Hierarchical porous MgBO2(OH)microspheres:Hydrothermal synthesis,thermal decomposition,and application as adsorbents for Congo red removal☆
2018-08-18PanpanSunLiyuanChenLinXuWanchengZhu
Panpan Sun,Liyuan Chen,Lin Xu,Wancheng Zhu*
Department of Chemical Engineering,Qufu Normal University,Shandong 273165,China
Keywords:Hierarchical porous microspheres Magnesium borate hydroxide Adsorption Congo red Hydrothermal
ABSTRACT A facile eco-friendly hydrothermal route(180°C,12.0 h)has been developed for the first time to the uniform hierarchical porous MgBO2(OH)microspheres without the aid of any organic additive,surfactant or template,by using the abundant MgCl2·6H2O,H3BO3and NaOH as the raw materials.The as-obtained porous microspheres exhibit a specific surface area of 94.752 mg·g−1,pore volume of 0.814 cm3·g−1,and ca.84.0%of which have a diameter of 2.25–3.40 μm.The thermal decomposition of the porous MgBO2(OH)microspheres(650 °C,2.5 °C·min−1)leads to the porous Mg2B2O5microspheres with well-retained morphology.When utilized as the adsorbents for the removal ofCRfrommimic waste water,the present porous MgBO2(OH)microspheres exhibit satisfactory adsorption capacity,with the maximum adsorption capacity qmof 309.1 mg·g−1,much higher than that derived from most of the referenced adsorbents.This opens a new window for the facile green hydrothermal synthesis of the hierarchical porous MgBO2(OH)microspheres,and extends the potential application of the 3D hierarchical porous metal borates as high-efficiency adsorbents for organic dyes removal.
1.Introduction
Three-dimensional(3D)hierarchical porous architectures self-assembled by one-dimensional(1D)or two-dimensional(2D)sub-units have drawn extensive interest due to their fantastic characteristics,such as unique porous structures,high specific surface area and good mechanicalproperties[1–4].Among the varieties of advanced functional 1D nanostructures or 1D nanostructured building blocks constituted hierarchical architectures,metal oxides(e.g.MgO[5],Al2O3[6],Fe2O3[7],ZnO[8],etc.),carbonates,silicates,and especially 1D nanostructured magnesium borates(e.g.Mg2B2O5nano whiskers[9],nano wires[10],nanorods[11],and nanobelts[12],Mg3B2O6nanotubes[13],MgB4O7nanowires[14])have attracted much research attention and considerable efforts for their widespread applications when employed as reinforcements in electronic ceramics[14],whisker-reinforced composites[15],and antiwear additives[16],etc.So far,however,the reported 1D magnesium borates nanostructures were mostly obtained via the conventional high temperature based techniques,including the chemical vapor deposition(CVD)[10,13,14]and molten salt synthesis(MSS)[17,18].Although the CVD and MSS can easily yield high crystallinity products,the inevitably encountered problems such as high temperature[10,14],and corresponding high energy consumption[14],or even requisite large amount of flux agent in some cases[18],etc.,will in turn limit the practical applications of these techniques.
During the past few years,in addition to 1D magnesium borates nanostructures,there have been very few works in literature on the 3D nanostructured metal borates.As reported,3D flower-like Mg3B2O6microspheres consisted of nanosheets were synthesized through a polyvinyl pyrrolidone(PVP)assisted precipitation followed by a calcination(650 °C,4 h)[19];and also,mesoporous 2MgO·B2O3microspheres of low crystallinity were acquired by a precipitation and subsequent annealing(500°C,4.0 h),with sodium dodecyl sulfate(SDS)as the capping agent[20].Besides,oval-like 4CaO·5B2O3·7H2O micro spheres consisted of nanoplates were fabricated via a hydrothermal method(120°C)in the presence of the surfactant polyethylene glycol(PEG-300)[21].As mentioned above,in order to obtain 3D hierarchical porous alkaline-earth metal borates,high-molecular polymer additives or organic solvents are usually required during the controllable self-assembly of the subunits for the desired hierarchical architectures.Notably,however,it not only leads to the relatively low solubility of the inorganic reactants but also suffers from the underlying security issues,when using organic species for the usually unavoidable toxic, flammable,and even explosive nature of the high-molecular polymer additives or organic solvents[3,22].Most recently,we reported hierarchical Laminar Ca4B10O19·7H2O[23]superstructures and porous Ca(BO2)2micros pheres[4]via the facile hydrothermal procedure,without the aid of anytem plate,surfactant,orcapping agent.In addition,weal so developed an ionothermal con fined self-assembly approach for the hierarchical porous MgBO2(OH)superstructures,and the facile thermal decomposition process(600°C,12.0 h)thereafter rendered the Mg2B2O5microspheres with high crystallinity.Impressively,both MgBO2(OH)and Mg2B2O5microspheres presented outstanding adsorption efficiency for Congo red(CR)[3].As known,CR is abenzidine-based anionic diazo dye,usually applied in wood pulp,cotton textile,hair colorant,photosensitizer and as a redox indicator in analytical chemistry.To date,nevertheless,it still remains an enormous challenge to develop a rational,mild and controllable green synthesis for the 3D hierarchical nanoarchitectures of metal borates.
