Degradation analysis of A2/O combined with AgNO3+K2FeO4on coking wastewater
2018-08-18PengyuZhuKaijinZhuRobPuzeyXiaoliRen
Pengyu Zhu,Kaijin Zhu*,Rob Puzey,Xiaoli Ren
1School of Engineering,University of Hull,Cottingham Road,Hull,the United Kingdom
2Department of Environmental Engineering,Taiyuan Institute of Technology,Taiyuan 030008,China
Keywords:Coking wastewater Biochemical treatment AgNO3+K2FeO4catalytic oxidation Cyclic organics Degradation characteristics
ABSTRACT In this work,a coking wastewater was selected and a biochemical A2/O treatment device for fractional degradation was designed and employed.After each stage of the treatment,the products were analyzed through gas chromatography–mass spectroscopy(GC–MS)to determine their composition.Finally,AgNO3+K2FeO4was used as an advanced deep catalytic oxidation treatment.It was concluded from the analysis that cyclic organics could be degraded and the chemical oxygen demand(COD)was controlled within 50 mg·L−1,in line with the targetvalue.Meanwhile,the spectra obtained from the GC–MS were in accordance with the conclusion sreached based on the COD.The research results showed that all hard-degradable organics in coking wastewater could be eliminated through the A2/O bio-membrane treatment and the advanced treatment of making use of K2FeO4as anoxid ant and Ag+asacatalyst,the catalytic efficiency with Ag+as acatalyst of K2FeO4was very high.Ag+could evidently improve the oxidation capacity of K2FeO4to waste water in its short stability time,and this isan important innovation.
1.Introduction
Coking wastewater,a kind of hard-treated industrial wastewater[1,2],contains large amounts of aromatic and heterocyclic organics which are hard-degradable.At present,an anaerobic acidification–anoxic–aerobic process(A2/O)is commonly applied in the treatment of coking wastewater,but the chemical oxygen demand(COD)of the discharged tail water is still high(>250 mg·L−1)[3,4],this is mainly due to the higher COD background value of cyclic substances.This research found that an effective process leading to basic complete degradation of cyclic organics and final COD value below 50 mg·L−1was the use of potassium ferrate(K2FeO4)as an oxidant and AgNO3as a catalyst.In particular,due to the promotion action of silver ion,it is very effective to increase the oxidation efficiency of potassium fer rate,which easily decomposes in water in a short time,however all current studies of this respect are not involved.In order to further understand the degradation process of cyclic organics aimed at A2/O treatment process as well as the oxidation method of coking wastewater,it is necessary to analyze and compare the degradation characteristics of some main species involved by means of GC/MS analysis.
2.Experimental
2.1.Experimental setup for coking wastewater degradation
Fig.1 shows a drawing of the device employed for the treatment of coking wastewater.The setup includes the A2/O biomembrane and the advanced oxidation process,in which an up- flow cylindrical reactor is adopted as anaerobic column,followed by anoxic column and aerobic tank,which is integrated with a sedimentation tank.The final flocculation tank is also integrated with a sedimentation tank.Combined packings are placed inside all the reaction tanks.
The wastewater used in the experiments was sampled from the coking factory of Taiyuan Iron&Steel Company.Its COD and total organic carbon(TOC)values were 2350 mg·L−1and 1432 mg·L−1,respectively.
By comparing the removal efficiency of pollutants under different parameters,the operating conditions of each stage were determined as follows:
(1)Anaerobic segment:Dissolved oxygen(DO)≤ 0.2 mg·L−1;re flux ratio(R)1.5;hydraulic residence time(HRT)9.5 h;mixed liquid suspension solid concentration(MLSS)< 4.0 g·L−1,pH 7.0–8.0.
(2)Anoxic segment:DO 0.5 mg·L−1;R 1.5;HRT 11 h;MLSS< 4.0 g·L−1;pH 7.0–8.0.
(3)Aerobic segment:DO 5.0–6.0 mg·L−1;R 1.8;HRT 10.5 h;MLSS< 2.0 g·L−1;pH 7.5–8.0,KH2PO4regulation.
(4)Ambient temperatures above were at 20°C.

