Nitrogen Budget in Recirculating Aquaculture and WaterExchange Systemsfor Culturing Litopenaeusvannamei
2018-08-24CHENZhaoGEHongxingCHANGZhiqiangSONGXiefaZHAOFazhenandLIJian
CHEN Zhao, GE Hongxing, CHANG Zhiqiang, SONG Xiefa,ZHAO Fazhen, and LI Jian,
Nitrogen Budget in Recirculating Aquaculture and WaterExchange Systemsfor Culturing
CHEN Zhao1), 2), GE Hongxing1), 2), CHANG Zhiqiang2), SONG Xiefa1),ZHAO Fazhen2), and LI Jian2),*
1),,266003,2),,,,266071,
In order to investigate the culture characteristics of two indoor intensivefarming modes, recirculating aquaculture system (RAS) and water exchange system (WES), this study was carried out to analyze the water quality and nitrogen budget including various forms of nitrogen, microorganismand chlorophyll-. Nitrogen budget was calculated based on feed input, shrimp harvest, water quality and renewal rate, and collection of bottom mud. Input nitrogen retained in shrimp was 23.58% and 19.10% respectively for WES and RAS, and most of nitrogen waste retained in water and bottom mud. In addition, most of nitrogen in the water of WES was TAN (21.32%) and nitrite (15.30%), while in RAS was nitrate (25.97%), which means that more than 76% of ammonia and nitrite were removed. The effect of microalgae in RAS and WES was negligible. However, bacteria played a great role in the culture system considering the highest cultivable cultivable bacterial populations in RAS and WES were 1.03×1010cfumL−1and 2.92×109cfumL−1, respectively. Meanwhile the proportion of bacteria in nitrogen budget was29.61% and 24.61% in RAS and WES, respectively. RAS and WES could realize shrimp high stocking culture with water consuming rate of 1.25m3per kgshrimp and 3.89m3per kgshrimp, and power consuming rates of 3.60kwh per kg shrimp and 2.51kwh per kg shrimp, respectively. This study revealed the aquatic environment and nitrogen budget of intensive shrimp farming in detail, which provided the scientific basis for improving the industrial shrimp farming.
RAS; shrimp; water quality; nitrogen budget; microorganism
1 Introduction
Shrimp aquaculture has been an important fishery production in China. In 2015, the total production of shrimp was 1161340 metric tons, accounting for 80.93% of crustacean yields. The pacific white shrimp () was the major aquaculture species due to its advantages over other species, such as high yield, strong adaptability, fast growth, among others, with production of 893182 metric tons, accounting for 76.90% of total shrimp production (CFSY, 2016).
Traditional extensive shrimp farming contributes a lot to providing high-quality protein. But it is with the cost of water pollution and habitat destruction. In addition, it is susceptible to the change of climate. Compared to the traditional extensive pond culture mode, the indoor intensive shrimp culture mode, namely industrialized culture mode, could provide a relatively stable culture environment for shrimp which is necessary for continuous high yield. Water exchange system (WES) and recirculating aquaculture system (RAS) are two typical indoor intensive culture modes. The object of both of them is to provide a stable and controllable condition for the cultured species. WES maintains the culture condition by water exchange. It would consume plenty of fresh water. However, water in RAS can be (partially) reused after the treatment (Rosen- thal, 1986). RAS is a water saving and environment-friendly aquaculture system, and it has already been applied successfully to culture fish, including African catfish, eel, trout, turbot, seabass and sole (Martins, 2010). Many scientific reports have been published to illustrate the application of RAS in fish aquaculture, and it has been found that RAS has many advantages in terms of reducing water consumption(Verdegem, 2006), improving waste reuse and nutrient recycling (Piedrahita, 2003), providing a better hygiene and disease management (Summerfelt, 2009; Tal, 2009) in comparison with WES(d’Orbcastel, 2009a; Kolarevic, 2014; Colson, 2015; d’Orbcastel, 2009b; Attramadal, 2012). As to shrimp aquaculture, RAS has initially been used in parent shrimp culture (Otoshi, 2003), and recently it has also been applied in industrialized culture of the Pacific white shrimp (Holl, 2011).
Nitrogen is the most important nutrient element in aquaculture system. Nitrogen budget is not merely related to the assimilation efficiency of cultured species for feed nitrogen (Thoman, 2001), but also affects the culture condition and adjacent environment, since inorganic nitrogen is toxic to aquatic organism (Lin and Chen, 2001; Lin and Chen, 2003; Kuhn, 2010) and responsible for water eutrophication (Dierberg and Kiattisimkul, 1996; Naylor, 1998). Previous studies (Muthuwan, 1991; Briggs and Fvnge-Smith, 1994; Jackson, 2003; Tha- kur and Lin, 2003) showed that less than 31% of feed nitrogen could be harvested as shrimp biomass. Much of it was discharged to adjacent water area, causing eutrophication, and reducing farming profitability. By now, there are a lot of reports on nitrogen budget of shrimp pond culture system, while only a few reports are with respect to industrialized shrimp culture system.
The nitrogen budget and water quality change in RAS and WES for shrimp aquaculture were investigated in the present study in order to understand nitrogen output in detail and seek strategies to improve the use efficiency of nutrients in the industrialized shrimp culture system.
2 Materials and Methods
2.1 Experimental Site and Condition
The study was carried out from July to September, 2016, in Weifang New Earth Aquiculture Co., Ltd., Shandong, China (37˚01΄N, 119˚13΄E). Water used for shrimp culture is a mixture of underground freshwater and brine, and the final salinity (Sal) was adjusted to 17gL−1. Water was disposed with ozone and heated to 28℃ before pumped into shrimp culture system. Roots blower was used for aeration, while pure oxygen was prepared for emergencies.
