Higher Dietary Arachidonic Acid Levels Improved the GrowthPerformance, Gonad Development, Nutritional Value, andAntioxidant Enzyme Activities of Adult Sea Urchin(Strongylocentrotus intermedius)
2018-08-24ZUORantaoLIMinDINGJunandCHANGYaqing
ZUO Rantao, LI Min, DING Jun, and CHANG Yaqing
Higher Dietary Arachidonic Acid Levels Improved the GrowthPerformance, Gonad Development, Nutritional Value, andAntioxidant Enzyme Activities of Adult Sea Urchin()
ZUO Rantao, LI Min, DING Jun, and CHANG Yaqing*
,,116023,
The gonads of sea urchins () are characterized by high levels of arachidonic acid (ARA, 20:4n-6) and eicosapentaenoic acid(EPA, 20:5n-3). However, to our knowledge, little information is available regarding the physiological response of adult sea urchins to dietary ARA. In the present study, four dietary feeds were formulated with graded ARA (0, 0.5%, 1%, and 2% dry diet). Each diet was randomly allocated to three cages during a 56-day feeding experiment. The results showed that the sea urchin weight gain rate (WGR) and the gonadosomatic index (GI) significantly increased as ARA was equal to or above 1.0% ofdry diet (<0.05). The activities of superoxide dismutase (SOD), catalase (CAT), and total anti-oxidative capacity (T-AOC) were the highest in the coelomic fluid of sea urchins that were fed diets with 1% ARA. The total essential amino acid (TEAA) and its ratio to total non-essential amino acid (TNEAA) showed a similar tendency to WGR and GI as dietary ARA increased,and the highest TEAA and TEAA/TNEAA were observed in the gonads of sea urchins that were fed diets with 1% ARA. Levels of ARA and ARA/EPA of the gonads increased while n-3/n-6 polyunsaturated fatty acid (PUFA) decreased with the increase of dietary ARA(<0.05). EPA in the gonads of experimental animals fed with formulated feeds showed no significant differences (>0.05), but was significantly lower than those fed with kelp (<0.05). These results suggested that relatively higher levels of ARA (1% dry diet) significantly promoted growth, gonad development, activities of antioxidant enzymes, as well as nutritional values (TEAA, TEAA/TNEAA, and PUFA) of adult.
arachidonic acid; sea urchin; weight gain rate; fatty acid; amino acid; gonad development; antioxidant enzyme
1 Introduction
Sea urchins have been listed as one of the most popular sea food candidates worldwide for centuries due to their delicious roe (gonad) (Rubilar, 2016). During the past decade, wild sea urchins have been over-fished to meet the continuously increasing demands of consumers. This has posed serious threats to the wild sea urchin resources and subsequent roe production (Lawrence, 2007). Aquaculture has proven to be a potentially effective means for solving this problem, and several sea urchin species have been extensively studied as potential culture objects (Watts, 2007; Phillips, 2010; Lawrence, 2011; Zhao, 2013; Wei, 2016; Zuo, 2017a).
,which was introduced to China from Japan in 1989, is one of the most important culture species in North China’s sea (Chang and Wang, 1997; Chang, 2012). Conventionally, two kinds ofalgae,and, comprise the primary food sources in the culture and production of(Chang, 2005), but they cannot be obtained during late summer and autumn seasons when juvenile sea urchins require abundant high-quality food to sustain fast growth and to mature into adult sea urchins (Chang, 1999). Artificially formulated feeds have been successfully used as alternatives to large algae from the perspective of food consumption, growth performance, as well as gonad quality (Liyana-Pathirana, 2002; Pearce, 2004; McBride, 2004; Daggett, 2005; Chang, 2005; Phillips, 2010; Lawrence, 2011). Specifically, the requirements of proteins (Hammer, 2006; Cook, 2008; Heflin, 2012; Zuo, 2017a), carotenoids (Shpigel, 2006), phospholipids (Gibbs, 2009), and phosphates (Böttger and McClintock, 2001) have been ascertained for several sea urchin species. However, to our knowledge, no information is available regarding the physiological role of dietary fatty acids in any sea urchin species.
Gonad is the sole edible part of sea urchins. Notably, arachidonic acid (ARA, 20:4n-6) and eicosapentaenoic acid(EPA, 20:5n-3) are the most abundant long-chain polyunsaturated fatty acids (LC-PUFA) in the gonads of sea urchins (Liyana-Pathirana, 2002). ARA has also been noted to be the long-chain polyunsaturated fatty acid (LC-PUFA) that is clearly accumulated into the eggs of sea urchins () (Carboni, 2012). This indicates that ARA could be critical in the regulation of gonad development and reproduction. Indeed, numerous previous studies have verified that ARA had positive effects on the gonad development, spawning performance, egg quality, and offspring quality of several fish species including European sea bass () (Bruce, 1999), Japanese flounder () (Furuita, 2003), Atlantic halibut () (Mazorra, 2003), rice field eel () (Zhou, 2011), and tongue sole () (Xu, 2017a), and various crustacean species including cladoceran () (Ginjupalli, 2015), tiger shrimp () (Coman, 2011), and Pacific white shrimp () (Xu, 2017b).
However, the specific role of dietary ARA in any sea urchin species remains unknown. Thus, this study was conducted to investigate the effects of dietary ARA on the growth performance, gonad development, nutritional value, and antioxidant enzyme activities in. We aimed to provide some preliminary clues for formulating feeds when adult sea urchins are in early maturation stages and when they require high-quality feeds to achieve maturation.
2 Materials and Methods
2.1 Experimental Diets and Feeding Experiment
Ingredients and nutrient composition of the experimental diets are described in Tables 1 and 2. Four iso- proteic (24%) and iso-lipidic (4.5%) experimental diets were formulated to contain the graded levels of ARA (0%, 0.5%, 1%, and 2% dry diet) by the supplementation of ARA powder (100gkg−1, in the form of triglyceride, Cabio Biotechnology Co., Ltd., Wuhan, China), which were named ARA-0, ARA-0.5, ARA-1, and ARA-2. Next, different amounts of soybean oil were added to reach a final lipid concentration of 4.5% dry diet. Seaweed meal, soybean meal, and fish meal were included as the primary protein sources of the diets.
All ingredients were ground into fine powder through a 320-μm mesh. Ingredients in each diet were first thoroughly blended. Then soybean oil was thoroughly mixed with other ingredients of the corresponding diet. Finally, 25% water was incorporated to make stiff dough. Pellets (2mm×5mm) were automatically made by a pellet- making machine (Jinan Dingrun Machinery Company, Jinan, China) and dried for about 12h in a ventilated oven at 40℃. After drying, feeds were packed in plastic bags and stored at −20℃ until use.
