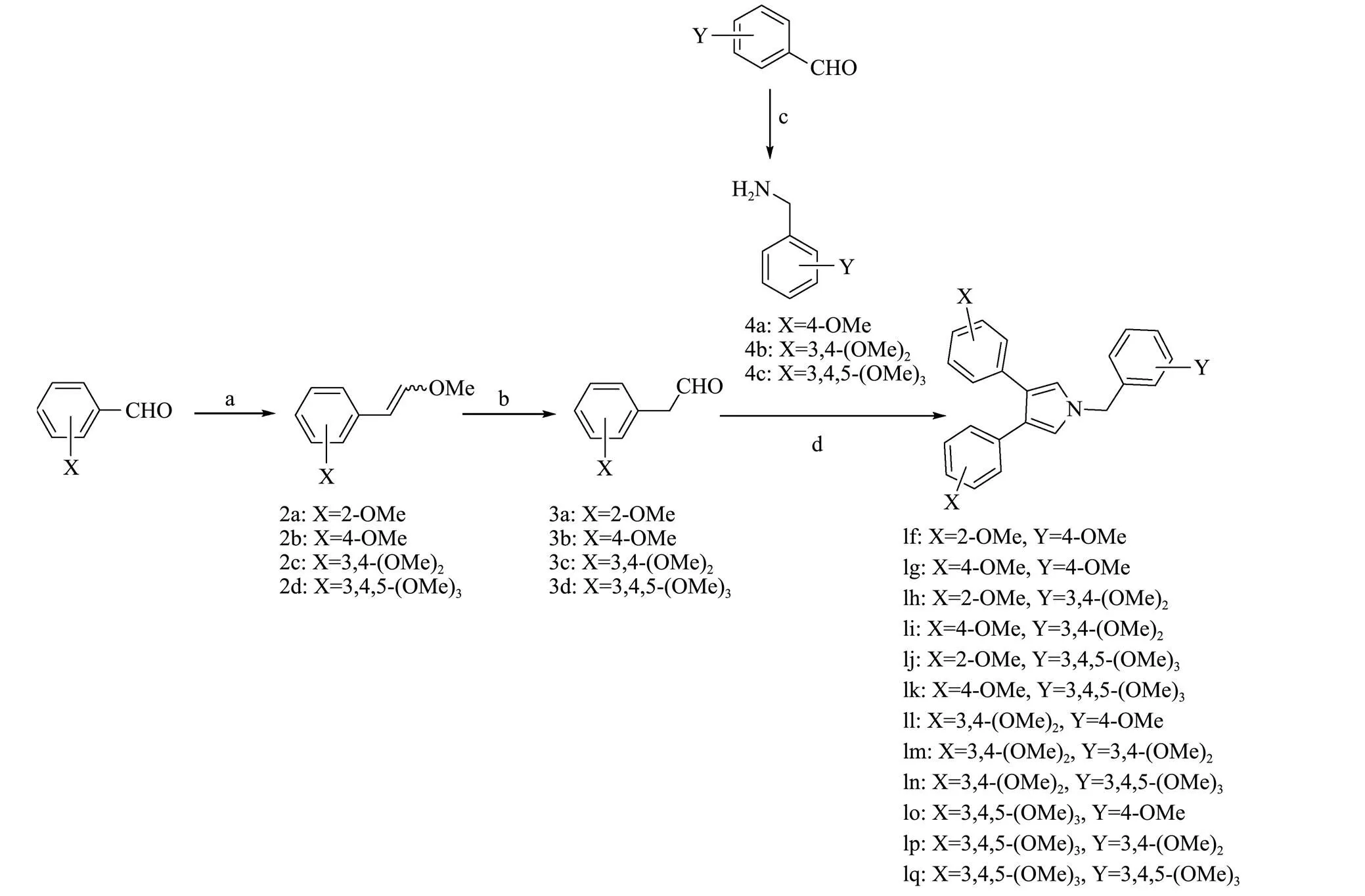
Scheme 1 Synthetic route of permethyl neolamellarin A analogues (1f-1q). Reactants and conditions: a) Ph3P+CH2OCH3Cl−, t-BuOK, THF, 0℃ then r.t., 4h; b) TAF, H2O, CH2Cl2, r.t.,18h; c) a. NH2OH.HCl, NaOH, H2O, CH3OH, 70℃, 1h; b. Zn, HOAc, 70℃, 2h; d) benzylamine, AgOAc, NaOAc, THF, 60℃, 8 h.