2.2 Experimental Rearing Systems
RAS and WES were in aquaculture plant that covered with double-deck transparent plastic film and black felt for partial lighting and thermal insulation. Both culture systems included 10 culture tanks (6m×6m×1.1m, actual use area was 32m2) in parallel, and the water depth was 0.85m.
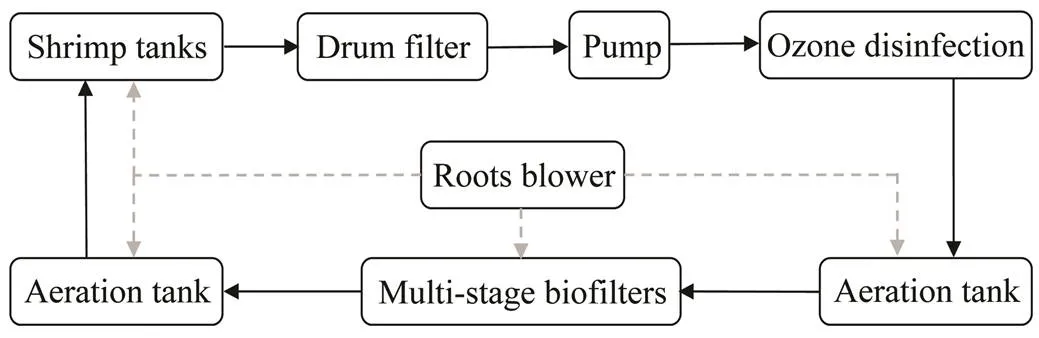
Fig.1 Composition and flow chart of RAS.
The composition and running process of RAS are shown in Fig.1. The multi-stage biofilters included seven tanks, while four of them were filled with elastic brush media and the other three ones were filled with suspended porous media. Daily cycle rate of RAS was about 7d−1. The culture tanks and inlet/outlet structure of WES was the same as RAS. The culture periods of RAS and WES were both 50 days. For WES there was a low daily water exchange rate (about 12%) in the first 19 days, thereafter, daily water exchange rate was 60%. Water flow rate of RAS was 80m3h−1, with seven daily hydrologic cycles. There were three manage periods for RAS with the water exchange rates of 5%, 12% and 20%, respectively. In summary, the mean water exchange rate of WES and RAS was44% and 10%, respectively.
2.3 Experimental Design
larvae were purchased from Hainan Province, China, with the initial body length approximately 5mm. They were reared in shrimp larvae plant for about one month before experiment. The average weight of the shrimps was 0.87±0.03g.
cultured in RAS was compared to those in WES for a raised period. Three days before the juvenile prawn were shifted into RAS, probiotics were inoculated into the biofilters with some fish meal as nutrition for accelerating biofilm development. Both rearing systems were stocked with the same initial density of 656 shrimps per m2(18kg shrimp for each tank). Shrimps were fed six times a day (at 1:00, 5:00, 9:00, 13:00, 17:00 and 21:00) with formulated feed (41% proteins) and some organisms including artemia, clam meat and scallop skirt. Daily feeding rate was 6% of the biomass at the beginning and adjusted according to the actual feeding condition. The total feed, residual feed and feces, as well as water exchange quantity and nitrogen concentration were monitored to calculate nitrogen input and output. Furthermore, chlorophyll-, total cultivable bacteria, total vibrio and turbidity were measured to assess the culture condition and estimate the effect of microalgae and bacteria on nitrogen budget.
2.4 Measurements
O2, pH, temperature and salinity were recorded with YSI (YSI Incorporated, Yellow Springs, OH, USA). Turbidity was measured by turbidimeter (Yuefeng, Shanghai, China). Other physicochemical parameters, chlorophyll, total cultivable bacterial amount and total vibrio amount were monitored every ten days. During the ten days, the bottom mud were collected three times.
Some shrimp samples (about 100 individuals) were taken randomly at the beginning and end of the experiment to measure the mean weight and water content. Bottom mud was collectedfilter screen (48μm in mesh size) at drain outlet and drum filter, and measured its water content. Nitrogen content in shrimp, bottom mud and feed were determined through elemental analyzer (Vario EL cube; Elementar, Germany).
Water samples were taken 3h after the morning meal between 8:00 and 9:00 from three tanks and the inlet/outlet of biofilter. Some water was filtered through 0.22-µm-pore-diameter filter membrane for the detection of nitrite, ammonia, nitrate and dissolved nitrogen. The remaining water was left for detection of total nitrogen, chlorophyll-, total cultivable bacteria, total vibrio and turbidity. Equal quantity of water was collected from three tanks of RAS and WES, respectively, every day, and was frozen at −20℃. The water from each culture systems was melted and mixed, respectively, every ten days, and various forms of nitrogen were determined to estimate the nitrogen discharge in different forms.
Nitrite nitrogen (NO2−-N) was measured by spectrophotometric method (Bendschneider and Robinson, 1952).Total ammonia nitrogen (TAN), nitrate nitrogen (NO3−-N)and total nitrogen (TN) were measured by standard methods for the examination of water and wastewater(APHA., 1981). Dissolved organic nitrogen was calculated by the margin calculation between total dissolved nitrogen and inorganic nitrogen (., nitrite, total ammonia and nitrate). The nitrogen of suspended particles was figured out by the difference between total nitrogen and total dissolved nitrogen.
Chlorophyll-concentration was measured by hot-ethanol method (Chen, 2006), then nitrogen content of microalgae was estimated through carbon chlorophyll-ratio (Lue, 2009) and carbon nitrogen molar ratio (Mei, 2011). Total cultivable bacteria amount was enumerated by spread plate method (APHA, 1981) with marine agar 2216E media (OPPENHEIMER, 1952), and bacteria nitrogen biomass was estimated through its popu- lation (Lee and Fuhrman, 1987). Vibrio number was e- numerated by TCBS agar media (Turner, 2009; Thompson, 2004; Pfeffer, 2003) for reflecting the status of disease. In addition, the bacteria and vibrio number in bottom mud were also monitored by spread plate method.