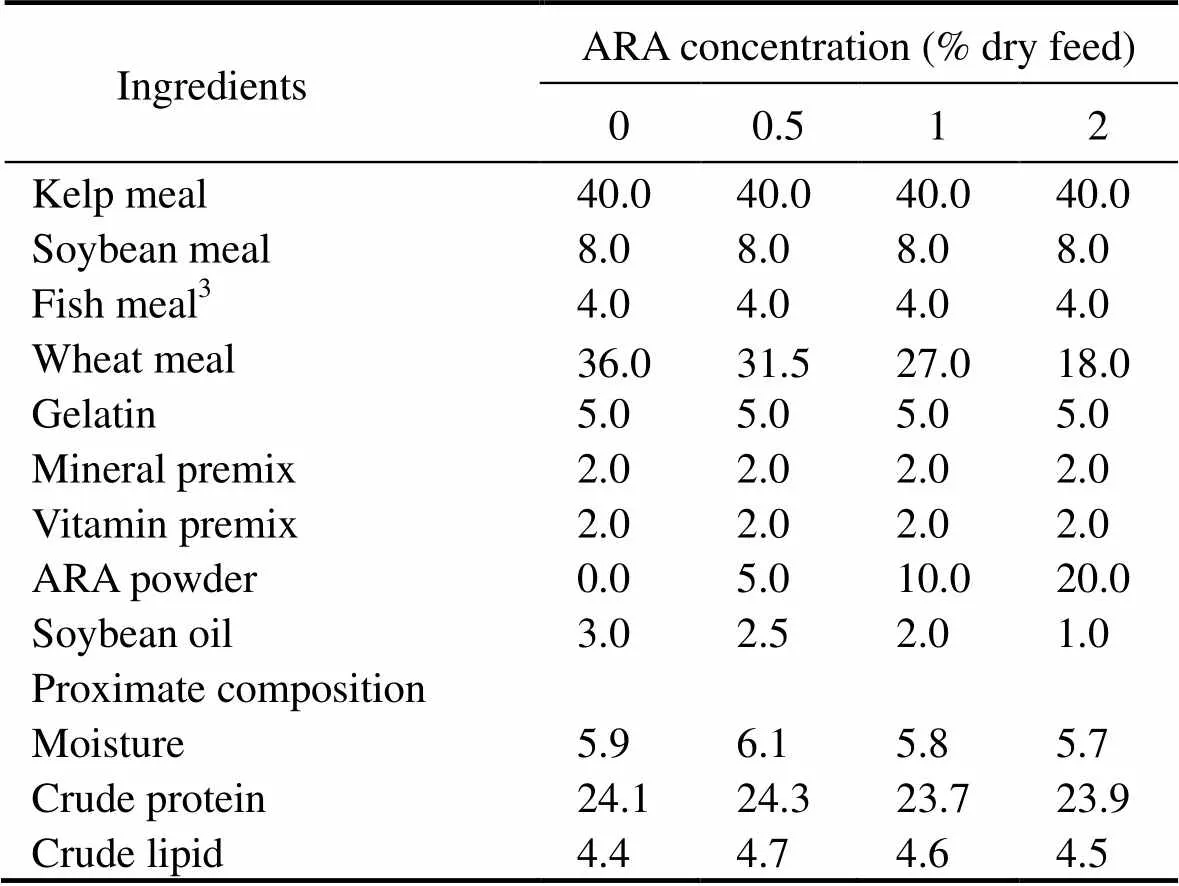
Table 1 Formulation and proximate composition of the experimental diets (% dry feed)
Notes: Seaweed meal: Mixture ofand Ruppiaceae(m:m=1:1), crude protein 18% dry matter, crude lipid 0.9% dry matter, Yingkou Xiandaiyuye Biotechnology Company (Yingkou, Liaoning Province, China). Soybean meal: crude protein 43.4% dry matter, crude lipid 1.9% dry matter, Yingkou Xiandaiyuye Biotechnology Company (Yingkou, Liaoning Province, China). Fish meal: crude protein 68.1% dry matter, crude lipid 10.2% dry matter, Qingdao Qihao Biotechnology Company (Qingdao, Shandong Province, China). Wheat meal: crude protein 16.4% dry matter, crude lipid 1.0% dry matter, Yingkou Xiandaiyuye Biotechnology Company (Ying- kou, Liaoning Province, China). Mineral premix (mg or gkg−1diet): CuSO4·5H2O, 10mg; Na2SeO3(1%), 25mg; ZnSO4·H2O, 50mg; CoCl2·6H2O (1%), 50mg; MnSO4·H2O, 60mg; FeSO4·H2O, 80mg; Ca (IO3)2, 180mg; MgSO4·7H2O, 1200mg; zeolite, 18.35g. Vitamin premix (mg or gkg−1diet): vitamin D, 5mg; vitamin K, 10mg; vitamin B12, 10mg; vitamin B6, 20 mg; folic acid, 20mg; vitamin B1, 25mg; vitamin A, 32mg; vitamin B2, 45mg; pantothenic acid, 60mg; biotin, 60mg; niacin acid, 200mg; α-tocopherol, 240mg; inositol, 800mg; ascorbic acid, 2000mg; microcrystalline cellulose, 16.47g. ARA powder: 100mgg−1ARA, in the form of triglyceride, Cabio Biotechnology Company (Wuhan, Hubei Province, China).
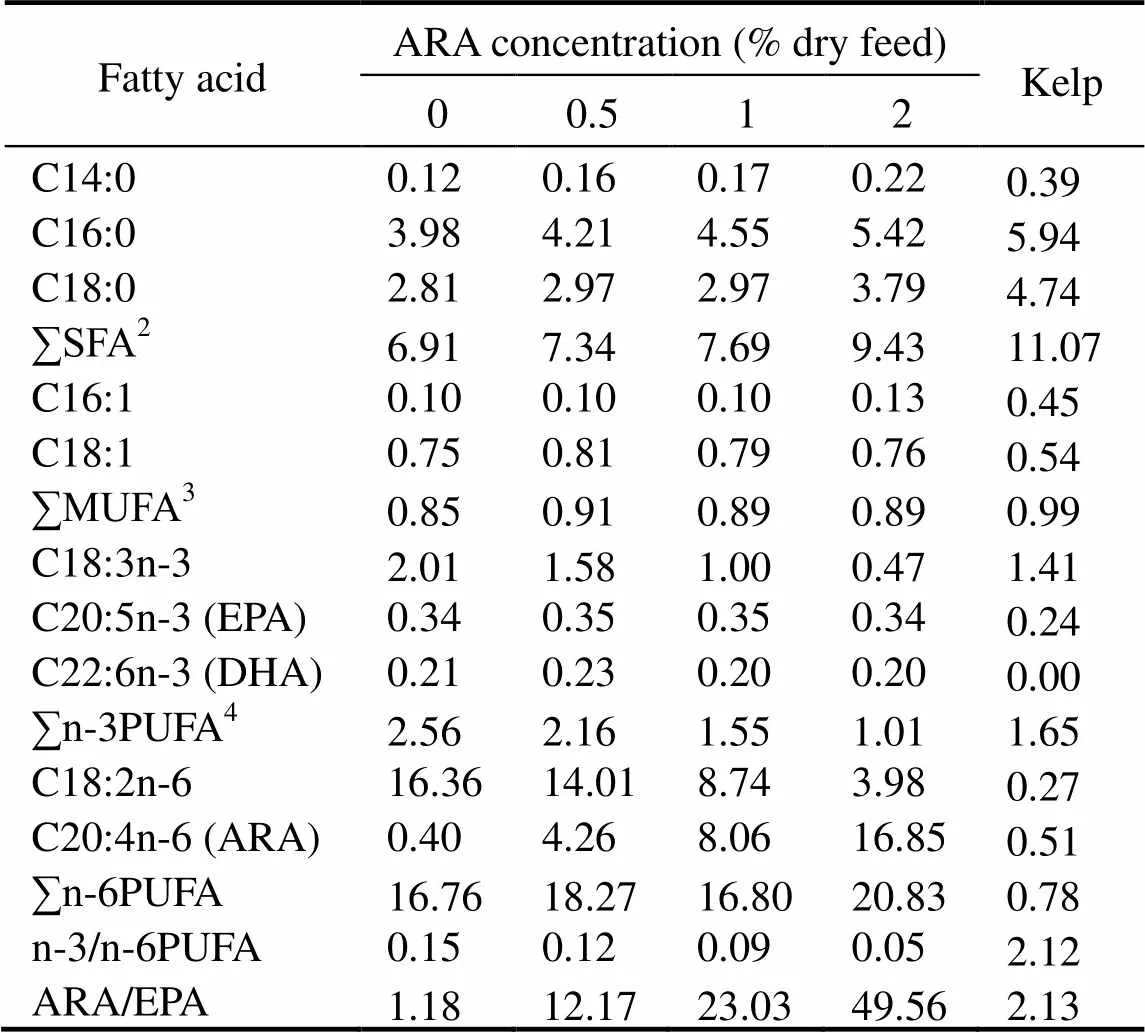
Table 2 Fatty acid content in kelp and formulated feeds(gkg−1 dry weight) experiment†
Notes:†Some fatty acids, of which the contents are minor, trace amount or not detected, such as C22:0, C20:0, C24:0, C14:1, C20:1n−9, C22:1n−11, C20:2n−6, C20:3n−6, C22:5n−3, were not listed in the table. SFA, saturated fatty acids; MUFA, mono-un- saturated fatty acids; PUFA, polyunsaturated fatty acids.