In this contribution,we reported, for the first time, a successful strategy for the hierarchical porous MgBO2(OH)microspheres through a facile,green and reliable hydrothermal process,without the aid of any organic additives or templates.Meanwhile, influences of the process parameters (e.g. reactant concentration, temperature, and time) on the hydrothermal products were explored in detail. Moreover, the thermal decomposition properties and latent employment of the hierarchical porous MgBO2(OH)microspheres as adsorbents for elimination of CR from mimic waste water were also evaluated. Compared with the previous work,the present mild hydrothermal route to the hierarchical porous MgBO2(OH)/Mg2B2O5micros pheres is distinct of the green and eco-friendly characteristic.
2.Experimental
2.1.Synthesis of hierarchical porous MgBO2(OH)microspheres
All chemicals were of analytical grade and used directly without further purification.The MgBO2(OH)microspheres were fabricated by a co-precipitationof MgCl2·6H2O,H3BO3and NaOH at room temperature(R.T.),followed by a facile hydrothermal treatment.Typically,1.529 g(7.5 mmol)of MgCl2·6H2O,0.928 g(15 mmol)of H3BO3and 0.300 g(7.5 mmol)of NaOH(molar ratio of Mg:B:OH=1:2:1)were individually dissolved into 10.0,30.0 and 10.0 ml of deionized(DI)water.Subsequently,the solutions of H3BO3and NaOH were successively dropwise added to the above MgCl2·6H2O solution at R.T.under vigorous magnetic stirring.Having been agitated for 10 min,the as-formed precursor slurry wastransferred into a Te flon-lined stainless steelautoclave(capacity:65 ml),which was sealed,then heated to 180°C(heating rate:5 °C·min−1),and kept in the constant temperature for 12.0 h.With the hydrothermal conversion completed,the autoclave was naturally cooled down to R.T.After that,the product was collected,and washed for thre etimes with DI water and ethanol,respectively,and finally dried at70°C for 12.0 h.Toevaluate the effects of the process para meters on the hydrothermal products,the reactant concentration,temperature,and time were tuned within the range of 0.075–0.225 mol·L−1,120–210 °C and 6.0–24.0 h,respectively,with other conditions kept the same.
2.2.Thermal decomposition of the porous MgBO2(OH)microspheres
The hydrothermally synthesized MgBO2(OH)microspheres were transferred into a porcelain boat,which was then located in a tube furnace and heated to 600–700 °C in air,with a heating rate of 2.5 °C·min−1.The furnace was then kept under the preset calcination temperature for2.0 h.After the thermal decomposition,the system was cooled down to R.T.naturally,and the samples were collected,rinsed with DI water and ethanol for three times,and then dried at 70°C for 12.0 h.
2.3.Hierarchical porous MgBO2(OH)microspheres as the adsorbents for CR removal
The as-obtained MgBO2(OH)microspheres were used as the adsorbents for the removal of CR from the mimic waste water.In a typical procedure,within each 50 ml conical flask,20.0 mg of the MgBO2(OH)microspheres were added to 40.0 ml of the CR solution with various initial concentrations(50.0–500.0 mg·L−1)at R.T.After an enough long time such as 300 min for the adsorption to reach equilibrium,the flasks were sampled,and the adsorbents were separated from the filtrate by a disposable syringe needle filter(pore diameter:0.22 μm,applicable for aqueous phase).The concentration of the residual CR in the filtrate was determined by UV–vis absorption spectroscopy at a wavelength of 496 nm.In order to understand the kinetics,the adsorption experiment was carried out by keeping the initial concentration of the CR containing solution as 50.0 mg·L−1.Within the whole adsorption time range of 0–300 min,for each time,4.0 ml of the suspension solution was taken out from the flaskat the preset intervals.The adsorption capacity of CR for MgBO2(OH)micros pheres could be calculated by the Eqs.(1)and(2):

whereqt(mg·g−1)andqe(mg·g−1)aretheamountoftheadsorbedCR on the adsorbents at time t and the equilibrium,respectively;V is the total volume of CR solution(L);c0,ctand ceindicate the concentration of CR solution(mg·L−1)at initial,real-time t and equilibrium,respectively;m(mg)is the mass of the employed adsorbents,i.e.the MgBO2(OH)microspheres.
2.4.Characterization
Phase and crystal structure of the sampleswere determined by an Xray powder diffractometer (XRD,Mini Flex600, Rigaku, Japan) at a scanning rate of 10(°)·min−1,witha fixed power source(30.0 kV,10.0 mA)and a Cu-Kαradiation(λ =0.15406 nm).Morphology and microstructure of the productswere recorded on a field emission scanning electron microscopy(SEM,JSM 7401F,JEOL,Japan)operated at 3.0 kV,aswell as a high resolution transmission electron microscopy(TEM,JEM-2010,JEOL,Japan)worked at 120.0 kV.Diameter distribution of the as-obtained micros pheres was approximately estimated by directly measuring ca.100 typical particles from the representative SEM images.Porous structure of the product was identified by the N2adsorption–desorption isotherms,by using a chemisorption-physisorption analyzer(SSA-4200,Beijing Builder Company,China)at 77 K after the sample had been degassed at 200 °C for 2.0 h.Brunauer–Emmett–Teller(BET)specific surface areas(SBET)for the samples were calculated from the N2adsorption–desorption data within the relative pressure range of P/P0=0.05–1.0.Pore size distribution(PSD)was determined from the adsorption branch of the isotherms using the Barrett–Joyner–Halenda(BJH)model.Concentrations of CR containing or supernatant solution originated from filtration were analyzed according to the variation of absorbance,and the corresponding absorption spectra were recorded by using a UV–vis spectrophotometer(UV-756 CRT,Shanghai Yoke Instrument and Meter Co.,LTD,China),by monitoring the characteristic absorption peak at ca.λ=496 nm.