2.5 Statistical Analysis
The statistical analysis was performed using SPSS program version 13.0 (SPSS, Chicago, IL, USA), an independent samples T test was conducted to compare the significant differences between RAS and WES on shrimp growth performance and water quality, and one-way ANOVA was conducted on the change of bacteria number.
3 Results
3.1 General Water Quality
The make-up water quality was roughly constant during the whole experiment (Table 1). The DO, pH, temperature,turbidity, salinity of rearing water are presented in Table 2. The pH value was significantly lower in RAS than in WES. Due to the relatively lower water renewal rate, the water temperature in RAS was significantly higher than that in WES. Although WES had high water exchange rate, the turbidity of WES was significantly higher than that of RAS.

Table 1 General quality of make-up water
Note:†Standard deviation.

Table 2 O2, pH, temperature, turbidity and salinity of rearing water
Notes: RAS, recirculating aquaculture system; WES, water exchange system; * Significant difference with<0.05.
3.2 Water Quality and Rearing Condition
The general rearing water condition is shown in Table 2. The various forms of nitrogen in RAS and WES are presented in Figs.2 and 3. On account of the high stocking density and low water renewal rate in earlier period, TAN in WES reached a high level (10.11mgL−1) on the tenth day. Increasing water renewal rate (60%) could reduce TAN, but its effect was limited. TAN was increased continuously from day 20 to day 50 and reached 11.9mgL−1at the end of the breed period. TAN also reached a high level (6.44mgL−1) in the first 10 days of RAS due to the immaturity of biofilm in biofilter. Ten days later, the biofilter could control TAN and keep it at a relatively low level, with an average concentration of 1.02mgL−1.

Fig.2 Fluctuation of various forms of nitrogen in WES during culture period (50 days). WES, water exchange system; SSN, suspended solid nitrogen; DON, dissolved organic nitrogen.

Fig.3 Fluctuation of various forms of nitrogen in RAS during culture period (50 days). RAS, recirculating aquaculture system; SSN, suspended solid nitrogen; DON, dissolved organic nitrogen.
Nitrite was at low concentration during the first ten days in WES. After that, nitrite increased quickly and reached the peak (7.72mgL−1) on day 20. The final concentration of nitrite in the WES was 6.14mgL−1. Since probiotics were inoculated into the biofilter could work fast. In the first ten days a large quantity of ammonia was transformed into nitrite and nitrite concentration reached the peak (8.80mgL−1) at the 10th day. Furthermore, 20 days later, nitrite dropped to a low and safe level. Thus RAS could control harmful nitrogen efficiently.
Nitrate in WES was at a low level during the culture period with the mean concentration of 0.68mgL−1. But in RAS, nitrate continuously increased and reached 32.4mgL−1in the end. Most of the toxic ammonia and nitrite in RAS were transformed into less toxic nitrate.
Dissolved organic nitrogen (DON) and suspended solid nitrogen (SSN) were relatively stable in WES and RAS. The mean DON of WES and RAS were 1.66mgL−1and 2.40mgL−1respectively. The mean SSN of RAS was significantly (<0.01) lower than that of WES due to the effect of drum filter in RAS.
The culture plant was adopted to partial lighting, and the light intensity was ranged from 1000 to 10000 Lux in the daytime. In general, except during the first ten days that chlorophyll-in WES (0.1532μgL−1) was significantly (<0.01) higher than that in RAS (0.0029μgL−1), chlorophyll-was at a low level in both culture systems with the mean values of 0.0041μgL−1and 0.0039μgL−1respectively.
The cultivable bacterial population in culture systems fluctuated significantly during the culture period, as shown in Fig.4 and Fig.5. After the first 15 days, the cultivable bacteria population in WES and RAS were 3×107cfumL−1and 0.52×107cfumL−1respectively. Then it grew rapidly in RAS and reached 1.03×1010cfumL−1at the 25th day. Thereafter, the cultivable bacteria population declined but still at high level. The cultivable bacterial population in WES grew slowly and reached the peak (2.92×109cfumL−1) at the 35th day and declined sharply at the 45th day (1.04×108cfumL−1). The fluctuation of vibrio population in WES was similar to that of cultivable bacteria. At the 15th day, the vibrio concentration in WES was 0.13×104cfumL−1, and increased slowly to 2.23×104cfumL−1at the 35th day, then remained stable. The vibrio increased rapidly in RAS and reached 2.42×104cfumL−1at 15th day, and grew to 5.15×104cfumL−1at 25th day. In general, the bacteria population level in RAS was higher than that in WES.

Fig.4 Fluctuation of bacterial population in WES and RAS during culture. WES, water exchange system; RAS, recirculating aquaculture system. a and b indicate statistical differences at P<0.05.
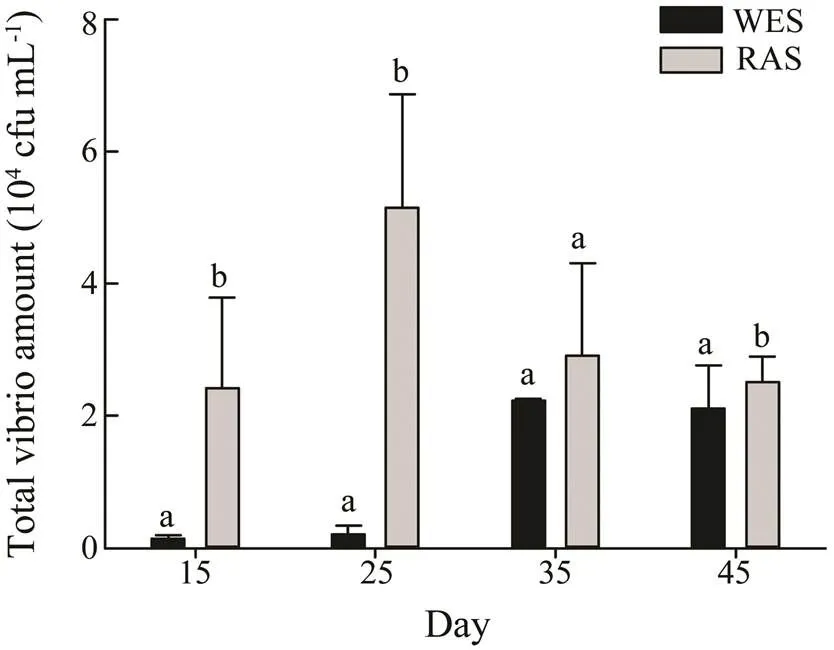
Fig.5 Fluctuation of vibrio population in WES and RAS during culture. WES, water exchange system; RAS, recirculating aquaculture system. a and b indicate statistical differences at P<0.05.