The feeding experiment was conducted from March to May at the Key Laboratory of Mariculture and Stock Enhancement in North China’s Sea, Ministry of Agriculture (Dalian, China). Adult sea urchins of similar sizes (initial weight: about 60g) were purchased from a local farm at Lvshun (Dalian, Liaoning Province). Prior to the feeding experiment, sea urchins of similar sizes were reared in holding tanks (180cm×100cm×80cm) and fed with kelp every day to adapt to the experimental conditions. After two weeks, sea urchins were distributed among 15 net cages (20cm×30cm×50cm), and in each cage 20 individuals were stocked. Each diet was randomly allocated to three cages. All cages were held in a flow-through tank with a total volume of 1440L and the water flow speed was maintained at 2.0Lmin−1. Feeds were supplied every day and the residual feeds and feces were siphoned out daily from the tank. The feeding experiment lasted for 56 days. During the feeding experiment, water temperature ranged from 14℃ to 17℃, salinity was 30, and sea water was aerated with dissolved oxygen above 7mgL−1.
2.2 Sampling
At the completion of the feeding experiment and following a 24h starvation period, all sea urchins in each cage were counted and individually weighed. Then, coelomic fluid from 10 sea urchins per cage was sampled from the cavity with 27-gauge needles and 1mL syringes. The upper fluid was obtained from the coelomic fluid by centrifugation (3000rmin−1, 4℃, 5min), and was frozen in liquid nitrogen and stored at –80℃ for determining the activities of superoxide dismutase (SOD), catalase (CAT), andantioxidant capacity (AOC). The gonads of 10 sea urchins in each cage were dissected, weighed, and stored at –80°C for subsequent analysis of crude proteins, crude lipids, amino acids, and fatty acids.
2.3 Chemical Analysis
Proximate analysis of feeds and gonads were conducted in accordance with AOAC (1995) guidelines. Samples of feed were dried to constant weight at 105℃ using the oven to determine moisture. Crude protein was determined by digestion using the Kjeldahl method and estimated by multiplying nitrogen by a factor of 6.25. Crude lipids were gravimetrically measured after ether extraction by the Soxhlet method.
The fatty acid profiles were analyzed using the procedures described by Norambuena. (2013). After lipid extraction, a known amount of methyl nonadecanoate (19:0) was added as an internal standard and fatty acids were esterified into methyl esters using the acid catalyzed methylation method (Zuo, 2015). Fatty acid methyl esters were separated using hexane, and then identified and quantified by gas chromatography using Thermo Fisher Trace 1310 ISQ (Thermo Fisher Scientific, Wal- tham, MA, USA) with a fused silica capillary column (TG-5MS) and a flame ionization detector. The oven temperature was programmed to rise from 80℃ to 200℃ at a rate of 10℃min−1; from 200℃ to 250℃ at a rate of 5℃min−1; and finally from 250℃ to 270℃ at a rate of 2℃min−1. The injector and the detector were both set at 290℃. The carrier gas helium is set at a constant flow of 1.2mLmin−1. The resulting peaks were corrected by the theoretical relative FID response factors (Ackman, 2002) and quantified relative to the internal standard. Fatty acids were eventually reported as gkg−1wet gonad.
Amino acids in protein sources and experimental diets were determined according to the method by Zhang(2017). Approximately 20mg gonad was hydrolyzed with 6molL−1HCl at 110℃ for 24h, and then metered to the final volume of 50mL using volumetric flask. After that, 2mL was drawn into a small bottle and placed in a vacuum drying Oven (Bander, USA) to evaporate the remaining HCl. Analysis of the amino acids was performed after adding 2mL of sodium citrate loading buffer (0.2molL−1, pH 2.2). Ultraviolet detection was performed at a wavelength of 248nm using Thermo Fisher U3000 (Thermo Fisher Scientific, Waltham, MA, USA). Amino acid contents were expressed as g/kg wet sample.
2.4 Antioxidant Enzyme Assays
SOD activity was spectro photochemically measured using the commercial kit (Nanjing Jiancheng Bio-engi- neering Institute, Nanjing, China). The reaction mixture consisted of 50mmolL−1potassium phosphate buffer (pH 7.8), 0.1mmolL−1EDTA, 0.1mmolL−1xanthine, 0.013mmolL−1cytochrome C and 0.024IUmL−1xanthine oxidase. The reaction was triggered by the addition of xanthine oxidase. Results were expressed in units of SOD per milligram of soluble protein and each unit was defined as the amount of enzyme necessary to produce 50% inhibition of ferricytochrome C reduction rate at 550nm (Mc- Cord and Fridovich, 1969).
CAT was assayed using the commercial kit (Nanjing Jiancheng Bio-engineering Institute, Nanjing, China). After one minute of CAT reaction with H2O2, ammonium molybdate was quickly added to stop the reaction (Aebi, 1984). The remaining H2O2was quantified by reaction with ammonium molybdate, which generates a light-yel- low complex. The CAT activity was calculated by assaying the absorbance at 405nm using distilled H2O as blank. Results were expressed in units of CAT per mg soluble protein and each unit was defined as the amount of enzyme necessary to resolve one μmol H2O2in one second at 37℃.
AOC activity was assayed using a commercial kit (Nanjing Jiancheng Bio-engineering Institute, Nanjing, China). The method is based on the reduction of Fe3+to Fe2+, and the subsequent Fe2+-phenanthroline complex formation. Absorbance was read at 520nm using distilled H2O as blank. Results were expressed in units of AOC per milligram soluble protein and each unit was defined as the amount of enzyme required to increase the absorbance by 0.01 in one minute at 37℃ (Zuo, 2017a).
2.5 Calculations and Statistical Analysis
The following variables were calculated with different methods:
whereiandfare the initial and final number of sea urchins during the feeding experiment;iandfare the initial and final average weight of sea urchins;andare the final wet gonad and body weights, respectively.
All statistical analyses were performed using SPSS 16.0 for Windows. Statistical data analysis was performed using one-way analysis of variance. If there is signifi-cance, differences between the group means were tested by Tukey’s multiple range test. The level of significance was chosen at<0.05 and the results were presented as means±SEM (standard error of the mean).
3 Results
3.1 Survival Rate, Growth Performance, and Gonadosomatic Index of Adult Sea Urchins
SR ranged from 93.3% to 98.3% (>0.05). WGR of sea urchins in ARA-1 (10.41%) and ARA-2 (9.20%) were comparable with those which were fed with kelp (9.38%), but significantly higher than that in ARA-0 (1.88%) and ARA-0.5 (7.94%) (<0.05) (Table 3).