3.Results and Discussion
3.1.Structure and morphology of the hydrothermally synthesized MgBO2(OH)microspheres

Fig.1.XRD pattern(a),SEM(b and b1)and TEM(c)images,and N2adsorption–desorption isotherms(d)of the 3D MgBO2(OH)microspheres hydrothermally synthesized at 180 °C for 12.0 h,with molar ratio of Mg:B:OH as 1:2:1,concentration of MgCl2·6H2O as 0.150 mol·L−1.Red vertical lines in(a)indicate the standard pattern of MgBO2(OH)(MBOH-1)(JCPDS No.39–1370).Insets(b1),(b2)and(d1)show the diameter distribution,high resolution SEM image and pore diameter distribution of the MBOH-1 microspheres,respectively.

Fig.2.Influence of concentration on the compositions(a)and morphologies(b and c)of the products hydrothermally synthesized at 180°C for 12.0 h,with molar ratio of Mg:B:OH as 1:2:1.Concentration of MgCl2·6H2O(mol·L−1):(a1,b)MBOH-2:0.075;(a2,c)MBOH-3:0.225.Blue vertical lines of(a1)and the red vertical lines of(a2)demonstrate the standard pattern of Mg7B4O13·7H2O(JCPDS No.19–0754)and MgBO2(OH)(JCPDS No.39–1370),respectively.
Chemical composition,morphology,and porous structures of the hydrothermal productsyn the sized at180°Cfor12.0hare presented in Fig.1,with the molar ratio of Mg:B:OH=1:2:1 and concentration of MgCl2as 0.150 mol·L−1.As can be seen in the XRD pattern(Fig.1(a)),all the diffraction peaks were in good agreement with those of the standard monoclinic MgBO2(OH)(MBOH-1)(JCPDS No.39–1370,a=1.2614nm,b=1.0418nmandc=0.3144nm),indicatingthehighpurity of the product.Obviously,the pure phase of the present MBOH-1 is quite similar to the hierarchical porous MgBO2(OH)superstructures originated from the ionothermal con fined self-organization[3];meanwhile,the diffraction peak with the strongest intensity of the two products is both located at 2θ =33.76°for the corresponding plane(121).Notably however,it is completely different from the strongest peak observed at 2θ =14.11°that corresponds to the(200)plane,indicating the preferential growth direction of the high aspect ratio MgBO2(OH)nanowhiskers parallel to the longitudinal direction of the axis[24].The typical SEM image(Fig.1(b))demonstrated that,the hydrothermal MBOH-1 particles revealed relatively uniform micros pherical pro file with a narrow diameter distribution(Fig.1(b1)),and ca.84%of which had a diameter within the range of 2.25–3.40 μm.Moreover,the high magnification SEM image(Fig.1(b2))distinctly displayed that,the as-formed MBOH-1 microspheres were composed of multitudes of loosely stacked nanorods.Furthermore,the TEM image(Fig.1(c))recon firmed the hydrothermal product particles of microspherelike morphology,with loosely constitutional nanorods in the spherical body.Apparently,the as-obtained porous MBOH-1 microspheres are significantly unlike the agglomerated urchin-like spherical MgBO2(OH)nano whiskers[25].
Typical nitrogen adsorption–desorption analysis was performed to obtain in-depth insight into the texture of the as-synthesized porous MBOH-1 microspheres(Fig.1(d,d1)).It can be seen from Fig.1(d)that,the sorption isotherms of the porous MBOH-1 microspheres exhibited a type IV with a H3-type hysteresis loop at a high relative pressure of P/P0as 0.75–1.00 according to the Brunauer–Deming–Deming–Teller classi fication,suggesting the predominant narrow slit like mesopores existed within the superstructures[26].And the slit like mesopores were largely owing to the stack of the constitutional MgBO2(OH)nanorods within the microspheres.In addition,the PSD pro file(Fig.1(d1))derived from the adsorption branch further evidenced the presence of the mesopores(average pore diameter:34.2 nm,within the range of 2–50 nm according to the definition on mesoporous materials by International Union of Pure and Applied Chemistry(IUPAC))and also multitudes of macropores(>50 nm,in accordance with the definition on macroporous materials by IUPAC)existed in the body,indicating the as-synthesized MgBO2(OH)microspheres of distinct hierarchical porous structures characteristic.Moreover,theporous MBOH-1microspherespossesseda specificsurface area of 94.752 m2·g−1,a pore volume of 0.814 cm3·g−1,and an average pore diameter of 34.2 nm.The present specific surface area is much larger than that of the reported hierarchical porous MgBO2(OH)microspheres(57.2 m2·g−1)[3]and mesoporous 2MgO·B2O3microspheres(53.0 m2·g−1)[20].In addition,the pore volume of the present hydrothermally synthesized microspheres is also larger than hierarchical porous MgBO2(OH)microspheres(0.32 cm3·g−1)[3]and mesoporous 2MgO·B2O3microspheres(0.37 cm3·g−1)[20].This implies the as-synthesized MBOH-1 microspheres as potential adsorbents for high-ef ficiency removal of organic dye.
