Effects of Salinity and Temperature on Growth and Survival ofJuvenileIwagaki Oyster Crassostrea nippona
2018-08-24WANGTaoandLIQi
WANG Tao, and LI Qi, 2), *
Effects of Salinity and Temperature on Growth and Survival ofJuvenileIwagaki Oyster
WANG Tao1), and LI Qi1), 2), *
1),,,266003,2),,266003,
Iwagaki oysteroccurs naturally along the coasts of Japan and Korea. Because of its unique flavor, delicious taste,edibility during the summer and high commercial value, it has been identified as a potential aquaculture species.To determine the optimum aquaculture conditions and provide necessary information for mass production of the juvenile, the effects of six salinities (15, 20, 25, 30, 35 and 40) and five temperatures (16, 20, 24, 28 and 32℃) on growth and survival of juvenilewere examined in this study. In the salinity experiment, the largest values of mean shell height and growth rate were observed at salinity 25 (20.96±0.36mm and 172.0µmd−1, respectively), which were significantlydifferent (<0.05)with those of other treatments, except at salinity 30 (20.56±1.05mm and 160.3µmd−1, respectively) (>0.05). The maximum survival rate 84.44% was always observed at salinity 20, and there was no significant difference (>0.05)in survival rate among salinities varying between 15 and 35. In the temperature-related experiments, the highest growth and survival rates of juvenile were observed at 24℃ (180.8µmd−1and 84.4%) and 28℃ (190.7µmd−1and 83.3%), respectively, on day 20, and showed significantly (<0.05) larger size and higher survival rate than any other groups. Both juvenile survival and growth were significantly depressed at extreme salinities (15, 40) and temperatures (16℃, 32℃). Based on the results of the present study, a salinity range from 25 to 30 and a temperature range from 24℃ to 28℃ are considered optimal conditions for survival and growth ofjuvenile.
; juvenile; salinity; temperature; survival; growth
1 Introduction
Iwagaki oysterbelongs to Bivalvia, Ostreidae, and is a large sessile oyster inhabiting intertidal hard grounds and reefs along the coast of East Asia including Japan and Korea (Itoh., 2004). Because of its unique flavor, delicious tasteand marketability in summer when Pacific oysteris unavailable, the commercial price ofis estimated as high as five folds ofin Japan (Itoh., 2004).Thus this species has a market prospect and high potential value of large scale farming. Traditionally,farming is largely dependent on natural seeds (Tanaka., 2010). However, such seed collection is labor intensive, often unreliable and available for a short season. Moreover, with the increasing interest ofculture, natural seed collection may not satisfy the demand of aquaculture, and seed production is always in short supply due to the deficiency of facilities (Tanaka., 2010; Fujiwara, 1995). These issues have significantly constrained the cultivation of, hence, devel- oping proper breeding, nursery, and aquaculture techniques for mass production of juveniles are crucial for meeting the success offarming.
Autecological study of bivalves has clearly demonstrated that environmental factors play an important role in the development, growth and survival of aquatic animals (Tang., 2012).Among them, salinity and temperature are considered to be the most important physical parameters affecting the physiological responses and survival of aquatic organisms,which have been described as ‘master factors’ (Re, 2005; Kinne,1964).Salinity imposes an additional metabolic load (Bao and You, 2004) and affects biological activities, including those related to immune responses (Taylor., 2007), fertilization (Verween, 2007), development of embryos (Davis and Calabrese, 1969), survival and growth of larvae and juvenile (Huo, 2014). Temperature modifies energy flow, which regulates the rate of biological processes (Scheltema, 1967).Accordingly, the effects of these two factors have been described for numerous marine species (Ko.,2014; Huo., 2014; Xu., 2011). For, although previous studies have documentedseasonal variations in reproductive activity and biochemical composition, karyotype, and culture method (Itoh., 2004; Okumura.,2005; Adachi., 2014), and information pertaining tofor spat production in hatchery is still limited. Large-scale production of an aquaculture species must be based on adequate knowledge of its ecological requirements for optimum development. Moreover,inhabits the low intertidal and shallow sub-tidal zones at depth of 10–20m (Li, 2007), making them susceptible to changes in environmental conditions. Therefore, evaluating the effects of exogenous factors, especially temperature and salinity, on the growth and survival of, not only improves our understanding of ecological habits of this species, but also provides basic data for the conservation and restocking of natural populations.