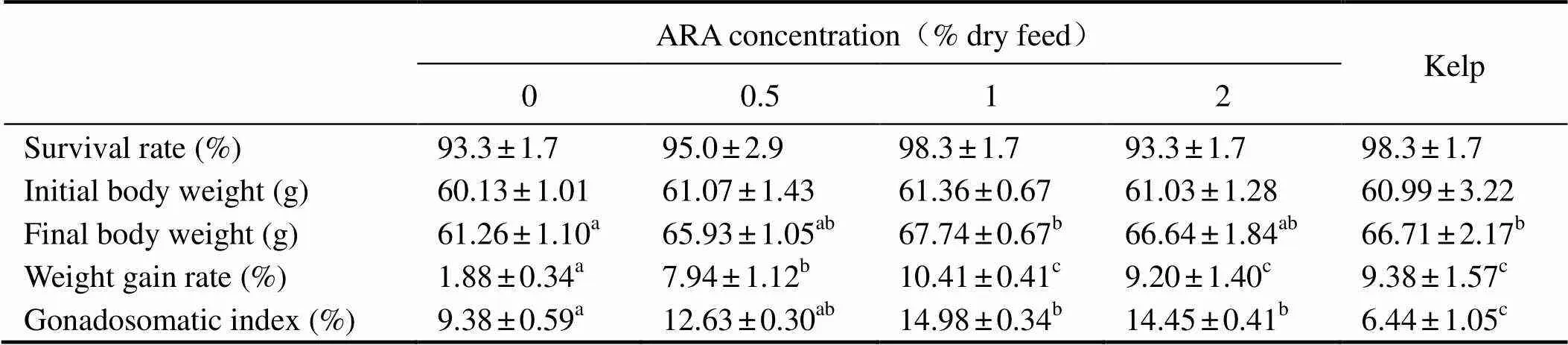
Table 3 Survival rate, growth performance and gonadosomatic index of adult sea urchin (Strongylocentrotus intermedius) in response to graded level of dietary ARA†
Note:†Mean values with the different superscript letters within the same row are significantly different at<0.05.
Sea urchins that fed with kelp showed the lowest GI (6.44%), which was significantly lower than those fed with formulated feeds (<0.05). GI of sea urchins in ARA-1 (14.98%) was comparable with that in ARA-0.5 (12.63%) and ARA-2 (14.45%), but significantly higher than that in ARA-0 (9.38%) (<0.05) (Table 3).
3.2 Activities of Antioxidant Enzymes
The activities of SOD, CAT, and AOC in the coelomic fluid of sea urchins first significantly increased as dietary ARA increased to 1% (<0.05), and then decreased with further increase of dietary ARA (>0.05). The activities of SOD, CAT, and AOC in sea urchins fed with kelp were slightly higher than those in ARA-0 (>0.05), but sig-nificantly lower than those in ARA-1 (<0.05) (Table 4).
The activity of SOD in ARA-1 was 234.4UmL−1, which was comparable with that in ARA-0.5 (233.8UmL−1) and ARA-2 (192.6UmL−1) (>0.05), but significantly higher than that in ARA-0 (175.1UmL−1) and those fed with kelp (183.3UmL−1) (<0.05). The activities of CAT in ARA-1 was 7.82UmL−1, which were comparable with that in ARA-0.5 (5.49UmL−1),ARA-2 (6.92UmL−1), and those fed with kelp (6.28UmL−1) (>0.05), but significantly higher than that in ARA-0 (4.26UmL−1) (<0.05). The activity of AOC in ARA-1 (8.88UmL−1) was comparable with that in ARA-0.5 (7.19UmL−1) and ARA-2 (8.21UmL−1) (>0.05), but was significantly higher than that in ARA-0 (1.29UmL−1) and those fed with kelp (4.52UmL−1) (<0.05) (Table 4).
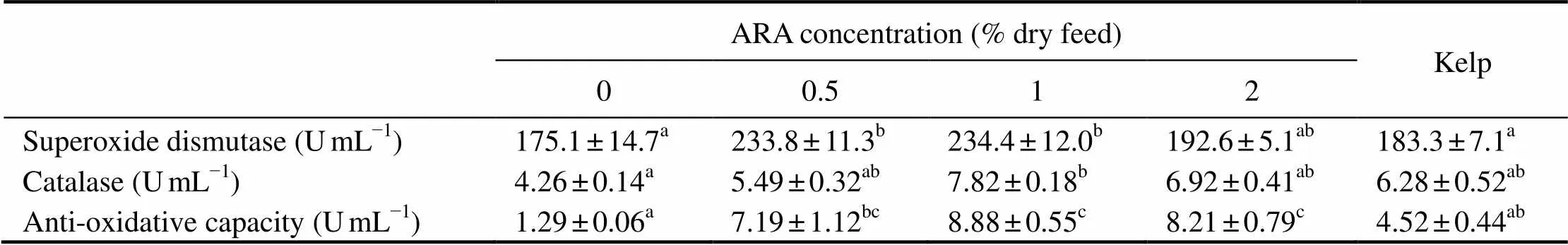
Table 4 Antioxidant enzyme activities in the coelomic fluid of adult sea urchin (Strongylocentrotus intermedius)in response to graded dietary ARA†
Note:†Mean values with the different superscript letters within the same row are significantly different at<0.05.
3.3 Proximate Analysis
The moisture of gonads ranged from 80.95% to 82.29% (>0.05). Crude proteins ranged from 9.74% to 12.20% (>0.05). Crude lipids in the gonad of sea urchin fed with kelp (0.60%) were slightly lower than those fed with formulated diets (0.7%–0.9%) (>0.05). However, crude lipids of gonads showed no significance in the sea urchins fed with formulated feeds (>0.05) (Table 5).
3.4 Fatty Acid composition in the Gonad of Adult Sea Urchins
Total saturated fatty acids (SFA) in the gonads of sea urchins significantly decreased from 15.08 to 9.72gkg−1wet weight as dietary ARA increased to 1% (<0.05). Total mono-unsaturated fatty acids (MUFA) in the gonads of sea urchins significantly decreased from 2.16 to 1.62gkg−1wet weight as dietary ARA increased to 2% (<0.05). MUFA was the lowest in the gonads of the sea urchins that were fed with kelp (1.36gkg−1), which was significantly lower than those fed with formulated feeds (1.62–2.16gkg−1) (<0.05) (Table 6).
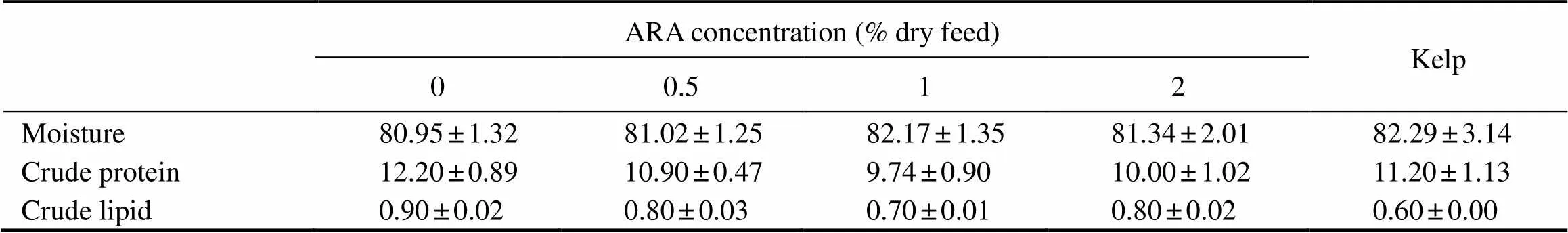
Table 5 Proximate analysis (% wet weight) on the gonad of adult sea urchin (Strongylocentrotus intermedius)in response to graded dietary ARA†
Note:†Mean values with the different superscript letters within the same row are significantly different at<0.05.