3.2.Effect of reactant concentration on the hydrothermal products
To explore the impact of the concentration of the reactant on the compositions and morphologies of the products,the concentration of MgCl2within the reaction system was modulated within 0.075–0.225mol·L−1,keeping other conditions unchanged.When the concentration was0.075mol·L−1,the hydrothermal product was con firmedas irregular Mg7B4O13·7H2O(MBOH-2)(JCPDS No.19-0754)nano flakes(Fig.2(a1,b))with relatively high crystallinity.Notably however,the present MBOH-2 nano flakes are distinctly different with our previously reported irregular poor crystallinity precipate Mg7B4O13·7H2O nanoparticles[24].With the concentration of the MgCl2changed from 0.150 to 0.225 mol·L−1,all the products were pure phase of MgBO2(OH)(Figs.1(a)and 2(a2)).In contrast with the uniform hierarchical porous MBOH-1 microspheres obtained at the concentration of 0.150 mol·L−1(Fig.1(b)),while the concentration further increased to 0.225 mol·L−1,nonuniform microspheres(MBOH-3)with relatively more compact structure emerged(Fig.2(c)).As known,the high reactant concentration generally leads to relatively high degree of supersaturation,which is believed bene ficial to the original 1D growth of the MgBO2(OH)subunits and their subsequent 3D self-assembly for compact microspheres[27].
3.3.Effects of temperature and time on the hydrothermal products

Fig.3.XRD patterns(a)and SEM images(b-d)of the hydrothermal products obtained at different temperatures for 12.0 h,with the molar ratio of Mg:B:OH as 1:2:1,concentration of MgCl2·6H2O as 0.150 mol·L−1.Temperature(°C):(a1,b)MBOH-4:120,(a2,c)MBOH-5:150,(a3,d)MBOH-6:210.Red vertical lines of(a)present the standard pattern of MgBO2(OH)(JCPDS No.39–1370).

Fig.4.XRD patterns(a)and SEM images(b-f)of the hydro the rmalproductsobtainedat180°Cfordifferenttime,withthemolarratioofMg:B:OHas1:2:1,concentrationofMgCl2·6H2Oas 0.150 mol·L−1.Time(min):(a1,b,b1)MBOH-7:1;(a2,c)MBOH-8:30;(a3,d)MBOH-9:120;(a4,e)MBOH-10:360;(a5,f)MBOH-11:1080.Red vertical lines of(a)denote the standard pattern of MgBO2(OH)(JCPDS No.39–1370).
To further study the hydrothermal formation of the hierarchical porous MgBO2(OH)microspheres,temperature and time-dependent experiments were carried out,as demonstrated in Figs.3 and 4,respectively.With the hydrothermal temperature ranging from 120°C to 210°C,all the products were well assigned to MgBO2(OH)(JCPDS No.39-1370),whereas a distinct variation in the product morphology emerged with the change of the temperature.When hydrothermally treated at120°C,the product was confirmed as nonuniform more compact microspheres as well as bulky particles(Fig.3(a1,b)).As shown in Fig.3(a2,c),with temperature increasing to 150°C,the product turned to pure phase of MgBO2(OH)structures,with preliminarily assembled microsphere morphology and some slender 1D nanorod subunits coexisted with some other irregular particles.As aforementioned,when treated at 180°C,uniform porous MgBO2(OH)microspheres were acquired.However,with the temperature further going up to 210°C,the morphology of the product evolved into nonuniform microspheres with relatively looser structure as well as smaller size(Fig.3(a3,d)).Thus in the present case,the optimum temperature for uniform hierarchical porous MgBO2(OH)microspheres was determined as 180°C.
Besides,the hydrothermal duration time also has a significant impact on the morphologies of the products.Fig.4 illustrates the phases and structures of the products hydrothermally synthesized at 180°C for different times.When hydrothermally treated for 0 min,no solid state product was available.Notably however,with the hydrothermal time increased from 1 min to 30 min,120 min,360 min and 1080 min,the products were all con firmed as the pure phase of monoclinic MgBO2(OH)(Fig.4(a)).After the hydrothermal treatment for 1 min,the low crystallinity and original spherical MBOH-7 assemblies were formed(Fig.4(a1,b)).The high-magnification SEM image(Fig.4(b1))clearly reveals that short tiny 1 D nanorods emerged on the surfaces of the initial MgBO2(OH)microspheres.When the hydrothermal time ranged from 30 min to 120 min,the crystallinities of MgBO2(OH)got higher and higher(Fig.4(a2,a3)).Simultaneously,the rudimentary constitutional tiny subunits of the nonuniform microspheres evolved from short nanorods(MBOH-8,Fig.4(c))to relatively long ones(MBOH-9,Fig.4(d)).When hydrothermally treated for 360 min,the MBOH-10 was pure phase of MgBO2(OH)microspheres whereas with nonuniform morphology and apparently partial aggregation(Fig.4(a4,e)).With the duration time extending to 720 min(12.0 h),uniform hierarchical porous MBOH-1microspheresconstructed by interconnected nanorods were obtained(Fig.1(b,c)).As the time increasing to 1080 min(18.0 h),somehow low crystallinity dense or almost bulky particles MBOH-11 were acquired(Fig.4(a5,f)).Consequently,taking the pure phase aswell as uniformmicrospheres of the product into consideration,the optimal hydrothermal time was fixed to 12.0 h.