This study investigated the effects of different salinity and temperature levels on the growth and survival of juvenile, aiming to determine the optimum rearing conditions ofin hatcheries. The information obtained in this study will be valuable for the further development ofaquaculture industry.
2 Materials and Methods
2.1 Maintenance of Juveniles Prior to the Experiments
At the beginning of the experiments, a total of 1200 juvenilecultivated at Rushan Bay (36˚43´– 37˚36´N and 121˚28´–121˚39´E), Shandong Province, China, were collected randomly in February 2016. The animals were transported to Haiyi Hatchery in Yantai, and acclimatized in 80-L plastic containers with static aerated seawater at a salinity of 33 and a temperature of 3℃ for two weeks. The acclimation conditions represented the generalcultivation conditions of the northern Chinese aquaculture areas in winter. Initial mean shell height (SH) each group at the beginning of temperature and salinity experiments were showed in Table 1.Juveniles were fed daily a mixture ofand(1:1) at a final concentration of 10 cellsmL−1d−1. All containers were aerated with air diffusers and seawater was changed daily.The temperature of seawater was increased to the desired level with thermostatic immersion heaters before being added to the con- tainers. A constant room temperature was maintained with an air conditioner. Only juveniles showing healthy signs and normal behavior were used in the experiments.

Table 1 Initial mean shell height for each group of juveniles at the beginning of temperature and salinity experiments
2.2 Experimental Design
Based on water temperature and salinity data along the coast of Shandong Province, juvenilewere acclimated at six salinities (15, 20, 25, 30, 35 and 40) and five temperatures (16, 20, 24, 28 and 32℃). Salinity was adjusted at a rate of 2d−1with dechlorinated freshwater (dilution) or with artificial sea salt (increase) until the required salinities (15, 20, 25, 30, 35, 40) with deviation ±0.5 were reached. Temperature was adjusted at a rate of 2℃d−1by holding the experimental containers in a water bath with thermostatic immersion heaters until the test temperatures (16, 20, 24, 28 and 32℃) were reached. Salinity treatments were carried out at a constant temperature of 22℃, when ambient seawater temperature in the field site was 20 to 24℃, while the salinity was kept stable at 32 in temperature treatments. The salinity of the experimental solutions was checked using a portable refractometer. Thermostatically-controlled heaters were used to maintain the temperature of seawater to be within 0.5℃. Each treatment group included three replicatesto ensure the accuracy of the results, and each replicate consisted of 30 individuals.
2.3 Measurement of Daily Growth Rate and Survival Rate
The daily growth rate was the ratio of the difference of the measured shell height (SH) and initial height divided by the number of days:
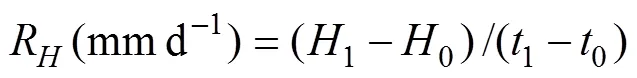
Survival rate (R) was the ratio of the measured survival and the initial stocking amount:
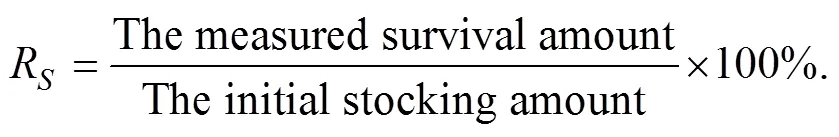
Oysters were exposed for five weeks to the different temperatures and salinities, whilethe growth and survival were measured every five days in each group of juveniles. Criteria for mortality of individuals were with a permanent wide valve gape and showing no response to the touch of a glass rod or fine brush. Individuals that did not survive the duration of the experiment were removed immediately.