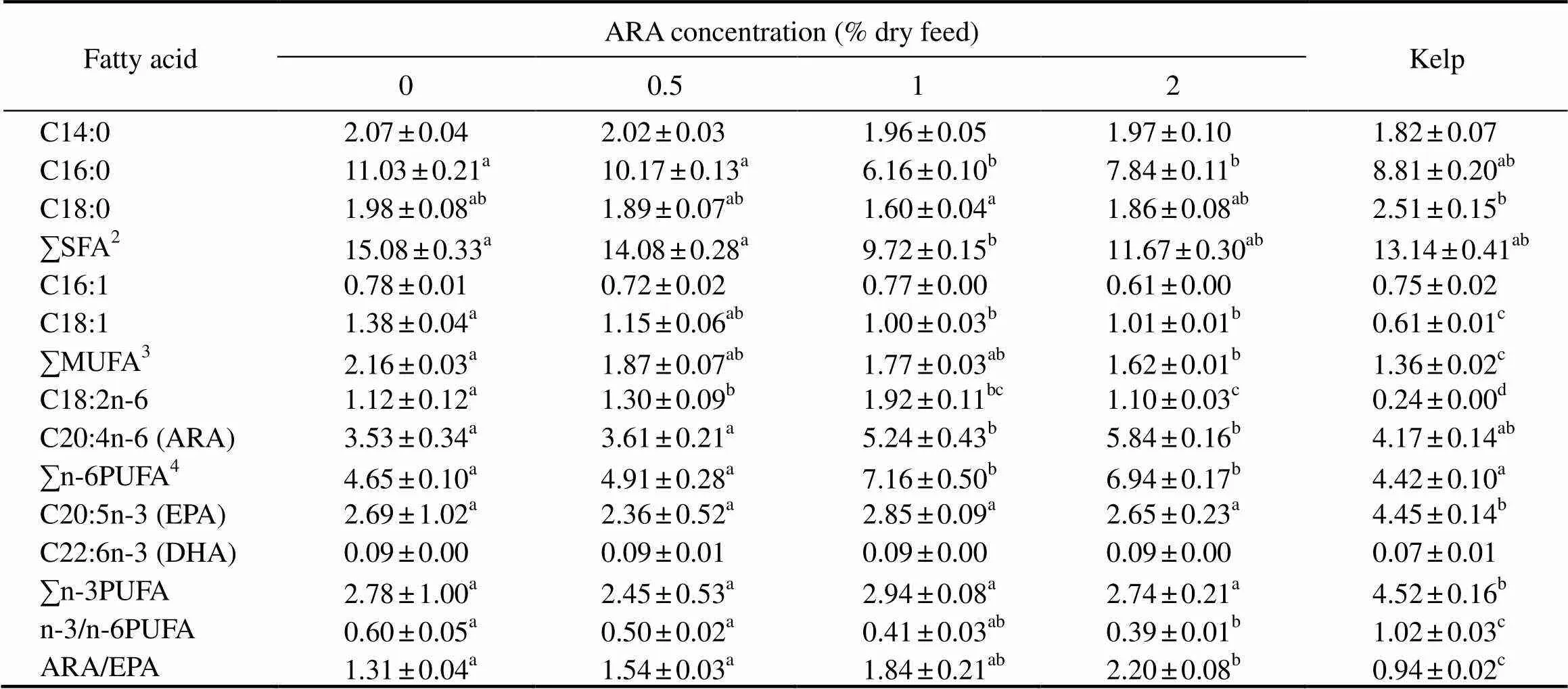
Table 6 Fatty acid composition (gkg−1 wet weight) in the gonad of adult sea urchin (Strongylocentrotus intermedius)in response to graded dietary ARA†
Notes:†Mean values with the different superscript letters within the same row are significantly different at< 0.05; Some fatty acids, of which the contents are minor, trace amount or not detected, such as C22:0, C20:0, C24:0, C14:1, C20:1n−9, C22:1n−11, C20:2n−6, C20:3n−6, C18:3n−3, C22:5n−3, were not listed in the table. SFA, saturated fatty acids; MUFA, mono-unsaturated fatty acids; PUFA, polyunsaturated fatty acid.
ARA content in the gonads significantly increased with the increase of dietary ARA (<0.05). ARA content of the gonad in ARA-0 (3.53gkg−1) was comparable with that in ARA-0.5 (3.61gkg−1) (>0.05) but significantly lower than that in ARA-1 (5.24gkg−1) and ARA-2 (5.84gkg−1) (<0.05). There was no significant difference in ARA content between the sea urchins fed with kelp and those fed with formulated feeds (>0.05). EPA, DHA, and n-3 PUFA showed no significant difference in the gonads of sea urchins that were fed with formulated feeds (>0.05), but EPA and n-3 PUFA in the gonads of sea urchins were significantly lower than those fed with kelp (<0.05). The n-6 PUFA in the gonad of ARA-1 was comparable with that in ARA-2, but was significantly higher than that in ARA-0, ARA-0.5 and those fed with kelp (<0.05) (Table 6).
ARA/EPA significantly increased as dietary ARA increased (<0.05). ARA/EPA in ARA-2 (2.20) was comparable to that in ARA-1 (1.84), but significantly higher than that in ARA-0 (1.31), ARA-0.5 (1.54), and those fed with kelp (0.94) (<0.05). On the contrary, the ratio of n-3/n-6 PUFA significantly decreased from 0.60 to 0.39 as dietary ARA increased to 2.0% (<0.05). The n-3/n-6 PUFA in the gonad of sea urchins fed with kelp showed the highest ratio (1.02), significantly higher than those fed with formulated feeds (0.39–0.60) (<0.05) (Table 6).
3.5 Amino Acid Composition in the Gonads of Adult Sea Urchins
The total non-essential amino acid (TNEAA) significantly decreased as dietary ARA increased (<0.05). The TNEAA of the gonads were significantly lower in ARA-1 (50.97gkg−1), ARA-2 (50.42gkg−1), and kelp (52.04gkg−1) than those in ARA-0 (68.40gkg−1) and ARA-0.5 (63.88gkg−1) (<0.05). The major TNEAA were cysteine, glutamate, aspartic acid, glycine, arginine, and tyrosine. Among them, cysteine, glutamate and aspartic acid values in the gonad showed a decreasing tendency as dietary ARA increased, with the lowest value observed in ARA-1(>0.05). Cysteine concentrations in the gonads of ARA-0 (14.49gkg−1) and ARA-0.5 (11.24gkg−1) were significantly higher than those in ARA-1 (2.43gkg−1g) and ARA-2 (2.77gkg−1) and those animals fed with kelp (2.87gkg−1) (<0.05) (Table 7).
The total essential amino acid (TEAA) and TEAA/ TNEAAfirstly increased as dietary ARA increased to 1.0%, and then decreased with further increase of this fatty acid. The TEAA in ARA-0.5 (36.71gkg−1) and ARA-1 (35.92gkg−1wet weight) were significantly higher than that in ARA-2 (30.72gkg−1) and those fed with kelp (28.58gkg−1) (<0.05). However, there was no significant difference in the contents of lysine and methionine among different treatments (>0.05) (Table 7).