3.4.Hydrothermal formation mechanism of the MgBO2(OH)microspheres
Based on the above control experimental results(Figs.1–4),and also the previous work on the hydrothermal synthesis of the 1D MgBO2(OH)nanowhiskers with a molar ratio of the reactants of Mg:B:OH=2:3:4[9],as well as the ionothermal con fined self-assembly for the porous 3D MgBO2(OH)superstructures[3],the hydrothermal formationof the present nanorods constructed MgBO2(OH)microspheres can be figured out,as demonstrated in Figs.5 and 6.Firstly,within the present specific molar ratio of the reactants of Mg:B:OH=1:2:1,the rudimentary short tiny MgBO2(OH)nanorods with lower crystallinity(Fig.5(a1))or in other words with higher active surface energy were acquired,compared with those obtained at the same temperature and treated for the same time whereas with a molar ratio of Mg:B:OH=2:3:4(MBOH-12,Fig.5(a2))which often leads to discrete 1D MgBO2(OH)nanostructures[9,24].Thus the tiny nanorods with lower crystallinity would be inclined toaggregateeachothertoreducetheoverallsurfaceenergyofthesystem,resulting in relatively compact microspheres(Figs.4(a1,b),6(a)).Secondly,with the further hydrothermal treatment going on,the constitutional short tiny 1D MgBO2(OH)nanorods(i.e.subunits)radially grew into longer nanorods due to the highly intrinsic anisotropic crystal structureofMgBO2(OH)(a=1.2614nm,b=1.0418nmandc=0.3144nm),giving rise to relatively loose MgBO2(OH)microsphere(Figs.4(a2–a4,c–e),6(b)).Finally,when the reaction time was further extended,the subunits further grew into high aspect ratio nanorods, attributed to the traditional Ostwald ripening,simultaneously bringing about uniform hierarchical porous MgBO2(OH)microspheres(Figs.1,6(c)).
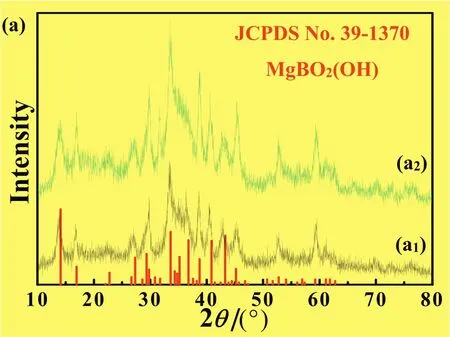
Fig.5.Influence of reactant molar ratio on the compositions of the products hydrothermally synthesized at 180°C for 12.0 h.Molar ratio of Mg:B:OH(mol):(a1)MBOH-1:1:2:1;(a2)MBOH-12:2:3:4.Red vertical lines demonstrate the standard pattern MgBO2(OH)(JCPDS No.39–1370).
3.5.Thermal decomposition of the MgBO2(OH)microspheres
The thermal decomposition properties of the MgBO2(OH)microspheres were evaluated,so as to get further in vestigation on the thermal stability.The calcination temperature,heating rate and time were determined based on our previous work[3,17].Fig.7 demonstrates the composition and morphology of the calcined product originated from the thermal decomposition of the hydrothermally formed MgBO2(OH)micro spheres at 650°C for 2.0 h.As presented in the XRD pattern(Fig.7(a)),all diffraction peaks could be readily indexed to those of the standard triclinic Mg2B2O5(JCPDS No.73-2232).The crystallinity of the asobtained Mg2B2O5microspheres was remarkably higher than that of the mesoporous 2MgO·B2O3micros pheres[20],whereas relatively lower than the triclinic Mg2B2O5nanowhiskers calcined by the MgBO2(OH)nano whiskers at 600–700 °C for 2.0 h[9].In addition,the as-calcined Mg2B2O5particles exhibited well-preserved uniform spherical pro file of the MgBO2(OH)microspheres(Fig.7(b)),indicating their good structural stability at elevated temperature.
3.6.Hierarchical porous MgBO2(OH)/Mg2B2O5microspheres as adsorbents for CR removal
As known,the adsorption of organic compounds is not only associated with additional interactions between the adsorbates and adsorbents,but also related to the textural characteristics(e.g.large specific surface area,and appropriate pore volume)of the adsorbents.When employed as adsorbents,the present MgBO2(OH)micros pheres with different morphologies exhibited the same interactions between the MgBO2(OH)and the selected adsor bates.Notably however,the uniform MgBO2(OH)micros pheres might provide higher specific surface area as well as more applicable pore size than the distinct nonuniform MgBO2(OH)ones.Particularly,for the future recycling and reusablility as well as easy operation purpose of the adsorbents,uniform microspheres should bepreferred tothosenonuniformones.Thus,the presenthierarchical porous MBOH-1 micros pheres(relatively high specific surface area:94.752 m2·g−1,pore volume:0.814 cm3·g−1,average pore diameter:34.2 nm)and corresponding calcinated Mg2B2O5microspheres were adopted as the adsorbents for elimination of CR from simulated waste water.