Our synthesis started from the known methoxyl substituted phenyl acetaldehydes 3, which was synthesized in 65%-90% yield by wittig reaction of methoxyl substituted benzaldehyde with (methoxymethyl)-triphenyl- phosphonium chloride followed hydrolysis with TFA (Rosowsky., 2003),and methoxyl substituted benzylamine 4, which was synthesized in 85%-95% yield by reduction of methoxyl substituted benzaldehyde oxime with zinc dust (Ayedi., 2013). One-pot AgOAc-mediated condensation of the methoxyl substituted phenyl acetaldehydes 3 with methoxyl substituted benzylamine 4 at 60℃ generated the 3,4-bisaryl-1-benzyl pyrrole 1f-1q in 55%-75%yield (Li., 2010).
In the preceding work, we evaluated the cytotoxicity of the Neolamellarin A analogues 1f-1q on three cancer cell lines (A549, HCT116 and HT29) at a concentration of 10 μmolL−1(data unpublished), finding almost no cytotoxic activity. Based on established neural protection model in our lab (Hao., 2015), we further tested their neuroprotective activity on PC12 cell line (Figs.2-3). Surprisingly, all the analogues were strongly against glutamate-induced PC12 cell death, and bell-shape curve could be observed with the increase of drug concentration.
Synthesis of series of 3,4-bisaryl-N-alkylated perme- thylated neolamellarin A analogues and their cytotoxicities and neuroprotective activity have been presented and discussed here. Although the series of compounds had little cytotoxic activity, their outstanding neuroprotective activity extends potential application of neolamellarin A analogues and enriches biological function of marine alkaloid.

Fig.3 Neuroprotective activity of the neolamellarin A analogues on PC12 cell line. Percentage of viable cells with 4h pretreatment of glutamate at a final concentration of 8mmolL−1, followed by co-cultured with or without neolamellarin A analogues (1f-1q) at final concentrations of 5, 10, 20, and 40μmolL−1 for 24h compared to the compound-free control received an equal volume of DMSO. The analogues were formulated initially in DMSO and then diluted in complete growth media. Each value was calculated from 3 independent experiments performed in triplicate. Data are shown as mean ±SD.
3 Experimental Protocols
3.1 Chemical Synthesis
3.1.1 General
THF was purified by dispersed KOH (about 50gL−1), boiling, and distillation over Na in the presence of benzophenone under argon atmosphere. All other solvents and materials were obtained from commercial sources and used without further purification. Thin-layer chromatography (TLC) was performed on precoated E. Merck silica-gel 60 F254 plates. Column chromatography was performed on silica gel (200-300mesh Qingdao China). Melting points were determined on a Mitamura-Riken micro-hot stage without correction. 1H NMR and 13C NMR spectra were obtained on a Bruker 600 spectrometer with tetramethylsilane (Me4Si) as internal standard, and chemical shifts were recorded in δ values. Mass spe- ctra were recorded on a Q-TOF Globalmass spectrometer.
3.1.2 General procedure for the synthesis of substituted phenyl acetaldehydes 3
Ph3P+CH2OCH3Cl−(3.42g, 10mmol) was placed in a sealed flask which was equipped with a thermometer and constant pressure dropping funnel and under nitrogen atmosphere, then cooled the flask to 0℃. The THF (6mL) solution of t-BuOK(1.35g, 12mmol) was slowly added and continue to react 0.5h. And then added the THF (6 mL) solution of substituted benzaldehyde (10mmol) dropwise and continue to stir for 1h, then reacted for 2h at room temperature. Then the reaction mixture was pour- ed into water (50mL) and extracted with EA (30×3mL). The combined organic extracts were washed with H2O (3×40mL), dried (MgSO4), and concentrated on a rotary evaporator at a bath temperature of about 20℃ to give a residue of compound 2, which was purified by flash chromatography on silica gel (PE: EA = 100: 1).