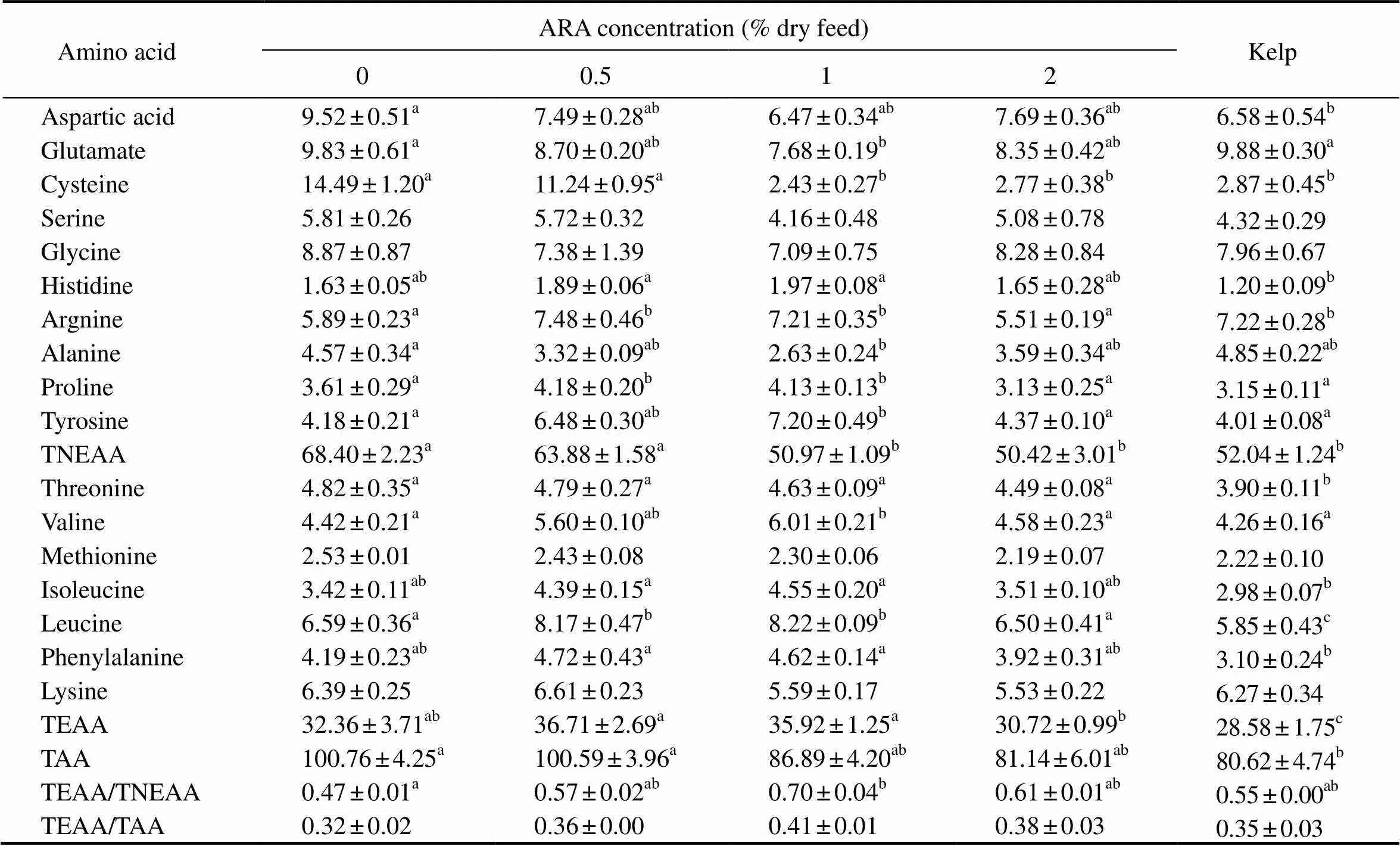
Table 7 Amino acid composition (gkg−1 wet weight) in the gonad of adult sea urchin (Strongylocentrotus intermedius)in response to graded dietary ARA†
Notes:†mean values with the different superscript letters within the same row are significantly different at<0.05. TNEAA, total non-essential amino acid; TEAA, total essential amino acid; TAA, total amino acid.
4 Discussion
In the present study, the WGR of adult sea urchins was approximately 1.88%–10.81%, lower than that of the juvenile counterparts (208.6%–264.9%) (Zuo, 2017a). The growth phase could probably be accounted for the lower WGR in this study. In the present study, 1.0% dietary ARA could significantly increase the WGR of experimental animals. This was consistent with that in adult sea cucumber () (0.8% dry diet) (Zuo, 2017b), but inconsistent with that in tongue sole () broodstocks (2.0% dry diet) (Xu, 2017a) and juvenile sea bass () (0.36% dry diet) (Xu, 2010). These different results could be attributed to different species, different experimental conditions, and different growth phases, where the requirement for essential fatty acids was usually higher in adults and broodstocks than in juveniles (Li, 2005). WGR of sea urchins in ARA-1 (10.41%) and ARA-2 (9.20%) were comparable with those fed with kelp (9.38%), but significantly higher than those fed with ARA-0 (1.88%) and ARA-0.5 (7.94%). On one hand, this suggests a requirement of ARA for the growth of adult sea urchins; on the other hand, it could be that other nutrients in kelp accounted for the relatively higher WGR with kelp diet (ARA-0.5).
In this study, sea urchins fed with kelp showed significantly lower GI than those fed with the various formulated feeds. This could be caused by the lower protein content of the kelp. Previous studies on several sea urchin species have verified that dietary protein is essential for their gonad development (Hammer, 2006; Heflin, 2012; Zuo, 2017a). In addition to proteins, lipids and fatty acid profiles of broodstock diets have been identified as major dietary factors that determine the successful reproduction and survival of offspring (Izquier- do, 2001). Xu(2017a) found that the sex steroid hormone synthesis in tongue sole () was significantly increased by dietary ARA at a concentration of 15.44% of TFA (130g lipid per kg dry diet). The increasing synthesis of sex steroid hormone could be accomplished by the ARA-derived eicosanoid (Norambuena, 2013; Xu, 2017a).
Oxidative stress has been found to exert negative effects on the normal gonad development of zebra fish () (Zhang, 2016), germ quality, and infertility of humans (Agarwal, 2006; Agarwal and Said, 2012). Anti-oxidants and antioxidant-related enzymes could exert protective responses to environmentally induced oxidative stress (Limón-Pachecoand Gonsebatt, 2009). Major yolk protein (MYP) is the main category of lipoprotein in the ovum of sea urchins, which could amount to 10%–15% of total protein in ovum or 9% wet weight of gonad (Marsh and Watts, 2001; Brooks and Wessel, 2003). In this study, the activities of SOD and CAT in coelomic fluid were significantly elevated by relatively higher dietary ARA (1% dry diet) but showed slight decrease in the activities in ARA-2. Since SOD and CAT could scavenge reactive oxygen species, they can protect phospholipids on the surface of MYP from hyperoxidation. ARA has been noted to regulate non-spe- cific immunity and decrease oxidative stress (Xu, 2010). However, excess ARA could be oxidized and produce a certain amount of oxyradical, which could thus consume some antioxidant enzymes (Xu, 2010). This could account for the slightly decreased activity of antioxidant enzymes in the coelomic fluid of sea urchins fed with ARA-2.
In the present study, ARA and EPA were the most abundant LC-PUFA in the gonads among all dietary treat- ments. ARA content in the gonads of ARA-0 was remarkably as high as that in ARA-0.5. Meanwhile, EPA content in the gonads of sea urchins fed with formulated feeds was approximately 10 times higher than that in the diets. Thus, sea urchins are assumed to have had strong capacity to deposit LC-PUFA that are essential for the cell membrane formation during gonad development (Liyana- Pathirana, 2002), whereas it may also imply that sea urchinspossess certain ability to synthesize LC-PUFA from linoleic acid (18:2n-6) and linolenic acid (18:3n-3). Previous studies have indeed detected some non-me- thylene interrupted fatty acids, such as 18:4n-3, 20:4n-3, 18:3n-6 and 20:3n-6 in the gonads of,and, but not in the corresponding diets (Cook, 2000; Castell, 2004; Carboni, 2012). However, on the basis of the present results, this confusion could not be eliminated and further studies are required to clone the critical enzymes (., elongase and desaturase) for LC-PUFA synthesis and to verify their specific functions by anyeast system (Li, 2010). In the present study, n-3/n-6 PUFA in the gonad of sea urchins decreased with the increase of dietary ARA. Sea urchins fed with kelp had the highest ratio of n-3/n-6 PUFA (1.02), which was significantly higher than those fed with formulated feeds (0.39–0.60). Recently, significant attention has been paid to the physiological role of n-3/n-6 PUFA. Lower ratio of n-3/n-6 PUFA could induce inflammation and subsequent oxidative stress, which could increase the risk of obesity, diabetes and cardiovascular diseases (Zuo, 2015). Thus, a balance between ARA and EPA should be quantified for sea urchins to reach an ideal ratio of n-3/n-6 PUFA in the gonads.