Fig.8(a)illustrates the variation of theadsorption rate or capacity of CR with the contact time(initial concentration of the solution:100 mg·L−1).As can be seen(Fig.8(a1,a2)),the adsorption rates of CR were abruptlydecreased within the first 10 min,rapidly approached ca.91.7%and90.9%within30min for MBOH-1andMg2B2O5porous microspheres,respectively.The removal efficiency of CR further increased ca.96.1%and 97.9%within the coming 60 min and 90 min for MBOH-1 microspheres,respectively,and remained relatively constant thereafter.Notably however,the as-calcined Mg2B2O5porous microspheres delivered unsatisfactory adsorption rate because the ct/c0value for the adsorption of CR declined within the following adsorption time(Fig.8(a2)).And the ultimate removal efficiencies of CR on the MBOH-1 microspheres and Mg2B2O5microspheres were con firmed as 99.1%and 79.8%within 300 min,respectively.Simultaneously,as depicted in Fig.8(a3,a4),the adsorption capacities of CR on the MBOH-1 microspheres and Mg2B2O5microspheres were up to 183.3 and 184.3 mg·g−1in the first 30 min,and then quickly leveled off to 198.5 mg·g−1of the MBOH-1 microspheres(Fig.8(a3)).Moreover,the corresponding adsorption capacity of CR onto the Mg2B2O5decreased from 184.3mg·g−1to161.9mg·g−1as adsorption time ranging from30min to 300 min(Fig.8(a4)).

Fig.6.Schematic illustration of the growth process of the MgBO2(OH)microspheres.
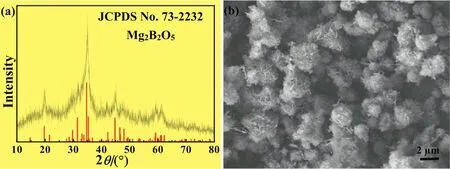
Fig.7.XRD pattern(a)as well as SEM image(b)of the Mg2B2O5microspheres calcined by MBOH-1 microspheres at 650 °C for 2.0 h(heating rate:2.5 °C·min−1).Red vertical lines in(a)present the standard pattern of triclinic Mg2B2O5(JCPDS No.73-2232).
As previously clarified[3],the high adsorption efficiency onto the MgBO2(OH)microspheres was attributed to the interactions between–OH and CR molecules.Thus,in the present case,we inferred that the adsorbed CR solute returned into the solution due to relatively weak inter action between the adsorbate CR and the adsorbent Mg2B2O5porous micro spheres in the course of long time adsorption,which was carried out under strong magnetic stirring.Consequently,the adsorption efficiency or adsorption capacity of CR onto the Mg2B2O5porous microspheres delivered a descending trend with the adsorption time prolonging,resulting in the namely abnormal desorption phenomenon.So,the further deep investigation on the adsorption was focused on the hierarchical porous MgBO2(OH)micros pheres,instead of the calcined ones.
Analysis on the adsorption isotherms is of vital importance for understanding the adsorption mechanism,such as the distribution of adsorbate molecules between the solution and adsorbent,as well as the possible interaction force among the adsorbate molecules[28].In the present case,the Langmuir and Freundlich isotherms were used to fit the experimental data.It is known that,the Langmuir isotherm is a powerful to oltostudy all sorption sites on the adsorbent,which are identical and energetically equivalent where an ideal monolayer and homogenized coverage on the adsorbent surface.In contrast,the Freundlich model is basically assumed multilayer and non-ideal adsorption with non-uniformly distributed sorption sites of different affinities[29].Mathematically,the models for Langmuir isotherm and Freundlich isotherm can be individually expressed as Eqs.(3)and(4).

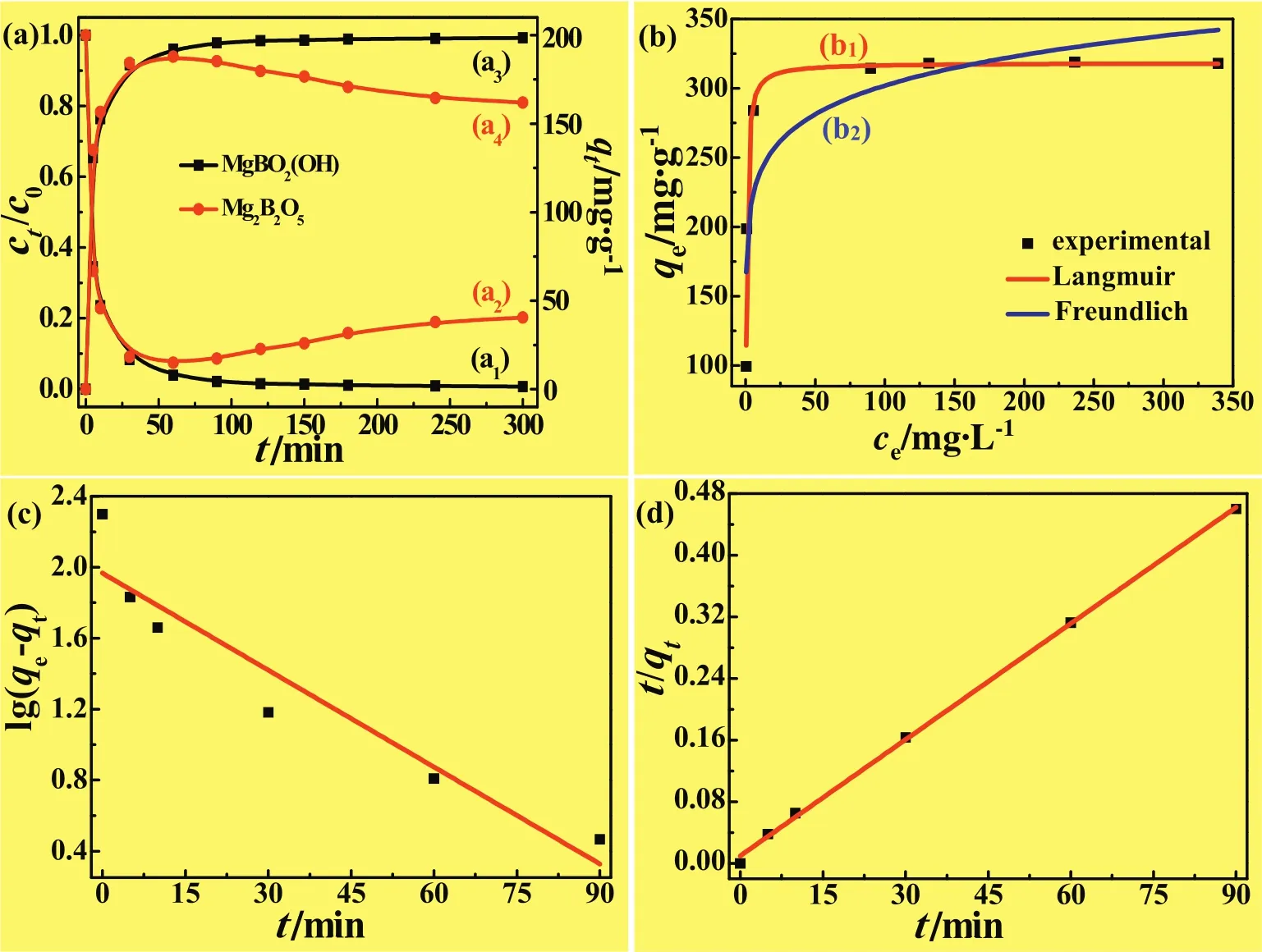
Fig.8.Adsorption rate(a1,a2)and capacity(a3,a4)of MBOH-1 microspheres(a1,a3)and Mg2B2O5microspheres(a2,a4)for CR removal as a function of the contact time(initial concentration/dosage of CR:100.0 mg·L−1/40 ml),as well as the adsorption isotherms(b)of MBOH-1 microspheres fitted according to the Langmuir(b1)and Freundlich(b2)models by non-linear regression,with the concentration of CR ranging from 50.0 mg·L−1to 500.0 mg·L−1.Pseudo- first-order(c)and pseudo-second-order(d)kinetic plots derived from the linear regression of the CR adsorption on the of MBOH-1 microspheres,by using a 40.0 ml of the adsorbate solution with an initial concentration of 100.0 mg·L−1.Dosage of the MBOH-1/Mg2B2O5microspheres:20.0 mg.