The above compound 2 (7.3mmol) was solved in CH2Cl2(73mL), then added water (1.5mL) and followed by CF3COOH (1.5mL) dropwise. Afer stirred for 24h, the mixture was quenched with sat. aq NaHCO3solution (60mL) and extracted with CH2Cl2(30×3mL). The combined organic extracts were washed with H2O (3×40 mL), dried (MgSO4), and concentrated on a rotary evaporator at a bath temperature of about 20℃ to give a residue of compound 3, which was purified by flash chro- matography on silica gel (PE:EA = 1:1).
3.1.2.1 2-(2-methoxyphenyl)acetaldehyde (3a)
Colourless liquid 0.74g, yield: 78.8%.
3.1.2.2 2-(3-methoxyphenyl)acetaldehyde (3b)
Light red liquid 0.68g, yield: 72.3%.
3.1.2.3 2-(3-methoxyphenyl)acetaldehyde (3c)
Colourless liquid 0.88g, yield: 80.0%.
3.1.2.4 2-(3,4-dimethoxyphenyl)acetaldehyde (3d)
Colourless liquid 0.93g, yield: 71.0%.
3.1.2.5 2-(3,4,5-trimethoxyphenyl)acetaldehyde (3e)
Light red liquid 1.39g, yield: 92.7%.
3.1.3 General procedure for the synthesis of (Y-phenyl) methanamine 4
A mixture of substituted hydroxylamine hydrochloride (2.80g, 40mmol), and NaOH (1.60g, 40mmol) was dissolved in water (15mL) , and the CH3OH solution (15 mL) of substituted benzaldehyde (20mmol) was added, then the mixture was heated at reflux for 1h. Then the mixture was coolded down to room temperature and extracted with EA (30×3mL). The combined organic ex- tracts were washed with H2O (3×40mL), dried (MgSO4), and concentrated on a rotary evaporator at a bath tempe- rature of about 20℃ to give a residue of benzaldehyde oximes.
The above crude benzaldehyde oximes was solved in HOAc (50mL) at 60℃, and zinc dust (6.5g, 100mmol) was added in batches. Then the mixture was heated at 70℃ for 1h and filtered through a pad of Celite®. Then the filtrate was concentrated and adjusted pH to 10, and extracted with EA (50×3mL). The combined organic extracts were then washed with H2O (3×50mL), dried (MgSO4), and concentrated on a rotary evaporator at a bath temperature of about 20℃ to give a residue of compound 4, which wad purified by flash chromato- graphy on silica gel (CH2Cl2: CH3OH = 30: 1).
3.1.3.1 (4-methoxyphenyl)methanamine (4a)
Sticky liquid 2.61g; Isolated yield 95.3%; and FCC (CH2Cl2: CH3OH = 30: 1).
3.1.3.2 (3,4-dimethoxyphenyl)methanamine (4b)
White solid 2.97 g. Isolated yield 88.9%; FCC (CH2Cl2: CH3OH = 30: 1).
3.1.3.3 (3,4,5-trimethoxyphenyl)methanamine (4c)
White solid 3.35 g. Isolated yield 85.0%; FCC (CH2Cl2: CH3OH = 30: 1).
3.1.4 General procedure for the synthesis of 1-(Y- benzyl)-3,4-di(X-phenyl)-1H-pyrrole 1f-1q
A mixture of substituted phenyl acetaldehydes 3 (3 mmol), and (Y-phenyl) methanamine 4 (3mmol) was stirred at room temperature in THF (15mL) for 0.5 h under argon. Then AgOAc (1.01g, 6mmol) and NaOAc (0.49g, 6mmol) was added successively under argon, then the solution was heated at 60℃ for 8h. The mixture was coolded down to room temperature, filtered through a pad of Celite® and the Celite® was washed with EtOAc. The filtrate was evaporated under reduced pressure and the resulting residue was purified by flash column chro- matography to give the pure 1-(Y-benzyl)-3,4-di(X- phenyl)-1H-pyrrole 1f-1q.