3.3 Shrimp Growth Performance
The growth performance of shrimp in RAS and WES systems is shown in Table 3. The growth rate of shrimp in RAS and WES systems was 0.184gd−1and 0.186gd−1, respectively. The survival rate of shrimp in RAS and WES systems was 68.97% and 75.45%, respectively. Comparing the results of growth rate, survival rate and final yield, the growth performance of shrimp in RAS was slightly inferior but comparable to that in WES.
Table 3 Growth performance of shrimp in RAS and WES systems

Notes: WES, water exchange system; RAS, recirculating aquaculture system.
3.4 Energy Consumption
In WES, the main power consumption was roots blower. In RAS, water-circulating pump, ozone disinfection, and biofilter aeration were also included in the energy consumption. In summary, the energy consumptions of WES and RAS during the experimental period (50 days) were 3540kwh and 4514kwh respectively. ·
3.5 Nitrogen Budget
Feed and juvenile shrimp were main nitrogen input resources. Feed nitrogen inputs were 155.57kg and 170.20 kg respectively in WES and RAS. Feed is taken a 100% of nitrogen input and net shrimp harvest (juvenile shrimp is subtracted) is taken as shrimp harvest nitrogen. The nitrogen output was shown in Fig.6. Only 23.58% and 19.10% of the input nitrogen were recovered in shrimp. The nitrogen remained in bottom mud in WES was 18.03%, which was significantly lower than the proportion of 26.64% in RAS. The largest proportion over the study was contained in water (51.09% in WES, 41.44% in RAS). In accordance with the result of water quality, the proportion of nitrite and TAN in WES were up to 15.30% and 21.32% respectively, however the proportion of nitrite and TAN in RAS were only 4.33% and 3.85% respectively. The nitrogen output of nitrate in WES was only 2.91%, while the proportion in RAS was 25.97%. Due to the filtration effect of drum filter, the turbidity in RAS (5.40±1.33 NTU) was significantly lower (<0.05) than that in WES (8.43±4.12 NTU) (Table 2). Therefore, the proportion of SSN in WES and RAS were 6.35% and 3.14%, respectively.
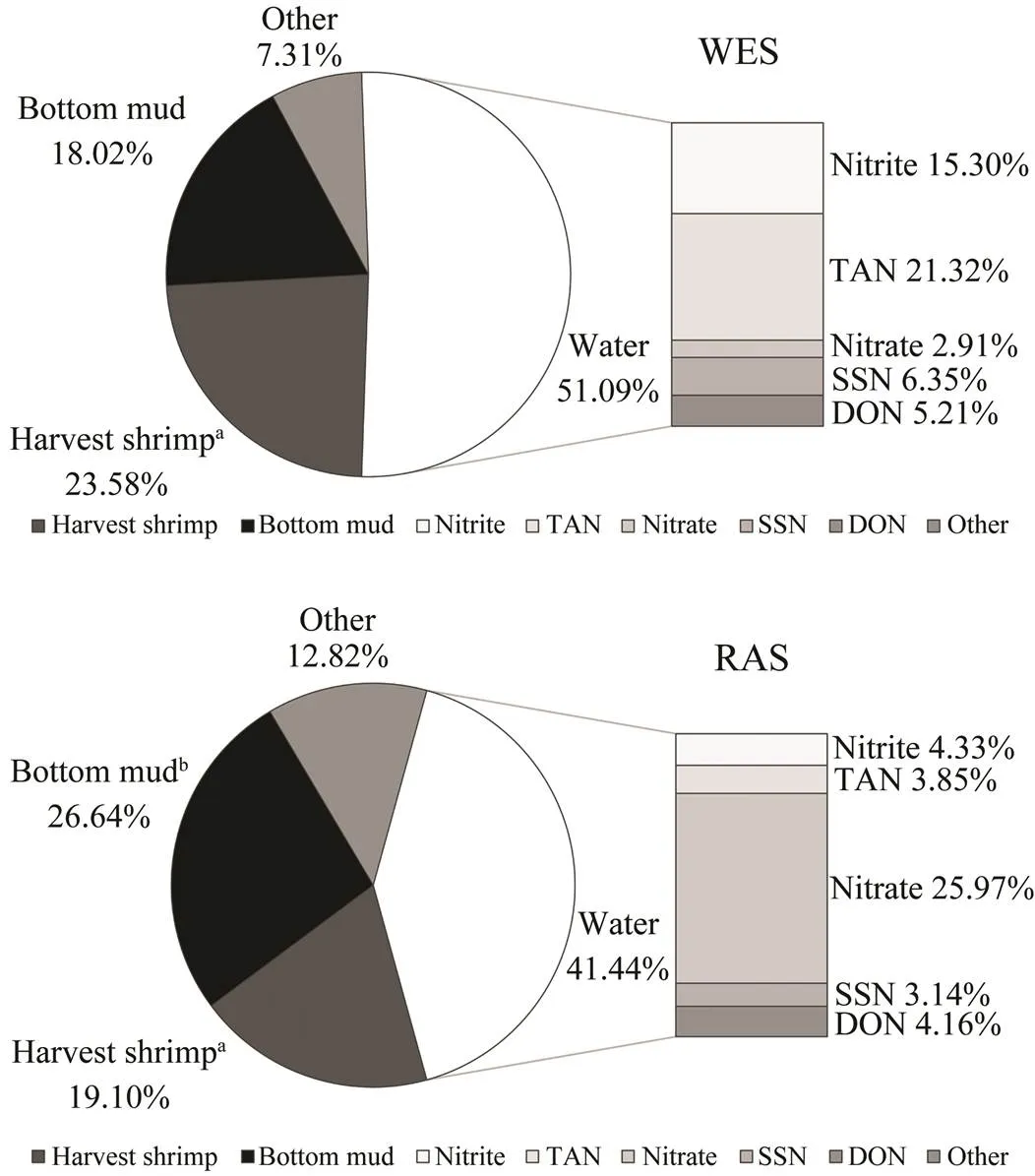
Fig.6 Nitrogen output account for nitrogen input of feed in WES and RAS. WES, water exchange system; RAS, recirculating aquaculture system; SSN, suspended solid nitrogen; DON, dissolved organic nitrogen. a Including dead shrimp but get rid of initial juvenile shrimp. b Including bottom mud of biofilters.