where qeis the equilibrium concentration of adsorbate on the adsorbent(mg·g−1),qmis the theoretical value for the maximum adsorption capacity of adsorbate on the adsorbent(mg·g−1),b is the coefficient relevant to the energy of adsorption(L·mg−1),and ceis the equilibrium concentration of adsorbate in solution(mg·L−1).For the Freundlich model,the con stantkfis related to the adsorption capacity per unit concentration,and constant 1/n is the adsorption intensity.
The adsorption data for the CR onto the MBOH-1 porous micros pheres were fitted with both Langmuir(Fig.8(b1))and Freundlich(Fig.8(b2))isotherm models by non-linear regression according to Eqs.(3)and(4),and the corresponding isotherm parameters were listed in Table 1.As depicted,the correlation coefficient(R2)via Langmuir model was 0.9766,considerably higher than that derived from the Freundlich model(0.7784).Clearly,from the correlation coefficient point of view,the Langmuir model presents a much more satisfactory fitting result for the adsorption of CR onto the hierarchical porous MBOH-1 microspheres than the Freundlich model does.In other words,the adsorption of CR on the present hierarchical porous MBOH-1 superstructures complies with monolayer adsorption process.In addition,the maximum adsorption capacity(qm)was calculated via the Langmuir model as 309.1 mg·g−1(Table 1).It is strongly believed that,the present prominent eliminationefficiency of CR is by and large attributed to the unique hierarchical porous structures of the MgBO2(OH)microspheres.

Table 1 Corresponding fitting parameters derived from the non-linear regression by using Langmuir isotherm model and Freundlich isotherm model of CR adsorption on the 3D MBOH-1 microspheres hydrothermally synthesized at 180°C for 12.0 h(molar ratio of Mg:B:OH=1:2:1,concentration of MgCl2·6H2O=0.150 mol·L−1)
To get a bigger picture on the application of the MgBO2(OH)microspheres as adsorbents,various adsorbents have been evaluated as candidates for the removal of CR and their maximum capacities were compared,as tabulated in Table 2.As shown,the hierarchical porous MBOH-1 microspheres exhibit relatively higherqmthanmost of there ferenced adsorbents do,such as urchin-like α-FeOOH hollow spheres[30],nanocrystalline Fe3O4[31]and esp.our previously reported ionothermally synthesized hierarchical porous MgBO2(OH)superstructures[3]with smaller specific surface areas(SBET).This somehow indicates the contribution of the intrinsically relatively large SBETto the adsorption of CR.In addition however,the present qmis even higher than that of MgO nanoplates[32],hierarchical porous CaCO3[33],and the nest-like Fe2O3[7]with significantly larger SBET.This reveals that the adsorption performance for CR on the adsorbents is not only owing to their specific surface area.As seen,the qmdoes not proportionally increase with the increase in the SBETof the adsorbents,suggesting possible extra electrostatic interactions between CR and the adsorbents[3].Notably however,the qmof the MBOH-1 microspheres is lower than that of microporous polymer/sulphonic-group carrying resin[34],porous La(OH)3nanowires[35]and γ-AlO(OH)/Mg-Al-LDH/C composite[36],probably owing to their much higher SBET[34,36]or stronger electrostatic interactions between the dye species and employed adsorbents.Thus,the high adsorption capacity for CR onto the hierarchical porous MBOH-1 superstructures can be ascribed to the relevantly larger specific surface area,pore volume and specific surface properties(e.g.electrostatic interactions).Nevertheless,taking the relatively complicated process or cost factor for those adsorbents into consideration,the present hierarchical porous MBOH-1 microspheres are promising and competitive adsorbents candidate for CR removal.