3.1.4.11-(4-methoxybenzyl)-3,4-bis(2-methoxyphenyl)-1H-pyrrole (1f)
White solid 0.48g. Isolated yield 80.0%; FCC (PE/ DCM/EA, 20:20:1).1H NMR (500MHz, CDCl3) δ (ppm) 7.25-7.23 (d,= 8.3 Hz, 2H), 7.11-7.10 (d,= 7.0 Hz, 4H), 6.89-6.87 (m, 4H), 6.82-6.78 (m, 4H), 5.03 (s, 2H), 3.80 (s, 3H), 3.47 (s, 6H).13C NMR (126 MHz, CDCl3) δ (ppm) 159.15 (C), 156.27 (2C), 130.43 (2CH), 129.43 (C), 129.26 (2CH), 126.57 (2CH), 126.13 (2C), 120.97 (2CH), 120.12 (2CH), 119.88 (2C), 113.99 (2CH), 110.65 (2CH), 55.28 (CH3), 54.95 (2CH3), 53.00 (CH2). HRMS (ESI)/: calcd for M+C26H26NO3, 400.1907; found, M+400.1905.
3.1.4.2 1-(4-methoxybenzyl)-3,4-bis(4-methoxyphenyl)- 1H-pyrrole (1g)
White solid 0.45g. Isolated yield 75.1%; FCC (PE/ DCM/EA, 20:20:1).1H NMR (500 MHz, CDCl3) δ (ppm) 7.21-7.16 (m, 6H), 6.89-6.88 (d,= 8.3Hz, 2H), 6.80- 6.678 (d,= 8.3Hz, 4H), 6.70 (s, 2H), 5.00 (s, 2H), 3.80 (s, 3H), 3.78 (s, 6H).13C NMR (126 MHz, CDCl3) δ (ppm) 159.23 (C), 157.61 (2C), 129.37 (6CH), 128.97 (2CH), 128.47 (C), 122.86 (2C), 119.78 (2C), 114.10 (2CH), 113.52 (4CH), 55.29(CH3), 55.26(2CH3), 52.98 (CH2). HRMS (ESI)/: calcd for M+C26H26NO3, 400.1907; found, M+400.1900.
3.1.4.31-(3,4-dimethoxybenzyl)-3,4-bis(2-methoxyphen-yl)-1H-pyrrole (1h)
White solid 0.54g. Isolated yield 83.8%; FCC (PE/ DCM/EA, 20:20:1).1H NMR (500 MHz, cdcl3) δ 7.21- 7.20 (d,= 8.0 Hz, 2H), 6.90-6.88 (d,= 8.0Hz, 2H), 6.83-6.81 (d,= 7.8Hz, 2H), 6.77-6.75 (m, 6H), 5.00 (s, 2H), 3.85 (s, 6H), 3.80 (s, 3H), 3.67 (s, 6H).13C NMR (126 MHz, cdcl3) δ 159.28 (C), 148.30 (2C), 147.02 (2C), 129.34 (C), 128.97 (2CH), 128.68 (2C), 123.08 (2C), 120.41 (2CH), 119.80 (2CH), 114.13 (2CH), 112.03 (2CH), 110.96 (2CH), 55.83 (2CH3), 55.61 (2CH3), 55.31 (CH3), 53.04 (CH2). HRMS (ESI)/: calcd for M+C28H30NO5, 460.2118; found, M+460.2113.
3.1.4.41-(3,4-dimethoxybenzyl)-3,4-bis(4-methoxyphen-yl)-1H-pyrrole (1i)
White solid 0.45g. Isolated yield 76.3%; FCC (PE/ DCM/EA, 20:20:1).1H NMR (500 MHz, CDCl3) δ (ppm) 7.17-7.16 (d,= 6.8 Hz, 4H), 6.83-6.78 (m, 7H), 6.71 (s, 2H), 4.99 (s, 2H), 3.87 (s, 3H), 3.85 (s, 3H), 3.78 (s, 6H).13C NMR (126 MHz, CDCl3) δ (ppm) 157.62 (2C), 149.12 (C), 148.66 (C), 129.72 (C), 129.35 (4CH), 128.41 (2C), 122.89 (CH), 120.11 (2C), 119.81 (2CH), 113.52 (4CH), 111.09 (CH), 110.74 (CH), 55.89 (2CH3), 55.14 (2CH3), 53.30 (CH2). HRMS (ESI)/: calcd for M+C27H28NO4, 430.2013; found, M+430.2006.