2.4 Statistical Analysis
One-way ANOVA was used to test the effects of salinity and temperature on the survival and growth of, and differences between means were compared using the least significant difference (LSD) test and Duncan’s multiple range test in SPSS (V.18) software. Significance level for all analysis was set at<0.05.
3 Results
3.1 Effects of Salinity on the Survival and Growth of Juvenile
Stock salinity had a significant effect on the size of juvenilein terms of shell height at the end of the experiment (Fig.1). In general, the shell height of juvenile progressively increased from salinities 15 to 25 and decreased from salinities 25 to 40.The largest value of shell height occurred at salinity 25 (20.96±0.36mm), and was significantly (<0.05) different from the values in other treatments, except at salinity 30 (20.56±1.05mm) (>0.05). At salinities higher than 30, differences in the shell height were not statistically significant (>0.05). The lowest value of SH was observed at extreme salinity of 40 (16.16±0.78mm), and was significantly (<0.05) lower than the values in other treatments, except at salinity of 15 (17.36±0.35mm) and 35 (17.06±0.91mm) (>0.05).

Fig.1 Effect of salinity on shell height of Crassostrea nippona juveniles at the end of the experiments.Each bar represents the mean±SEof three replicates. The same lowercase of same trait mean no significant difference (P>0.05).
Daily growth rate ofwas significantly affected by salinity (Table 2). With increasing salinity, the value of growth rate increased until the salinity was 25 (172.0μmd−1), while they were not significantly different from that at salinity 30 (160.3μmd−1), and then decreased. The lowest growth rate (35.0μmd−1) was observed at the highest salinity 40, was significantly (<0.05) different from the values in other treatments.
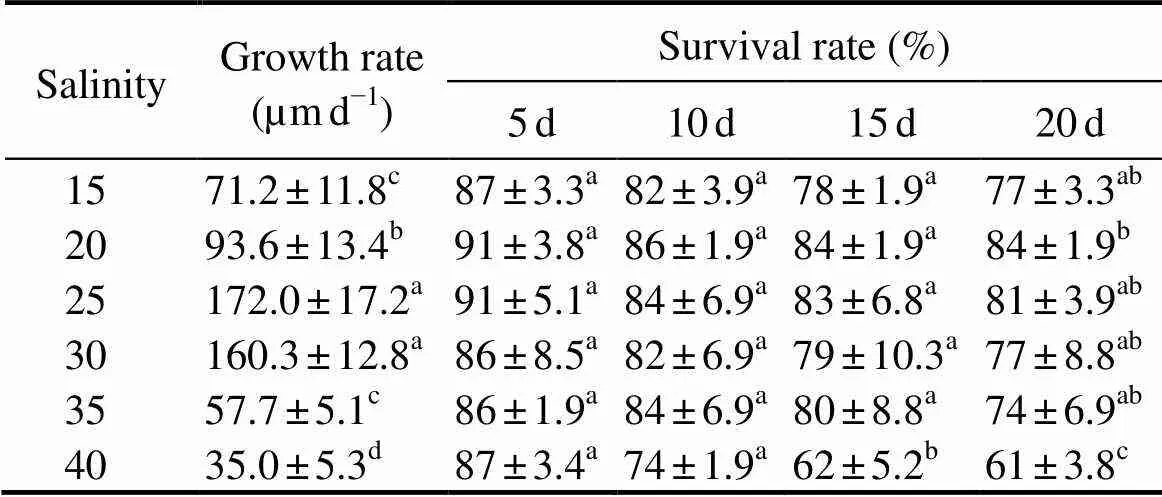
Table 2 Survival and growth rates of Crassostrea nippona juvenile held at different salinities
Notes: Within a column, values followed by different letters are sig- nificantly different (<0.05,=30). Data are given as Mean±SE.