To better ascertain the mechanism for the adsorption of CR onto the hierarchical porous MBOH-1 microspheres,the pseudo- first-order(PFO)and pseudo-second-order(PSO)were adopted to explain the experimental data,according to the following Eqs.(5)and(6),respectively.

where qeand qt(mg·g−1)are the quantity of CR adsorbed at equilibrium and time t(min),respectively;k1(min−1)and k2(g·mg−1·min−1)are the rate constants for the pseudo- first-order and pseudo-second-order kinetic models,respectively;t(min)is the contact time between the adsorbents and adsorbates.
Since the adsorption of the CR on the porous MBOH-1 microspheres reached equilibrium within 120 min(Fig.7(a)),the PFO(Fig.7(c))and PSO(Fig.7(d))kinetic plots were fitted via linear regression of the experimental data before equilibrium for the adsorption of CR(100 mg·L−1)on the present mesoporous microspheres.As depicted in Fig.7(c),when the PFO kinetic model was adopted,the value of k1was calculated from the plot of lg(qe−qt)vs.t,as listed in Table 3.The values of R2and qe,calc1were 0.8170 and 40.30(mg·g−1),respectively.Obviously,the R2and the qe,calc1were distinctly deviated from 1.0 and the experimental qe,exp(198.54 mg·g−1).Thus,the PFO kinetic model cannot well interpret the adsorption kinetics.Comparatively,when the PSO kinetic model was employed,a linear plot of t/qtvs.t was acquired(Fig.7(d)),and corresponding parameters were also given in Table 3.As listed,the fitted R2of the PSO kinetic model and the value of qe,calc2were con firmed as 0.9999 and 200.00(mg·g−1),respectively.Clearly,theR2ofthePSOkinetic modelin finitely approaches 1.0,dramatically higher than that derived from the PFO kinetic model.This reveals the adsorption process of CR on the hierarchical porous MBOH-1 microspheres can be more appropriately explained via the PSO kinetic model.In addition,the qe,calc2(200.00 mg·g−1)originated from the PSO kinetic mode is considerably close to the value of qe,exp(198.54 mg·g−1),in contrast with the qe,calc1(40.30 mg·g−1)originated from the PFO kinetic mode.It reveals that,the adsorption of CR onto the hierarchical porous MgBO2(OH)microspheres may belong to chemical adsorption,which is concerned with the valence forces between the MgBO2(OH)and CR by sharing or exchanging electrons[37].

Table 2 Comparison of the maximum adsorption capacities(qm,mg·g−1)for CR on various adsorbents

Table 3 Kinetic parameters fitted with pseudo- first-order model and pseudo-second-order model of CR adsorption on the 3D MBOH-1 microspheres for the initial concentration of 100 mg·L−1
4.Conclusions
Uniform hierarchical porous MgBO2(OH)microspheres consisted of slender nanorods sub-units were synthesized through a facile,green and reliable hydrothermal process(180°C,12.0 h),by using abundant the MgCl2·6H2O,H3BO3and NaOH as raw materials without aid of any organic additive,surfactant or template.The hierarchical porous MgBO2(OH)microspheres demonstrated a specific surface area and pore volume of 94.752 mg·g−1and 0.814 cm3·g−1,respectively,and ca.84.0%of which had a diameter within the range of 2.25–3.40 μm.A mild thermal decomposition(650 °C,2.5 °C·min−1,2.0 h)gives rise to the porous Mg2B2O5microspheres with well retained spherical morphology,indicating the good structural stability of the porous MgBO2(OH)microspheres.In addition,when utilized as the adsorbents for the elimination of CR from mimic waste water,the present hierarchical porous MgBO2(OH)microspheres exhibited satisfactory adsorption capacity.The maximum adsorption capacity qmfor CR was up to 309.1 mg·g−1,much higher than that derived from most of the referenced adsorbents.The adsorption of CR was well described by the Langmuir adsorption model and pseudo-second-order kinetic model,suggesting the monolayer and chemical adsorption of CR onto the present hierarchical porous MgBO2(OH)microspheres,respectively.This opens a new window for the facile green hydrothermal synthesis of the hierarchical porous MgBO2(OH)microspheres,and thus favorable for extending the potential application of the 3D metal borates with hierarchical porous structures as high-efficiency adsorbents for removal of CR or other organic dyes in water treatment.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- High efficiency production of ginsenoside compound K by catalyzing ginsenoside Rb1 using snailase☆
- Understanding the Influence of microwave on the relative volatility used in the pyrolysis of Indonesia oil sands☆
- Degradation and mineralization of aniline by O3/Fenton process enhanced using high-gravity technology☆
- Three-liquid-phase extraction and separation of V(V)and Cr(VI)from acidic leach solutions of high-chromium vanadium–titanium magnetite☆
- Insight into the degradation mechanism of cefixime under crystallization condition☆
- Experimental and simulation of the reactive dividing wall column based on ethyl acetate synthesis☆