3.1.4.53,4-bis(2-methoxyphenyl)-1-(3,4,5-trimethoxy- benzyl)-1H-pyrrole (1j)
White solid 0.54g. Isolated yield 78.3%; FCC (PE/ DCM/EA, 20:20:1).1H NMR (500 MHz, CDCl3) δ (ppm) 7.14-7.11 (m, 4H), 6.89 (s, 2H), 6.83-6.78 (m, 4H), 6.49 (s, 2H), 5.03 (s, 2H), 3.84 (s, 9H), 3.48 (s, 6H).13C NMR (126 MHz, CDCl3) δ (ppm) 156.28 (2C), 153.35 (2C), 137.30 (C), 133.10 (C), 130.43 (2CH), 126.69 (2CH), 125.95 (2C), 121.13 (2C), 120.12 (4CH), 110.66 (2CH), 104.70 (2CH), 60.83 (CH2), 56.04 (2CH3), 54.93 (2CH3), 53.71 (CH3). HRMS (ESI)/: calcd for M+C28H30NO5, 460.2118; found, M+460.2112.
3.1.4.63,4-bis(4-methoxyphenyl)-1-(3,4,5-trimethoxy- benzyl)-1H-pyrrole (1k)
White solid 0.62g. Isolated yield 89.9%; FCC (PE/ DCM/EA, 20:20:1).1H NMR (500 MHz, CDCl3) δ (ppm) 7.17-7.16 (d,= 7.1 Hz, 4H), 6.80-7.78 (d,= 7.5 Hz, 4H), 6.72 (s, 2H), 6.45 (s, 2H), 4.98 (s, 2H), 3.83 (s, 9H), 3.78 (s, 6H).13C NMR (126 MHz, CDCl3) δ (ppm) 157.70 (2C), 153.48 (2C), 133.04 (C), 130.19 (C), 129.40 (4CH), 128.37 (2C), 123.09 (2C), 119.96 (2CH), 113.58 (4CH), 104.54 (2CH), 60.88 (CH2), 56.13 (2CH3), 55.18 (2CH3), 53.75 (CH3). HRMS (ESI)/: calcd for M+C28H30NO5, 460.2118; found, M+460.2112.
3.1.4.7 3,4-bis(3,4-dimethoxyphenyl)-1-(4-methoxyben-zyl)-1H-pyrrole (1l)
White solid 0.58g. Isolated yield 84.2%; FCC (PE/ DCM/EA, 20:20:1).1H NMR (500 MHz, CDCl3) δ (ppm) 7.21-7.20 (d,= 8.0 Hz, 2H), 6.90-6.88 (d,= 8.0 Hz, 2H), 6.83-6.81 (d,= 7.8 Hz, 2H), 6.77-6.75 (m, 6H), 5.00 (s, 2H), 3.85 (s, 6H), 3.80 (s, 3H), 3.67 (s, 6H).13C NMR (126 MHz, CDCl3) δ (ppm) 159.28 (C), 148.30 (2C), 147.02 (2C), 129.34 (C), 128.97 (2CH), 128.68 (2C), 123.08 (2C), 120.41 (2CH), 119.80 (2CH), 114.13 (2CH), 112.03 (2CH), 110.96 (2CH), 55.83 (2CH3), 55.61 (2CH3), 55.31 (CH3), 53.04 (CH2). HRMS (ESI)/: calcd for M+C28H30NO5, 460.2118; found, M+460.2113.
3.1.4.81-(3,4-dimethoxybenzyl)-3,4-bis(3,4-dimethoxy-phenyl)-1H-pyrrole (1m)
White solid 0.64g. Isolated yield 87.2%; FCC (PE/ DCM/EA, 20:20:1).1H NMR (500 MHz, CDCl3) δ (ppm) 6.86-6.81 (m, 4H), 6.79-6.75 (m, 7H), 5.00 (s, 2H), 3.88 (s, 3H), 3.86 (s, 3H), 3.85 (s, 6H), 3.67 (s, 6H).13C NMR (126 MHz, CDCl3) δ (ppm) 149.12 (C), 148.71 (C), 148.27 (2C), 147.01 (2C), 129.58 (C), 128.61 (2C), 123.08 (2C), 120.37 (2CH), 120.15 (CH), 119.79 (2CH), 111.99 (2CH), 111.11 (CH), 110.92 (2CH), 110.80 (CH), 55.90 (2CH3), 55.80 (2CH3), 55.57 (2CH3), 53.35 (CH2). HRMS (ESI)/: calcd for M+C29H32NO6, 490.2224; found, M+490.2217.