4 Discussion
4.1 Present Intensive Farming Contrast to Traditional Extensive Farming
Compared with traditional pond culture, the industrialized shrimp culture generally has a higher culture density (Jackson, 2003; Thakur and Lin, 2003; Perez- Velazquez, 2008). High stocking density could improve culture efficiency and get more benefits, but high feed input and meticulous management on aquaculture environment are also necessary. In the present study, both TAN and nitrite could exceed safety level (Lin and Chen, 2001; Lin and Chen, 2003) in the culture period. The ammonia and nitrite reached up to 11.90 and 7.92mgL−1, respectively in WES, and reached up to 6.29 and 8.80mgL−1respectively in RAS, which was much higher than that of traditional pond farming (Thakur and Lin, 2003; Jackson, 2003). TAN and nitrite with high concentration have great threat to shrimp and they need to be controlled in secure level. It makes a great difference for the management of intensive and industrial shrimp farming.
High stocking density makes the water circumstance deteriorate rapidly. In order to maintain rearing environment, WES relies on water exchange and RAS relies on water purification. On the whole, the mean daily water renewal rate of WES was 44%, and 1kg shrimp yield consumed 3.89m3water. The mean daily water renewal rate of RAS was 10%, and 1kg shrimp yield consumed 1.25m3water. Compared to WES, RAS could realize the water saving rate of 68%. Meanwhile, high stocking density also means high power consumption. One kilogram shrimp yield consumed 3.60kWh and 2.51kWh power respectively for RAS and WES.
4.2 Aquatic Environment of WES and RAS
Water quality in WES was even worse than that in RAS, although a large amount of water was exchanged to maintain the circumstance. The most severe problem of WES was ammonia accumulation. The daily water exchange rate of sixty percent could not control ammonia level effectively. Since the 20th day, TAN was progressively increased and reached up to11.90mgL−1at the end of the experiment (Fig.2). It outdistanced the safety level and had serious threat to shrimp. Compared to ammonia, nitrite was easier to control for WES and below the safety level during most of the experimental time. RAS was adept in controlling ammonia and nitrite. Ammonia and nitrite accumulated in the early stage due to the immaturity of biofilter. But 20 days later, both TAN and nitrite were maintained at safety level. The lowest concentrations of TAN and nitrite in RAS was 0.43mgL−1and 0.50mgL−1, respectively. RAS could remove harmful nitrogen via nitrification and transform ammonia and nitrite into low toxic nitrate.
4.3 Intensive Shrimp Culture Contrast to Industrialized Fish Farming
Compared to industrialized fish culture, shrimp culture has a series of differences. The stocking density in this study was about 4.2–7.7kgm−3, which was obviously lower than intensive fish culture systems (Gelfand, 2003; d’Orbcastel, 2009c). However, compared with corresponding fish culture system, TAN and nitrite concentration in the present study were higher in general. It could be caused by the difference of metabolic characteristic between fish and shrimp. The feed nitrogen retained in shrimp is about 20%–31% (Muthuwan, 1991; Briggs and Fvnge-Smith, 1994; Jackson, 2003; Thakur and Lin, 2003), while the feed nitrogen retained in fish is about 25%–46% (Wang, 2007; Ai, 2007; Peres and Oliva-Teles, 2006). It means that compared to fish culture system more feed nitrogen can’t be retained in shrimp and they are released to environment. As the result, water quality in shrimp culture system is difficult to manage. In addition, water renewal rates in WES and RAS of fish are about 58m3per kg of feed and 9m3per kg of feed respectively (d’Orbcastel, 2009b), while in this study water renewal rate of WES and RAS are about 2m3per kg of feed and 0.6m3per kg of feed respectively. This is another reason resulting water quality of shrimp rearing system worse than fish rearing systems. It’s necessary to moderately increase water renewal rate to control water quality in shrimp rearing system.