In this study, the crude protein of gonads showed no significant differences in response to graded dietary ARA. This result was inconsistent with the findings by Zuo(2012) who reported that the protein content of large yellow croaker () decreased as dietary n-3 LC-PUFA increased. In addition to protein retention, amino acid profiles of gonad were also significantly affected by dietary ARA. TEAA and TEAA/TNEAA firstly increased as dietary ARA increased to 1%, but then decreased with further increase of ARA. This implies that relatively higher ARA (1.0% dry diet) could markedly enhance TEAA and TEAA/TNEAA in the gonads of sea urchins. Studies have shown that more lipids (soybean oil) in the diet resulted in a lower amino acid absorption and metabolism in Senegalese sole () larvae (Morais, 2005). Thus, in the present study, different absorption and metabolism rates could account for the different retention of EAA and NEAA in response to graded dietary ARA. Further research is necessary to find direct evidences to answer the questions.
In conclusion, relatively higher dietary ARA (1.0%) significantly increased the growth performance, gonad development, activities of antioxidant enzymes, as well as nutritional values reflected by TEAA, TEAA/TNEAA and PUFA in adult. Further studies are required to make clear the specific role of antioxidant enzymes in the gonad development, whether LC-PUFA could be synthesized, and how dietary ARA regulates the deposition of amino acids in the gonad of sea urchins.
Acknowledgements
This research was supported by the National Natural Science Foundation of China (No. 41606180) and Young Elite Scientists Sponsorship (No. YESS20150157). We thank Drs. G. Li, W. P. Fang, Q. H. Liang, M. J. Sun, and S. J. Jin for their help during the culture and sampling.
Ackman, R. G., 2002. The gas chromatograph in practical analyses of common and uncommon fatty acids for the 21st century., 465: 175-192.
Aebi, H., 1984. Catalase., 105: 121- 126.
Agarwal, A., and Said, T. M., 2012. Oxidative stress, DNA damage and apoptosis in male infertility: A clinical approach., 95 (4): 503-507.
Agarwal, A., Gupta, S., and Sikka, S., 2006.The role of free radicals and antioxidants in reproduction., 18 (3): 325-332.
Association of Official Analytical Chemists (AOAC), 1995.. 16th edition. Association of Official Analytical Chemists, Arlington, VA, 16-26.
Böttger, S. A., and Mcclintock, J. B., 2001. The effects of organic and inorganic phosphates on fertilization and early development in the sea urchin(Echinodermata: Echinoidea)., 129 (4): 307-315.
Brooks, J. M., and Wessel, G. M., 2003. Selective transport and packaging of the major yolk protein in the sea urchin., 261 (2): 353-370.
Bruce, M. P., Oyen, F., Bell, J. G., Farndale, B. M., Asturiano, J. F., Bromage, N. R., Carrillo, M., Zanuy, S., and Ramos, J., 1999. Development of broodstock diets for the European sea bass () with special emphasis on the importance of n-3 and n-6 HUFA to reproductive performance.,177 (1-4): 85-98.
Carboni, S., Hughes, A. D., Atack, T., Tocher, D. R., and Migaud, H., 2012. Fatty acid profiles during gametogenesis in sea urchin (): Effects of dietary inputs on gonad, egg and embryo profiles., 164 (2): 376-382.
Castell, J. D., Kennedy, E. J., Robinson, S. M. C., Parsons, G. J., Blair, T. J., and Gonzalez-Duran, E., 2004. Effect of dietary lipids on fatty acid composition and metabolism in juvenile green sea urchins ()., 242 (1-4): 417-435.
Chang, Y. Q., and Wang, Z. C., 1997. The raft culture of the sea urchin., 12 (2): 7-14 (in Chinese with English ab- stract).
Chang, Y. Q., Lawrence, J. M., Cao, X. B., and Lawrence, A. L., 2005. Food consumption, absorption, assimilation and growth of the sea urchinfed a prepared feed and the alga.,36 (1): 68-75.
Chang, Y. Q., Wang, Z. C., and Wang, G. J., 1999. Effect of temperature and algae on feeding and growth in sea urchin,., 23 (1): 69-76 (in Chinese with English abstract).
Chang, Y. Q., Zhang, W. J., Zhao, C., and Song, J., 2012. Estimates of heritabilities and genetic correlations for growth and gonad traits in the sea urchin., 43 (2): 271-280.
Coman, G. J., Arnold, S. J., Barclay, M., and Smith, D. M., 2011. Effects of arachidonic acid supplementation on reproductive performance of tank-domesticated., 17 (2): 141-151.
Cook, E. J., Bell, M. V., Black, K. D., and Kelly, M. S., 2000. Fatty acid compositions of gonadal material and diets of the sea urchin,: Trophic and nutritional implications., 255 (2): 261-274.
Cook, E. J., Hughes, A. D., Orr, H., Kelly, M. S., and Black, K. D., 2008. Influence of dietary protein on essential fatty acids in the gonadal tissue of the sea urchins, and, (Echinodermata)., 273 (4): 586-594.
Daggett, T. L., Pearce, C. M., Tingley, M., Smc, R., and Chopin, T., 2005. Effect of prepared and macroalgal diets and seed stock source on somatic growth of juvenile green sea urchins ()., 244 (1-4): 263-281.
Furuita, H., Yamamoto, T., Shima, T., Suzuki, N., and Takeuchi, T., 2003. Effect of arachidonic acid levels in broodstock diet on larval and egg quality of Japanese flounder., 220 (1-4): 725-735.
Gibbs, V. K., Watts, S. A., Lawrance, A. L., and Lawrance, J. M., 2009. Dietary phospholipids affect growth and production of juvenile sea urchin., 292 (1-2): 95-103.
Ginjupalli, G. K., Gerard, P. D., and Baldwin, W. S., 2015. Ara- chidonic acid enhances reproduction in Daphnia magna and mitigates changes in sex ratios induced by pyriproxyfen. Environ., 34 (3): 527-535.
Hammer, H., Hammer, B., Watts, S., Lawrance, A. L., and Lawrance, J. M., 2006. The effect of dietary protein and carbohydrate concentration on the biochemical composition and gametogenic condition of the sea urchin., 334 (1): 109-121.
Heflin, L. E., Gibbs, V. K., Powell, M. L., Makowsky, R., Lawrence, J. M., Lawrence, A. L., and Watts, S. A., 2012. Effect of dietary protein and carbohydrate levels on weight gain and gonad production in the sea urchin., s358-359 (2): 253-261.
Izquierdo, M. S., Fernández-Palacios, H., and Tacon, A. G. J., 2001. Effect of broodstock nutrition on reproductive performance of fish., 197 (s1-4): 25-42.
Lawrence, J. M., Chang, Y. Q., Cao, X. B., Lawrence, A. L., and Watts, S. A., 2011. Potential for production of uni byusing dry formulated feeds.,42 (2): 253-260.
Lawrence, J. M., Lawrence, A. L., and Watts, S. A., 2007. Feeding, digestion, and digestibility. In:Lawrence, J. M., ed., Elsevier, Amsterdam, 135-158.
Liyana-Pathirana, C., Shahidi, F., and Whittick, A., 2002. The effect of an artificial diet on the biochemical composition of the gonads of the sea urchin ()., 79 (4): 461-472.
Li, Y., Monroig, O., Zhang, L., Wang, S., Zheng, X., Dick, J. R., You, C., and Tocher, D. R., 2010. Vertebrate fatty acid desaturase with Δ4 activity., 107 (39): 16840-16845.
Li, Y. Y., Chen, W. Z., Sun, Z. W., Chen, J. H., and Wu, K. G., 2005. Effects of n-3 HUFA content in broodstock diet on spawning performance and fatty acid composition of eggs and larvae in., 245 (1): 263- 272.