3.1.4.93,4-bis(3,4-dimethoxyphenyl)-1-(3,4,5-trimeth- oxybenzyl)-1H-pyrrole (1n)
White solid 0.69g. Isolated yield 88.6%; FCC (PE/ DCM/EA, 20:20:1).1H NMR (500 MHz, CDCl3) δ (ppm) 7.10-7.08 (d,= 7.9 Hz, 1H), 7.06 (s, 1H), 6.98 (s, 1H), 6.93-6.92 (d,= 7.9 Hz, 1H), 6.87-6.84 (m, 3H), 6.47 (s, 1H), 6.28 (s, 2H), 5.06 (s, 2H), 3.92 (s, 3H), 3.88 (s, 6H), 3.80 (s, 3H), 3.75 (s, 6H), 3.72 (s, 3H).13C NMR (126 MHz, CDCl3) δ (ppm) 153.48 (2C), 149.02 (C), 148.59 (C), 148.39 (C), 147.21 (C), 137.00 (C), 135.60 (C), 134.48 (C), 128.72 (C), 125.62 (C), 124.62 (C), 121.29 (CH), 118.49 (CH), 117.13 (CH), 112.18 (CH), 111.50 (CH), 111.00 (CH), 108.61 (CH), 106.45 (CH), 103.27 (2CH), 60.83 (CH3), 56.06 (2CH3), 55.93 (CH3), 55.88(CH3), 55.83 (CH3), 55.60 (CH3), 50.90 (CH2). HRMS (ESI)/: calcd for M+C30H34NO7, 520.2330; found, M+520.2324.
3.1.4.10 1-(4-methoxybenzyl)-3,4-bis(3,4,5-trimethoxy-phenyl)-1H-pyrrole (1o)
White solid 0.67g. Isolated yield 86.0%; FCC (PE/ DCM/EA, 20:20:1).1H NMR (500 MHz, CDCl3) δ (ppm) 7.22-7.20 (d,= 8.2 Hz, 2H), 6.90-6.89 (d,= 8.2 Hz, 2H), 6.79 (s, 2H), 6.47 (s, 4H), 5.02 (s, 2H), 3.81 (s, 9H), 3.68(s,12H).13CNMR (126 MHz, CDCl3) δ(ppm) 159.33 (C), 152.75 (4C), 136.05 (2C), 131.28 (2C), 129.18 (C), 128.93 (2CH), 123.42 (2C), 120.00 (2CH), 114.18 (2CH), 105.59 (4CH), 60.92 (2CH3), 55.90 (4CH3), 55.33 (CH3), 53.12 (CH2). HRMS (ESI)/: calcd for M+C30H34NO7, 520.2330; found, M+520.2315.
3.1.4.111-(3,4-dimethoxybenzyl)-3,4-bis(3,4,5-trimeth-oxyphenyl)-1H-pyrrole (1p)
White solid 0.69g. Isolated yield 83.7%; FCC (PE/ DCM/EA, 20:20:1).1H NMR (500 MHz, CDCl3) δ (ppm) 6.87-6.83 (m, 2H), 6.80 (s, 3H), 6.48 (s, 4H), 5.03 (s, 2H), 3.89(s, 3H), 3.88 (s, 3H), 3.81 (s, 6H), 3.69 (s, 12H).13C NMR (126MHz, CDCl3) δ (ppm) 152.72 (4C), 149.15 (C), 148.80 (C), 136.04 (2C), 131.22 (2C), 129.40 (C), 123.40 (2C), 120.16 (CH), 119.98 (2CH), 111.14 (CH), 110.81 (CH), 105.56 (4CH), 60.88 (2CH3), 55.93(CH3), 55.87 (5CH3), 53.43 (CH2). HRMS (ESI)/: calcd for M+C31H36NO8, 550.2435; found, M+550.2426.
3.1.4.12 1-(3,4,5-trimethoxybenzyl)-3,4-bis(3,4,5-trime- thoxy- phenyl)-1H-pyrrole (1q)
White solid 0.76g. Isolated yield 87.5%; FCC (PE/ DCM/EA, 20:20:1).1H NMR (500MHz, CDCl3) δ (ppm) 6.81 (s, 2H), 6.48 (s, 6H), 5.01 (s, 2H), 3.84 (s, 9H), 3.81 (s, 6H), 3.69 (s, 12H).13C NMR (126MHz, CDCl3) δ (ppm) 153.53 (2C), 152.76 (4C), 137.61 (C), 136.12 (2C), 132.61 (C), 131.15 (2C), 123.54 (2C), 120.08 (4CH), 105.60 (2CH), 104.76 (2CH), 60.89 (CH3), 56.19 (2CH3), 55.90 (6CH3), 53.87 (CH2).