4.4 Shrimp Performance Comparison Between RAS and WES
In terms of aquatic environment, RAS was better than WES. But the shrimp performance in WES was better than those in RAS. The yield and shrimp weight of WES were better than that of RAS, but the one-way ANOVA showed that the differences were not significant (>0.05) due to the large difference among shrimp tanks and shrimp weight. It could be the microorganisms that caused the difference between RAS and WES systems. Both total cultivable bacteria and vibrio in RAS were higher than those in WES. Though microorganisms play a great role in adjusting water environment, excessive bacteria, especially the vibrio, might have a negative impact on shrimp, considering most of the shrimp disease are caused by vibrio (Lightner and Redman, 1998). In the whole culture period, cultivable bacteria population and vibrio population in RAS were larger than those in WES. The population peak of total cultivable bacteria and vibrio in the RAS were 1.03×1010cfumL−1and 5.15×104cfumL−1. Vibrio populations in WES and RAS both reached the magnitude of 104cfumL−1, which had a severe threat to the health of shrimp. It need further means to control harmful germs especially for the RAS. The morbidity in RAS was severer than that in WES, and the mean survival rate in RAS was 68.99% significantly (<0.05) lower than that in WES (75.48%). The water quality in shrimp tanks was adaptive for microorganisms. Low water renewal rate made it more serious for RAS. Though RAS was equipped with disinfection device, the function of the disinfection was inadequate as the power of ozonator was only 500W and its work time less than 12h each day. RAS need more efficient disinfection measure or other measures, such as probiotics application, to inhibit harmful germs.
4.5 Nitrogen Budget
The nitrogen retained in shrimp in WES was more than that in RAS, and both of them were comparable to former study (Muthuwan, 1991; Briggs and Fvnge-Smith, 1994; Jackson, 2003; Thakur and Lin, 2003). As residual feeds in RAS were removed from culture system timely, lower utilization of bait was possibly caused. Therefore, more residual feeds generated in RAS and the proportion of bottom mud in nitrogen output was higher. In addition, if residual feeds and feces could not be discharged from WES timely, they would be decomposed by bacteria, and the quantity of bottom mud would be reduced. This was another reason that the proportion of nitrogen in bottom mud in WES was lower than that in RAS.
The proportion of nitrite and TAN in nitrogen output of WES was 36.62%, which was much higher than the proportion of 14.45% in RAS. Ammonia is nitrogen metabolite of shrimp, and it could be transformed into nitrite and nitrate successively by nitrobacteria. Most of nitrite and ammonia in RAS was transformed into nitrate finally. It was accordant to the result of water quality. In the present study, nitrate in RAS was 32.4mgL−1in the end, which meant more than 76% of toxic nitrogen was removed by bacteria in RAS according to the percentage of inorganic nitrogen (ammonia, nitrite and nitrate) in nitrogen output. However, in WES only 7.37% toxic nitrogen could be transformed into nitrate because the nitrifying process was broken by frequent water exchange, and most of them could be removed by water exchange.
There were 7.31% and 12.82% of nitrogen in WES and RAS respectively that didn’t belong to the seven types of nitrogen output. They were caused by ammonia volatilization, denitrification and statistical error. According to the ammonia loss rate from water to atmosphere (Weiler, 1979) and rearing water environment, it could be estimated that the quantity of ammonia volatilization nitrogen were about 7.95kg (5.11%) and 2.01kg (1.18%), respectively in WES and RAS. Because of the high ammonia concentration, ammonia volatilization was significant in WES. Denitrification is another way that nitrogen transfer from water to atmosphere. Some bacteria could take advantage of nitrate and successively transform nitrate to NO2−, NO, N2O and N2(Ferguson, 1994). In RAS, nitrate accumulated continuously and finally reached 32.4mgL−1. Denitrifiers are common in nature, and it could be speculated that there was denitrification in biofilter or in gut of shrimp (Heisterkamp, 2016) to some extent. It was coincident to the nitrogen budget gap of 12.82% (21.82kg). In the present study, no measure was adopted to figure up the exact proportion of denitrification.
4.6 The Effect of Microalgae and Germ in RASand WES
In the earlier stage of WES, microalgae could have a certain extent of growth with the chlorophyll-concentration of 0.1532μgL−1. However, chlorophyll-declined sharply due to the subsequent high water renewal rate. In RAS microalgae could not get mass propagation because of disinfection. In addition, partial lighting of the plant and high turbidity of aquatic water were other reasons for the low chlorophyll-. Therefore microalgae couldn’t achieve the same great effect as in the previous studies (Samocha, 2015; Ge, 2016) in RAS and WES. Calculated microalgae nitrogen biomass via chlorophyll-(Lue, 2009) and figured up the total microalgae nitrogen biomass based on rearing water volume and renewal rate. Finally, microalgae nitrogen biomass in WES and RAS were 0.25g and 0.06g respectively. Their proportion in nitrogen output were negligible. Microalgae couldn not play a significant role in WES and RAS.
Bacteria play a great role in aquaculture. Such as digestion promotion, immunity enhancement and water quality improvement (Loh, 2017). In this study, the cultivable bacteria population in RAS was more than that in WES, and both of them had a significant fluctuation during the rearing period. Bacterial nitrogen biomass was calculated by bacteria population, which was figured outbacteria amount and water renewal rate, and the bacteria nitrogen biomass of 5.6fgN cell−1(Lee and Fuhrman, 1987). There were 33.76kg and 6.20kg nitrogen retained in cultivable bacteria in WES and RAS, respectively. In addition, bacteria had a great effect on nitrogen output, especially for inorganic nitrogen, and 7.37% (4.53kg) and 76.04% (44.20kg) of toxic nitrogen (ammonia and nitrite) was transformed into nitrate in WES and RAS, respectively. In this study, marine agar 2216E media was used to measure the population of cultivable bacteria, but it was not equal to the total bacteria as most of the bacteria in nature was uncultivable. Whereas comparing the result of spread plate method to fluorescence microscope method (Hobbie, 1977), there was no difference on the order of magnitudes. It could be particularity of shrimp tanks that made it dramatically different from natural environment. Therefore the result of spread plate method can be the representative for the bacteria population of shrimp tank. In view of most bacteria is uncultured (Rappé and Giovannoni, 2003), the proportion of bacteria in nitrogen output were more than 24.61% (38.29kg) and 29.61% (50.40kg) in WES and RAS, respectively. The effect of bacteria on nitrogen output was outstanding.