The survival rates of juvenile were not significantly different among salinities 15 to 40 on days 5 and 10, but a significant effect of salinity was observed after day 15 (Table 2). The survival rate at salinity 40 was high during the first 5 days and dropped sharply from day 10 to day 15. Therefore, salinity 40 was not included in the comparison between treatments in relation to optimum survival rates and optimum growth.Survival rate on day 15 was the highest at salinity 20, but there was no significant (>0.05) differences in survival between different salinities during any trail period, except at salinity 40 (<0.05). Survival rate decreased progressively from salinities 35 to 40 on day 20.
3.2 Effects of Temperature on the Survival and Growth of Juvenile
From 16 to 28℃, the shell height ofincreased progressively as temperature increased, and decreased sharply at temperature of 32℃ (Fig.2). The maxi- mal shell heightswere observed at temperatures of 24℃ (21.27±0.41mm) and 28℃ (22.31±0.69mm), which were significantly (<0.05) different from those measured at all other temperatures. The minimum shell heightswere observed at temperatures of 16℃ (17.45±0.35mm) and 32℃ (17.38±1.35mm),which were significantly dif- ferent from the values measured at other temperatures (<0.05).

Fig.2 Effect of temperature on shell height ofjuveniles at the end of the experiments. Each bar represents the mean±SEof three replicates. The same lowercase of same trait mean no significant difference (>0.05).
In general, daily growth rate of the shell height progressively increased from 16 to 28℃ and decreased from 28 to 32℃ (Table 3). Daily growth rate was the highest temperaturesof 24℃ (180.8μmd−1) and 28℃ (190.7μmd−1). At temperature of 32℃, daily growth rate was 73.0μmd−1, significantly (<0.05) slower than any other groups, except at temperature of 16℃ (73.3μmd−1) (>0.05).
The survival rate of juvenile decreased progressively with increasing experiment period (Table 3). The survival rate at16℃ was high during the first 5 days and dropped sharply from day 10 to day 20. Therefore, 16℃ was not included in the comparison between treatments in relation to optimum survival rates and optimum growth. Table 3 showed that there was no significant difference among different temperatures of 20℃ to 32℃ on day 5, 10 and 15, but a significant effect of temperature was observed after day 20. Among all treatments, it was noted that on day 20, survival rate was significantly (<0.05) higher at 24 and 28℃ than that at 20 and 32℃, ranging from 83% to 84%. Survival rate of the juvenile was longer at 20℃ than that at 32℃, whereas survival rate displayed no significant (>0.05) difference between these two treatments.

Table 3 Survival and growth rates of Crassostrea nippona juvenile held at different temperatures
Notes:Within a column, values followed by different letters are significantly different (<0.05,=30). Data are given as Mean±SE.
4 Discussion
According to the observed mortalities and daily growth rates, different salinities have significant effects on juvenileOur findings agree with those of Yao. (2015) who studied the effect of salinity on larval growth, survival and development of the oystersand. Previous studies have demonstrated that oysters are generally euryhaline species, although different species may differ in the optimal salinity that they prefer (Xu, 2011). For example, European flat oysterlarvae had the high growth rates and survival at an optimum salinities of 24–33 (Walne, 1956). Sydney rock oysterlarvae had the highest growth rates at salinities 23–39, and the highest survival rates at salinities 27–39 (Nell and Holliday, 1988). Mangrove oysterhad the highest embryo development rates at salinities of 25–37 (Dos Santos and Nascimento, 1985). For, there was no significant difference among different salinities of 15–35 after 20 days of cultivation, indicating that 15–35 is the optimum salinity for the survival of juvenile. However, the highest growth rates were observed at salinity of 25 and 30. When the salinity were adjusted to be lower or higher than this range, growth rate of juvenile was significantly affected, resulting in a decreased shell length. Therefore, a salinity range of 25–30 is the optimum salinity for the growth of juvenile. Similarly, in Kumamoto oyster, salinities higher than 25 had significant negative effects on the larvae development and growth (Xu., 2011). The survival and growth of spat, juvenile and adultwere observed to be depressed directly with lower salinity.Combined withsub- optimal elevated temperature, lower salinities have negative effects in all size classes of, resulting in dramatically greater mortality (Rybovich., 2016).