3.2 Bioactivity Study
3.2.1 Cell culture
Rat PC12 cells (adrenal gland; pheochromocytoma) were obtained from Cell Bank of Chinese Academy of Sciences (Shanghai, China). PC12 cells were cultured in Dulbecco’s Modified Eagle’s medium (high glucose, Gibco, CA, USA) supplemented with 10% fetal bovine serum (FBS) (Gibco, USA), 100UmL−1penicillin and 100ugmL−1streptomycin in a 95% humidified atmosphere with 5% CO2at 37℃. Cells were passaged with trypsin (0.025% EDTA) every 3 days with the subcultivation ratio of 1:6. Cell used was within 5-20 passages.
3.2.2 Cell viability assay
All synthesized analogues were dissolved in DMSO (Solarbio, CN) in a final concentration of 0.1% initially and further diluted with cell culture medium to desired molL−1concentrations for cellular viability assay.
For cellular viability assay (Yin., 2013; He., 2016; Tong., 2016), a 96-wells plate was seeded with 5000cellswell−1 in 100μL complete cell culture medium. After 24h, 100μL complete medium containing serial concentrations of each compound was added to each well. After another 24h cultured, cell viability was measured by MTT assay as described (Mosmann, 1983).
For the neuroprotective assay, PC12 cells were seeded into 96-well plates at a density of 5000cellswell−1, and cultivated for 24h. Cells were 4h prior exposure to 8mmolL−1glutamate, followed by compounds added at a final concentration of 5, 10, 20 and 40μmolL−1respectively, and then treating for another 24h. Cell viability was measured by MTT assay.
Acknowledgements
This study is supported by the National Natural Science Foundation of China (Nos. 21171154 and 81672585), the Key Technology Fund of Shandong Province (No. 2016ZDJS07 A07), and the Taishan Scholar Fellowship of Shandong Province in China to L. Z.
Ayedi, M. A., Le Bigot, Y., Ammar, H., Abid, S., El Gharbi, R., and Delmas, M., 2013. ChemInform abstract: Synthesis of primary amines by one-pot reductive amination of aldehydes., 43 (44): 2127-2133, DOI: 10.1002/chin.201343 053.
Bailly, C., 2014. Lamellarins: A tribe of bioactive marine natural products. In:. LA Barre, S., and Kornprobst, J., eds., Wiley-VCH Verlag GmbH & Co. KGaA, 377-386.
Bharate, J. B., Singh, S., Wani, A., Sharma, S., Joshi, P., Khan, I. A., Kumar, A., Vishwakarma, R. A., and Bharate, S. B., 2015. Discovery of 4-acetyl-3-(4-fluorophenyl)-1-(p-tolyl)-5- methy-lpyrrole as a dual inhibitor of human P-glycoprotein andNor A efflux pump., 19 (13): 5424-5431, DOI: 10.1039/ c5ob00246j.
Fan, A. L., Lin, W. H., and Jia, Y. X., 2011. Recent progress in the research on lamellarins and related pyrrole-derived alkaloids from marine organisms., 5 (20): M5, DOI: 10.5246/jcps.2011.05. 054.
Fan, H., Peng, J., Hamann, M. T., and Hu, J., 2008. ChemInform abstract: Lamellarins and related pyrrole-derived alkaloids from marine organisms., 18 (39): 264-287, DOI: 10.1002/chin.200818251.
Hao, C., Gao, L., Zhang, Y., Wang, W., Yu, G., Guan, H., Zhang, L., and Li, C., 2015. Acetylated chitosan oligosaccharides act as antagonists against glutamate-induced PC12 cell deathBcl-2/Bax signal pathway., 3 (13): 1267-1289, DOI: 10.3390/md13031267.
He, Y., Lan, Y., Liu, Y., Yu, H., Han, Z., Li, X., and Zhang, L., 2016. Pingyangmycin and bleomycin share the same cyto-cytotoxicity pathway. Molecules, 7 (21): DOI: 10.3390/molecules 21070862.