In conclusion, the intensive and industrial farming systems WES and RAS could improve shrimp yield. RAS could maintain acceptable water quality with a lower water renewal rate than WES, while the growth rate and yield of shrimp were comparable to WES. The nitrogen budget results provided detailed data on nitrogen output. The proportion of shrimp in nitrogen output were 19.10% and 23.58% respectively in RAS and WES. Most of the nitrogen was retained in water, and it was mainly in the forms of ammonia and nitrite in WES while in the form of nitrate in RAS. Microalgae had a little effect on nitrogen budget, while bacteria had a great role in shrimp farming and nitrogen budget. It’s beneficial to take advantage of the bacteria in industrial shrimp farming, while control them within a safe level.
Acknowledgements
This study was supported by the China Agriculture Research System (No. CARS-47), the Taishan Industrial Leader Talent Project of Shandong Province (No. LJNY 2015002) and the Aoshan Innovation Project of Qingdao National Laboratory for Marine Science and Technology (No. 2015ASKJ02).We thank the manager and staff of the shrimp farm for providing experimental site, offering management data and the facilities.
Ai, Q., Mai, K., Zhang, W., Xu, W., Tan, B., Zhang, C., and Li, H., 2007. Effects of exogenous enzymes (phytase, non-starch polysaccharide enzyme) in diets on growth, feed utilization, nitrogen and phosphorus excretion of Japanese seabass,., 147: 502- 508.
APHA, 1981.APHA American Public Health Association.
Attramadal, K. J. K., Salvesen, I., Xue, R., Oie, G., Storseth, T. R., Vadstein, O., and Olsen, Y., 2012. Recirculation as a possible microbial control strategy in the production of ma- rine larvae., 46: 27-39.
Bendschneider, K., and Robinson, R. J., 1952. A new spectro- photometric method for the determination of nitrite in sea water., 2: 87-96.
Briggs, M. R. P., and Fvnge-Smith, S. J., 1994. A nutrient budget of some intensive marine shrimp ponds in Thailand., 25: 789-811.
CFSY, 2016. China Fishery Statistical Yearbook.China Agri- culture Publishing House, Beijing.
Chen, Y., Chen, K., and Hu, Y., 2006. Discussion on possible error for phytoplankton chlorophyll-concentration analysis using hot-ethanol extraction method., 5: 550-552.
Colson, V., Sadoul, B., Valotaire, C., Prunet, P., Gaume, M., and Labbe, L., 2015. Welfare assessment of rainbow trout reared in a Recirculating Aquaculture System: Comparison with a Flow-Through System., 436: 151-159.
Dierberg, F. E., and Kiattisimkul, W., 1996. Issues, impacts, and implications of shrimp aquaculture in Thailand., 20: 649-666.
d’Orbcastel, E. R., Blancheton, J. P., and Aubin, J., 2009a. Towards environmentally sustainable aquaculture: Comparison between two trout farming systems using Life Cycle A- ssessment., 40: 113-119.
d’Orbcastel, E. R., Blancheton, J. P., and Belaud, A., 2009b. Water quality and rainbow trout performance in a Danish Model Farm recirculating system: Comparison with a flow through system., 40: 135-143.
d’Orbcastel, E. R., Ruyet, P. L., J., Bayon, N. L, and Blancheton, J. P., 2009c. Comparative growth and welfare in rainbow trout reared in recirculating and flow through rearing systems., 40: 79-86.
Ferguson, S. J., 1994. Denitrification and its control., 66: 89-110.
Ge, H., Li, J., Chang, Z., Chen, P., Shen, M., and Zhao, F., 2016. Effect of microalgae with semicontinuous harvesting on water quality and zootechnical performance of white shrimp reared in the zero water exchange system., 72: 70-76.
Gelfand, I., Barak, Y., Even-Chen, Z., Cytryn, E., van Rijn, J., Krom, M. D., and Neori, A., 2003. A novel zero discharge intensive Seawater recirculating system for the culture of marine fish., 34: 344-358.
Heisterkamp, I. M., Schramm, A., de Beer, D., and Stief, P., 2016. Direct nitrous oxide emission from the aquacultured Pacific white shrimp ()., 82: 4028-4034.
Hobbie, J. E., Daley, R. J., and Jasper, S., 1977. Use of nu- clepore filters for counting bacteria by fluorescence micro- scopy., 33: 1225- 1228.
Holl, C. M., Glazer, C. T., and Moss, S. M., 2011. Nitrogen stable isotopes in recirculating aquaculture for super-intensive shrimp production: Tracing the effects of water filtration on microbial nitrogen cycling., 311: 146-154.
Jackson, C., Preston, N., Thompson, P. J., and Burford, M., 2003. Nitrogen budget and effluent nitrogen components at an intensive shrimp farm., 218: 397-411.
Kolarevic, J., Baeverfjord, G., Takle, H., Ytteborg, E., Reiten, B. K. M., Nergard, S., and Terjesen, B. F., 2014. Performance and welfare of Atlantic salmon smolt reared in recirculating or flow through aquaculture systems., 432: 15- 25.
Kuhn, D. D., Smith, S. A., Boardman, G. D., Angier, M. W., Marsh, L., and Flick Jr., G. J., 2010. Chronic toxicity of nitrate to Pacific white shrimp,: Im- pacts on survival, growth, antennae length, and pathology., 309: 109-114.
Lee, S., and Fuhrman, J. A., 1987. Relationships between bio- volume and biomass of naturally derived marine bacterio- plankton., 53: 1298- 1303.
Lightner, D. V., and Redman, R., 1998. Shrimp diseases and current diagnostic methods., 164: 201-220.
Lin, Y. C., and Chen, J. C., 2001. Acute toxicity of ammonia onBoone juveniles at different salinity levels., 259: 109-119.