In the present study, rearingin the lowest (15 and 20), medium (25 and 30), and the highest salinities (35 and 40) had significant effects on juvenile growth. A similar result was also observed inlarvae, in which the lowest growth rate and survival rate were measured at salinities of 14, 18 and 34 on day 13. The mechanisms through which salinity affects marine mollusks remain unclear. However, previous studies indicate that there were two possible reasons for decreased growth rates and increased mortality under extreme salinity conditions. Firstly, reduced feeding rates could be a major driver for reduced growth rates in extreme salinity since oysters completely seal themselves off by closing their shells when salinity drops too low or increases too high (Berger and Kharazova, 1997). For example, in the European flat oyster, salinity were found to significantly affect absorption efficiency, filtration rate, and metabolic response, further lend to retarder growth of larvae. (Hutchinson and Hawkins, 1992). Low energy utilization efficiency of the body could be the other reason. Like other marine molluscs,was an osmotic conformer (Berger and Kharazova, 1997). Additional energy is required for the maintenance of water and mineral balance in body fluids and cells when juvenilewere cultivated at suboptimal salinity conditions (Berger and Kharazova, 1997). This conclusion agrees with that of Forcucci and Lawrence (1986), who unequivocally demonstrated that either additional energy is required for the maintenance or energy is used inefficiently that can cause the slow growth ofwhen salinity was adjusted to a level that was lower or higher than optimal salinity ranges.
Temperature is another important environmental variable that can influence juvenile survival and growth. In the present study, minimum growth and survival rate were observed at 16℃, and as temperature increased, these values reached the maximum at 24 and 28℃. This agrees with many other studies on the growth of mollusks that juveniles generally grew more quickly at the higher temperatures (Klinzing and Pechenik, 2000; Pechenik and Heyman, 1987). Davis and Calabrese (1969) suggested that the failure of marine bivalve species to grow at low temperatures appears to be a result of their inability to digest the available food, although they can survive and ingest food for a long time. The appropriate improvement of cultivation temperature could promote the activities of certain digestive enzymes, and increase the assimilation efficiency of the organism, subsequently leading to an increase in somatic growth (Bayne., 1999). However, with the temperature rising from 28 to 32℃, survival and growth of juvenile decreased sharply. This has been explained by the increase in growth of potential pathogenic ciliates and bacteria in cultures, leading to the proliferation of invaders or fouling organisms that bring their own microflora and severe disease problems, and further reducing oxygen availability (Gruffydd and Beaumont, 1972). Moreover, along with the increase of temperature, more energy was allocated to the osmoregulation and less to the protein synthesis, which resulted in the depletion of glycogen reserves and protein content in tissues, and eventually led to less favorable energy balance and smaller maximum size (Ivanina., 2013). Therefore, the temperature of 24–28℃ is optimum for the growth and survival of juvenile. Similarly, optimum larval development and settlement of the oysteroccurred at 27℃ (Rico-Villa., 2009). For, the average rate of growth of larvae increased progressively as the temperature increased from 10to 25or 27.5℃ and then decreased at 30and 32.5℃ (Davis and Calabrese, 1969).
Another notable result in the present study is that juvenile cultivated at 16℃ displayed lower survival rates than at 32℃, even though there was no significant difference between these two extreme temperatures on growth rates. The present result indicated that juvenile C.had greater adaptability to high temperature than low temperature. This may be related to the summer breeding habits of C.. The parentalhas a sexual maturation between August and September, which is the period with the highest water temperature of the year (Okumura., 2005). Similar phenomenon also shown in other marine bivalves, includingand(Parker., 2009; You., 2001).