Li, Q., Fan, A., Lu, Z., Cui, Y., Lin, W., and Jia, Y., 2010. One-pot AgOAc-mediated synthesis of polysubstituted pyr- roles from primary amines and aldehydes: Application to the total synthesis of purpurone., 18 (12): 4066- 4069, DOI: 10.1021/ol101644g.
Liu, R., Liu, Y., Zhou, Y. D., and Nagle, D. G., 2007. Molecular targeted antitumor agents. 15. Neolamellarins from the marine spongeinhibit hypoxia-inducible factor-1 activation and secreted vascular endothelial growth factor production in breast tumor cells., 11 (70): 1741-1745, DOI: 10.1021/np070206e.
Mosmann, T., 1983. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays., 65 (1-2): 55-63, DOI: 10.1016/0022-1759(83)90303-4.
Plisson, F., Huang, X. C., Zhang, H., Khalil, Z., and Capon, R. J., 2012. Lamellarins as inhibitors of P-glycoprotein-mediated multidrug resistance in a human colon cancer cell line., 7 (7): 1616-1623, DOI: 10.1002/ asia.201101049.
Quesada, A. R., Grávalos, M. G., and Puentes, J. F., 1996. Polyaromatic alkaloids from marine invertebrates as cytotoxic compounds and inhibitors of multidrug resistance caused by P-glycoprotein., 74 (5): 677-682, DOI: 10.1038/bjc.1996.421.
Rosowsky, A., Chen, H., Fu, H., and Queener, S. F., 2003. Synthesis of new 2,4-Diaminopyrido[2,3-d]pyrimidine and 2,4- Diaminopyrrolo[2,3-d]pyrimidine inhibitors of,, anddihydrofolate reductase., 1 (11): 59-67, DOI: 10.1016/S0968-0896(02)00325-5.
Tong, S., Zhang, M., Wang, S., Yin, R., Yu, R., Wan, S., Jiang, T., and Zhang, L., 2016. Isothiouronium modification empowers pyrimidine-substituted curcumin analogs potent cytotoxicity and Golgi localization., (123): 849-857, DOI: 10.1016/j.ejmech.2016. 07.071.
Yin, R., Jiang, L., Wan, S., and Jiang, T., 2015. Efficient syntheses of permethylated derivatives of neolamellarin A, a pyrrolic marine natural product., 2 (14): 329-334, DOI: 10.1007/s11802-015-2372-z.
Yin, R., Zhang, M., Hao, C., Wang, W., Qiu, P., Wan, S., Zhang, L., and Jiang, T., 2013. Different cytotoxicities and cellular localizations of novel quindoline derivatives with or without boronic acid modifications in cancer cells.(), 76 (49): 8516-8518, DOI: 10.1039/c3cc 45203d.
Zhang, P. Y., Wong, I. L., Yan, C. S., Zhang, X. Y., Jiang, T., Chow, L. M., and Wan, S. B., 2010. Design and syntheses of permethyl ningalin B analogues: Potent multidrug resistance (MDR) reversal agents of cancer cells., 14 (53): 5108-5120, DOI: 10.1021/jm100035c.
(Edited by Ji Dechun)
(Received April 24, 2017; revised June 8, 2017; accepted October 22, 2017)
© Ocean University of China, Science Press and Springer-Verlag GmbH Germany 2018
# These authors contributed equally to this work.
Tel:0086-532-82033054E-mail: zhanglj@qduhospital.cn; jiangtao@ouc.edu.cn
杂志排行
Journal of Ocean University of China的其它文章
- Offshore Fault Geometrics in the Pearl River Estuary, Southeastern China: Evidence from Seismic Reflection Data
- Application of Geoid Anomalies to the Tectonic Research in the East Asian Continental Margin
- Middle Holocene Organic Carbon and Biomarker Records from the South Yellow Sea: Relationship to the East Asian Monsoon
- Mesozoic Deformation and Its Geological Significance in the Southern Margin of the South China Sea
- Optimization of Shanghai Marine Environmental Monitoring Sites in the Identification of Boundaries of Different Water Quality Grades
- Seasonal Variation of Environmental Variables and Phytoplankton Community Structure and Their Relationship in Liaodong Bay of Bohai Sea, China