Lin, Y. C., and Chen, J. C., 2003. Acute toxicity of nitrite on(Boone) juveniles at different salinity levels., 224: 193-201.
Loh, J. Y., 2017. The role of probiotics and their mechanisms of action: An aquaculture perspective., 19-23.
Lue, S., Wang, X., and Han, B., 2009. A field study on the conversion ratio of phytoplankton biomass carbon to chlo- rophyll-in Jiaozhou Bay, China., 27: 793-805.
Martins, C. I. M., Eding, E. H., Verdegem, M. C. J., Heinsbroek, L. T. N., Schneider, O., Blancheton, J. P., d’Orbcastel, E. R., and Verreth, J. A. J., 2010. New developments in recircu- lating aquaculture systems in Europe: A perspective on en- vironmental sustainability., 43: 83- 93.
Mei, Z. P., Finkel, Z. V., and Irwin, A. J., 2011. Phytoplankton growth allometry and size-dependent C:N stoichiometry revealed by a variable quota model., 434: 29-43.
Muthuwan, V., 1991. Nutrient budget and water quality in in- tensive marine shrimp culture ponds. Master thesis. Asian Institute of Technology, Bangkok.
Naylor, R. L., Goldburg, R. J., Mooney, H., Beveridge, M., Clay, J., Folke, C., Kautsky, N., Lubchenco, J., Primavera, J., and Williams, M., 1998. Nature’s subsidies to shrimp and salmon farming., 282: 883-884.
Oppenheimer, C. H., 1952. The growth and viability of sixty- three species of marine bacteria as influenced by hydro-
static pressure., 11: 10-18.
Otoshi, C. A., Arce, S. M., and Moss, S. M., 2003. Growth and reproductive performance of broodstock shrimp reared in a biosecure recirculating aquaculture system versus a flow- through pond., 29: 93-107.
Peres, H., and Oliva-Teles, A., 2006. Effect of the dietary essential to non-essential amino acid ratio on growth, feed utilization and nitrogen metabolism of European sea bass ()., 256: 395-402.
Perez-Velazquez, M., Gonzalez-Felix, M. L., Gomez-Jimenez, S., Davis, D. A., and Miramontes-Higuera, N., 2008. Nitro- gen budget for a low-salinity, zero-water exchange culture system: II. Evaluation of isonitrogenous feeding of various dietary protein levels to(Boone)., 39: 995-1004.
Pfeffer, C. S., Hite, M. F., and Oliver, J. D., 2003. Ecology ofin estuarine waters of eastern North Carolina., 69: 3526-3531.
Piedrahita, R. H., 2003. Reducing the potential environmental impact of tank aquaculture effluents through intensification and recirculation., 226: 35-44.
Rappé, M. S., and Giovannoni, S. J., 2003. The uncultured mi- crobial majority., 57: 369- 394.
Rosenthal, H., Castell, J., Chiba, K., Forster, J., Hilge, V., Hogendoorn, H., Mayo, R., Muir, J., Murray, K., and Petit, J., 1986. Flow-through and recirculation systems., 100.
Samocha, T., Fricker, J., Ali, A., Shpigel, M., and Neori, A., 2015. Growth and nutrient uptake of the macroalga Gracilaria tikvahiae cultured with the shrimpin an Integrated Multi-Trophic Aquaculture (IMTA) system., 446: 263-271.
Summerfelt, S., Sharrer, M., Gearheart, M., Gillette, K., and Vinci, B., 2009. Evaluation of partial water reuse systems used for Atlantic salmon smolt production at the White River National Fish Hatchery., 41: 78- 84.
Tal, Y., Schreier, H. J., Sowers, K. R., Stubblefield, J. D., Place, A. R., and Zohar, Y., 2009. Environmentally sustainable land- based marine aquaculture., 286: 28-35.
Thakur, D. P., and Lin, C. K., 2003. Water quality and nutrient budget in closed shrimp () culture systems., 27: 159-176.
Thoman, E. S., Ingall, E. D., Davis, D. A., and Arnold, C. R., 2001. A nitrogen budget for a closed, recirculating mari- culture system., 24: 195-211.
Thompson, F. L., Iida, T., and Swings, J., 2004. Biodiversity of vibrios., 68: 403-431.
Turner, J. W., Good, B., Cole, D., and Lipp, E. K., 2009. Plank- ton composition and environmental factors contribute toseasonality., 3: 1082-1092.
Verdegem, M. C. J., Bosma, R. H., and Verreth, J. A. J., 2006. Reducing water use for animal production through aqua- culture., 22: 101-113.
Wang, Y., Kong, L. J., Li, K., and Bureau, D. P., 2007. Effects of feeding frequency and ration level on growth, feed utilization and nitrogen waste output of cuneate drum () reared in net pens., 271: 350-356.
Weiler, R., 1979. Rate of loss of ammonia from water to the atmosphere., 36: 685-689.
(Edited by Qiu Yantao)
Received July 9, 2017; revised September 20, 2017; accepted April 20, 2018)
© Ocean University of China, Science Press and Springer-Verlag GmbH Germany 2018
. Tel: 0086-532-85830183E-mail: lijian@ysfri.ac.cn
杂志排行
Journal of Ocean University of China的其它文章
- Offshore Fault Geometrics in the Pearl River Estuary, Southeastern China: Evidence from Seismic Reflection Data
- Application of Geoid Anomalies to the Tectonic Research in the East Asian Continental Margin
- Middle Holocene Organic Carbon and Biomarker Records from the South Yellow Sea: Relationship to the East Asian Monsoon
- Mesozoic Deformation and Its Geological Significance in the Southern Margin of the South China Sea
- Optimization of Shanghai Marine Environmental Monitoring Sites in the Identification of Boundaries of Different Water Quality Grades
- Seasonal Variation of Environmental Variables and Phytoplankton Community Structure and Their Relationship in Liaodong Bay of Bohai Sea, China