In summary, the results of this study clearly demonstrate that juveniles ofcan tolerate a wide range of salinities (15–35) and a wide range of temperatures (20–32℃),while exhibit larger shell height and better survival at salinities 25–30 and 24–28℃. Hence, culturing this species at lower salinities of 25–30 and higher temperatures of 24–28℃ will likely produce a high yield and benefit juvenile.
Acknowledgements
This study was supported by the grants from the Key Research and Development Program of Shandong Province (No. 2016ZDJS06A06), the National Natural Science Foundation of China (No. 31772843), and the Major Project for Tianjin Seed Technology (No. 15ZXZYNC00 050).
Adachi, K., Yokoi, T., Inoue, K., Yoshinaga, T., and Okumura, S. I., 2014. Karyotype revision in the Iwagaki oyster., 17: 9-10.
Bao, Y. B., and You, Z. J., 2004. Influences of several environmental factors on growth in marine shellfish larvae., 23: 39-41.
Bayne, B. L., Svensson, S., and Nell, J. A., 1999. The physiological basis for faster growth in the Sydney rock oyster,., 197: 377- 387.
Berger, V. J., and Kharazova, A. D., 1997. Mechanisms of salinity adaptations in marine molluscs., 355: 115-126.
Davis, H. C., and Calabrese, A., 1969. Survival and growth of larvae of the European oyster (L.) at different temperatures., 136: 193-199.
Dos Santos, A. E., and Nascimento, I. A., 1985. Influence of gamete density, salinity and temperature on the normal embryonic development of the mangrove oysterGuilding, 1828., 47: 335-352.
Forcucci, D., and Lawrence, J. M., 1986. Effect of low salinity on the activity, feeding, growth and absorption efficiency of(Echinodermata: Asteroidea)., 92: 315-321.
Fujiwara, M., 1995. The problems in seed production of Iwa oyster., 18: 14-21 (in Japanese).
Gruffydd, L. D., and Beaumont, A. R., 1972. A method for rearinglarvae in the laboratory., 15: 350-355.
Huo, Z. M., Wang, Z. P., Liang, J., Zhang, Y. H., Shen, J. P., Yao, T., Su, J. Q., and Yu, R. H., 2014. Effects of salinity on embryonic development, survival, and growth of., 13: 666-670.
Hutchinson, S., and Hawkins, L. E., 1992. Quantification of the physiological responses of the European flat oysterL. to temperature and salinity., 58: 215-226.
Itoh, N., Tun, K. L., Komiyama, H., Ueki, N., and Ogawa, K., 2004. An ovarian infection in the Iwagaki oyster,, with the protozoan parasite., 27: 311-314.
Ivanina, A. V., Dickinson, G. H., Matoo, O. B., Bagwe, R., Dickinson, A., Beniash, E., and Sokolova, I. M., 2013. Interactive effects of elevated temperature and CO2levels on energy metabolism and biomineralization of marine bivalvesand., 166: 101-111.
Kinne, O., 1964. The effects of temperature and salinity on marine and brackish water animals: II. Salinity and temperature-salinity combinations., 2: 281-339.
Klinzing, M. S. E., and Pechenik, J. A., 2000. Evaluating whether velar lobe size indicates food limitation among larvae of the marine gastropod., 252: 255-279.
Ko, G. W., Dineshram, R., Campanati, C., Chan, V. B., Havenhand, J., and Thiyagarajan, V., 2014. Interactive effects of ocean acidification, elevated temperature, and reduced salinity on early-life stages of the pacific oyster., 48: 10079-10088.
Li, W. J., 2007. Biology and cultivation of oyster, 12: 689-690 (in Chinese with Eng- lish abstract).
Nell, J. A., and Holliday, J. E., 1988. Effects of salinity on the growth and survival of Sydney rock oysterand Pacific oysterlarvae and spat., 68: 39-44.
Okumura, T., Miura, N., Semura, H., and Kishimoto, O., 2005. Seasonal changes in glycogen contents of the Iwagaki oyster,from the coast of Toga Bay, Konoura, Tomari, and Oki Islands in the Sea of Japan., 71: 363-368 (in Japanese).