Limón-Pacheco, J., and Gonsebatt, M. E., 2009. The role of antioxidants and antioxidant-related enzymes in protective responses to environmentally induced oxidative stress., 674 (1-2): 137-147.
Marsh, A. G., and Watts, S. A., 2001. Energy metabolism and gonad development., 32 (1): 27-42.
Mazorra, C., Bruce, M., Bell, J. G., Davie, A., Alorend, E., Jor- dan, N., Rees, J., Papanikos, N., Porter, M., and Bromage, N., 2003. Dietary lipid enhancement of broodstock reproductive performance and egg and larval quality in Atlantic halibut ()., 227 (s1-4): 21-33.
Mcbride, S. C., Price, R. J., Tom, P. D., Lawrence, J. M., and Lawrence, A. L., 2004. Comparison of gonad quality factors: Color, hardness and resilience, ofbetween sea urchins fed prepared feed or algal diets and sea urchins harvested from the Northern California fishery., 233 (1-4): 405-422.
Mccord, J. M., and Fridovich, I., 1969. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein)., 244: 6049-6055.
Morais, S., Koven, W., Ronnestad, I., Dinis, M. T., and Conceicao, L. E. C., 2005. Dietary protein/lipid ratio affects growth and amino acid and fatty acid absorption and metabolism in Senegalese sole (Kaup 1858) larvae., 246 (1-4): 347-357.
Norambuena, F., Estévez, A., Mañanós, E., Bell, J. G., Carazo, I., and Duncan, N., 2013. Effects of graded levels of arachidonic acid on the reproductive physiology of Senegalese sole (): Fatty acid composition, prostaglandins and steroid levels in the blood of broodstock bred in captivity., 191 (9): 92-101.
Pearce, C. M., Daggett, T. L., and Robinson, S. M. C., 2004. Effect of urchin size and diet on gonad yield and quality in the green sea urchin ()., 233 (1-4): 337-367.
Phillips, K., Hamid, N., Silcock, P., Sewell, M. A., Barker, M., Weaver, A., Then, S., Delahunty, C., and Bremer, P., 2010. Effect of manufactured diets on the yield, biochemical composition and sensory quality ofsea urchin gonads., 308 (1-2): 49-59.
Rubilar, T., Epherra, L., Deias-Spreng, J., Díaz-De-Vivar, M. E., Lawrence, A. L., and Lawrence, J. M., 2016. Ingestion, absorption and assimilation efficiencies and production in the sea urchinfed a formulated feed., 35 (4): 1083-1093.
Shpigel, M., Schlosser, S. C., Benamotz, A., Lawrence, A. L., and Lawrence, J. M., 2006. Effects of dietary carotenoid on the gut and the gonad of the sea urchin., 261 (4): 1269-1280.
Watts, S. A., Powell, M. L., Lawrence, A. L., and Lawrence, J. M., 2007. Sea urchin culture: Emerging species yields roe; supports medical modeling, environmental testing., 11: 76-77.
Wei, J., Zhao, C., Zhang, L., Yang, L., Zuo, R., Hou, S., and Chang, Y., 2016. Effects of short-term continuous and inter- mittent feeding regimes on food consumption, growth, gonad production and quality of sea urchinfed a formulated feed., 97 (2): 359-367.
Xu, H. G., Ai, Q. H., Mai, K. S., Xu, W., Wang, J., Ma, H. M., Zhang, W. B., Wang, X. J., and Liufu, Z. G., 2010. Effects of dietary arachidonic acid on growth performance, survival, immune response and tissue fatty acid composition of juvenile Japanese seabass,., 307 (1): 75-82.
Xu, H. G., Cao, L., Zhang, Y. Q., Johnson, R. B., Wei, Y. L., Zheng, K. K., and Liang, M. Q., 2017a. Dietary arachidonic acid differentially regulates the gonadal steroidogenesis in the marine teleost, tongue sole (), de- pending on fish gender and maturation stage., 468 (2): 378-385.
Xu, H. G., Zhang, Y. L., Luo, K., Meng, X. H., Luan, S., Cao, B. X., Chen, B. L., Liang, M. Q., and Kong, J., 2017b. Arachidonic acid in diets for early maturation stages enhances the final reproductive performances of Pacific white shrimp ()., 479 (10): 556-563.
Zhang, K. K., Mai, K. S., Xu, W., Liufu, Z. G., Zhang, Y. J., Peng, M., Chen, J. H., and Ai, Q. H., 2017. Effects of dietary arginine and glutamine on growth performance, nonspecific immunity, and disease resistance in relation to arginine catabolism in juvenile turbot (L.)., 468 (2): 246-254.
Zhang, Q. F., Li, Y. W., Liu, Z. H., and Chen, Q. L., 2016. Reproductive toxicity of inorganic mercury exposure in adult zebrafish: Histological damage, oxidative stress, and alterations of sex hormone and gene expression in the hypothalamic-pituitary-gonadal axis., 177 (8): 417- 424.
Zhao, C., Liu, P. J., Zhou, H. S., Tian, X. F., and Chang,Y. Q., 2013. Diel observation on the distribution of the sea urchinunder different food availability and shelter conditions in the laboratory., 45 (6): 357-364.
Zhou, Q. B., Wu, H. D., Zhu, C. S., and Yan, X. H., 2011. Effects of dietary lipids on tissue fatty acids profile, growth and reproductive performance of female rice field eel ()., 37 (3): 433- 445.
Zuo, R. T., Ai, Q. H., Mai, K. S., Xu, W., Wang, J., Liufu, Z. G., and Zhang, Y. J., 2012. Effects of dietary n-3 highly unsaturated fatty acids on growth, nonspecific immunity, expression of some immune related genes and disease resistance of large yellow croaker () following natural infestation of parasites ()., 32 (2): 249-258.
Zuo, R. T., Hou, S. Q., Wu, F. X., Song, J., Zhang, W. J., Zhao, C., and Chang, Y. Q., 2017a. Higher dietary protein increases growth performance, anti-oxidative enzymes activity and transcription of heat shock protein 70 in the juvenile sea urchin () under a heat stress., 2 (1): 18-23.
Zuo, R. T., Lv, D. L., Wu, F. X., Zhang, C. Y., Li, G., Li, M., Hao, Z. L., and Chang, Y. Q., 2017b. Effects of dietary arachidonic acid (ARA) on growth performance and nutrient composition of adult sea cucumber ()., 2017 (6): 30-34 (in Chinese with English abstract).
Zuo, R. T., Mai, K. S., Xu, W., Turchini, G. M., and Ai, Q. H., 2015. Dietary ALA, but not LNA, increase growth, reduce inflammatory processes, and increase anti-oxidant capacity in the marine finfish., 50 (2): 149- 163.
(Edited by Qiu Yantao)
(Received August 11, 2017; revised December 27, 2017; accepted May 15, 2018)
© Ocean University of China, Science Press and Springer-Verlag GmbH Germany 2018
. Tel: 0086-411-84762691E-mail: yqchang@dlou.edu.cn
杂志排行
Journal of Ocean University of China的其它文章
- Offshore Fault Geometrics in the Pearl River Estuary, Southeastern China: Evidence from Seismic Reflection Data
- Application of Geoid Anomalies to the Tectonic Research in the East Asian Continental Margin
- Middle Holocene Organic Carbon and Biomarker Records from the South Yellow Sea: Relationship to the East Asian Monsoon
- Mesozoic Deformation and Its Geological Significance in the Southern Margin of the South China Sea
- Optimization of Shanghai Marine Environmental Monitoring Sites in the Identification of Boundaries of Different Water Quality Grades
- Seasonal Variation of Environmental Variables and Phytoplankton Community Structure and Their Relationship in Liaodong Bay of Bohai Sea, China