Parker, L. M., Ross, P. M., and O’Connor, W. A., 2009. The effect of ocean acidification and temperature on the fertiliza- tion and embryonic development of the Sydney rock oyster(Gould 1850)., 15: 2123-2136.
Pechenik, J. A., and Heyman, W. D., 1987. Using KCI to determine size at competence for larvae the marine gastropod(L.)., 112: 27-38.
Re, A. D., Diaz, F., Sierra, E., Rodríguez, J., and Perez, E., 2005. Effect of salinity and temperature on thermal tolerance of brown shrimp(Ives) (Crustacea, Penaeidae)., 30: 618-622.
Rico-Villa, B., Pouvreau, S., and Robert, R., 2009. Influence of food density and temperature on ingestion, growth and settlement of Pacific oyster larvae,., 287: 395-401.
Rybovich, M., Peyre, M. K. L., Hall, S. G., and Peyre, J. F. L., 2016. Increased temperatures combined with lowered salinities differentially impact oyster size class growth and mortality., 35: 101-113.
Scheltema, R. S., 1967. The relationship of temperature to the larval development of(Gastropoda)., 132: 253-265.
Tanaka, M., Hisada, T., and Fujiwara, M., 2010. Possibility of natural spat collection of ‘Iwagaki’ oysterin western Wakasa Bay.,32: 17-22 (in Japanese).
Tang, B. J., Yin, F., Gui, C. S., Chen, X. Z., and Liu, B. Z., 2012. Hatching, growth and hatchling dietary preference in(Gmelin, 1791)., 326: 141- 147.
Taylor, J. F., Needham, M. P., North, B. P., Morgan, A., Thompson, K., and Migaud, H., 2007. The influence of ploidy on saltwater adaptation, acute stress response and immune function following seawater transfer in non-smolting rainbow trout., 152: 314-325.
Verween, A., Vincx, M., and Degraer, S., 2007. The effect of temperature and salinity on the survival oflarvae (Mollusca, Bivalvia): The search for environmental limits., 348: 111-120.
Walne, P. R., 1956. Experimental rearing of the larvae ofin the laboratory., 9: 23.
Xu, F., Guo, X. M., Li, L., and Zhang, G. F., 2011. Effects of salinity on larvae of the oysters,and the hybrid cross., 7: 796-803.
Yao, T., Wang, Z. P., Yan, X. W., Li, D. C., Zhang, Y. H., Huo, Z. M., Su, J. Q., and Yu, R. H., 2015. Effect of salinity on growth and survival of,and juvenile hybrids.,5: 1581-1586 (in Chinese with English abstract).
You, Z. J., Xu, S. L., Bian, P. J., and Chen, J., 2001. The effects of sea water temperature and salinity on the growth and survival oflarvae and juveniles., 23: 108-113.
(Edited by Qiu Yantao)
(Received May 9, 2017; revised May 25, 2017; accepted January 23, 2018)
© Ocean University of China, Science Press and Springer-Verlag GmbH Germany 2018
. Tel: 0086-532-82031622E-mail: qili66@ouc.edu.cn
杂志排行
Journal of Ocean University of China的其它文章
- Offshore Fault Geometrics in the Pearl River Estuary, Southeastern China: Evidence from Seismic Reflection Data
- Application of Geoid Anomalies to the Tectonic Research in the East Asian Continental Margin
- Middle Holocene Organic Carbon and Biomarker Records from the South Yellow Sea: Relationship to the East Asian Monsoon
- Mesozoic Deformation and Its Geological Significance in the Southern Margin of the South China Sea
- Optimization of Shanghai Marine Environmental Monitoring Sites in the Identification of Boundaries of Different Water Quality Grades
- Seasonal Variation of Environmental Variables and Phytoplankton Community Structure and Their Relationship in Liaodong Bay of Bohai Sea, China