Fig.1.Schematic diagram of the wastewater degradation process.
The analyses of wastewater composition were carried out by usinga Pegasus IV(American LECO Corporation)gas chromatograph–mass spectrometer(GC/MS,resolution>5000)with high sensitivity and good separation effect;it is the most frequently used method to analyze organic substances.
2.2.GC–MS analysis
2.2.1.GC analytic conditions
Chromatographic column:DB-5,0.32 mm×30 mm;Carrier gas:helium.
Flow rate:1 ml·min−1;Sampling temperature:260 °C.
Column temperature:initial temperature 45°C;Heating rate:10°C·min−1;Final temperature 300 °C.
Temperature hold:30 min.
Transferring curve temperature:285°C;Ion source temperature:240°C.
2.2.2.MS analytic conditions
Ionization mode:EI;Electron energy:70 eV;Mass range:40–450.
Sampling mode:full scans ampling;Multiplier tubevoltage:1600V;Emission current:235 μA.
The MS analysis was carried out through the following procedure:
(1)Extraction of wastewater sample with dichloromethane in a ratio of 1:1.
(2)Direct injection of 1 μl with a spilt ratio of 10:1.
The standard curve drawing was carried out in agreement with the conditions of wastewater sample analysis and treatment,and the minimum detection limit of all tested organic substances was 1 mg·L−1.
2.2.3.Extraction method
The extraction was carried out by using samples from the both of the untreated and treated wastewater.The procedure followed is summarized below:
(1)pH was adjusted to be lower than or equal to 2.0.
(2)Wastewater samples were extracted for two times by using 10 ml of dichloromethane.
(3)pH of the residual water was adjusted to be higher than or equal to 11.
(4)Extraction was repeated for two times with dichloromethane,combining acidic and alkaline extractions.
(5)Samples were subjected to filtration and emulsion breaking by using anhydrous Na2SO4.
(6)Wastewater was removed and the organic phase was concentrated by means of a rotary evaporator with purified nitrogen gas.
(7)Sample was diluted and prepared for GS–MS analysis.
2.3.Oxidation process
The oxidation was carried out by adding a fixed amount of KH2PO4to the coking wastewater to provide the needed nutrition for microorganisms[5].The overall hydraulic residence time of the system was 40.0 h,and it included the time required for the anaerobic(9.5 h),anoxic(11 h)and aerobic(10.5 h)processes.K2FeO4(0.5 wt%)and AgNO3(0.01 wt%)were added to the wastewater sample that had been previously treated by A2/O[6,7].The mixture was left to react under stirring for 2.0 h,the analyses were carried out on the wastewater resulting from the anaerobic,anoxic and aerobic processes,as well as the wastewaters to which K2FeO4only or K2FeO4and AgNO3had been added,to determine the contents of cyclic components.
3.Results and Discussion
3.1.Analytic test of cyclic organics aimed at untreated coking wastewater
The untreated wastewater was taken out to determine the contents of the main components.The results showed that the most abundant compounds were aromatics and heterocyclic organics[8,9].The basic results are listed in Table 1.
As can be seen from Table 1,aromatics make up 74%of all cyclic organics,77%of them are constituted by phenols.This indicates that the degradation of phenols is key to the overall COD reduction.Fig.2 shows the GC–MS spectrum circumstances of the untreated coking wastewater,from which we can see the peak order and contents of the main components.
Fig.2 shows that the main pollutants include methyl phenol,quinoline,indole,naphthol and other polycyclic or aromatic hydrocarbons or their substituent substances.The phenols are the highest and account for about 68.7%of all the detected components.The high contents ofpyridine,indole,quinoline,methylamine,and aniline lead to the resulting detection of ammonia nitrogen in the coking wastewater.

Table 1 Analytic results of components and contents of cyclic organics in untreated coking wastewater
3.2.Analysis of cyclic organic components in coking wastewater after anaerobic treatment
After anaerobic treatment,the simple phenols were mostly eliminated,but the increment in the methyl-phenol substitutional group caused a gradual decrease in the elimination rate and hindered the decomposition of trimethyl phenol[10–12].Heterocyclic compounds such asphenyl amine,quinoline,is oquinoline,in dole and pyridine were mostly eliminated,while the content of the intermediate methylquinoline,produced by the degradation of other substances,increased on the contrary,this shows that easily-degraded compound sarepreferentiallyeliminated during anaerobic treatment process.Meanwhile,other new compounds are produced during this process too,including crylic acid,methylaniline and carboxylic ester.The analytic results are shown in Table 2.
Comparing Table 2 with Table 1 shows that aromatic substances decrease by 43%,and 30%of them are phenol substances after anaerobictreatment.The coking wastewater analysis also showed that the COD value was 1468 mg·L−1,having decreased by 38%compared to the untreated water.This shows that the treatment has reached a significant effect and the contribution of cyclic organics to the COD is very distinct[13,14].

Table 2 Analytic results of cyclics and intermediate organics in coking wastewater after anaerobic treatment
3.3.Test analysis of organic components in coking wastewater after anoxic treatment
The anoxic treatment of the wastewater had only a small effect on the degradation of some complex organics such as 5-phenyl-oanisidine,2,6-dimethylphenylisocyanate,4-methyl-(1H)indole,and N-hydroxymethylcarbazole.On the other hand,the contents of nitrogenouscompoundssuchaspyridine,quinolineandindoledecreasedobviously.At the sametime,a smallamountof intermediatesubstances was produced,including oleic acid 12-alkene,octanal,5-methyl-3-hexyl alcohol and 2,6-butylated hydroxytoluene.The results con firm the capability of denitrifying bacteria which may significantly degrade nitrogenousorganics.Thecontentsofcyclicorganicsoftheanoxictreatment ef fluent are shown in Table 3.

Fig.2.Peak order of main cyclic organics in untreated coking wastewater.

Table 3 Analytic results of cyclic and intermediate organics in coking wastewater after anoxic treatment
From the comparison between Tables 3 and 2,it can be concluded that the anoxic treatment achieves a reduction result of 31%of the aromatics and47%of nitrogenous compounds through comparing to the effluent of the anaerobic treatment.The COD value is 987 mg·L−1after the anoxic process,58%lower than the corresponding value of the untreated wastewater.
3.4.Test analysis of relevant organic components in coking wastewater after aerobic stage
The analysis carried out on the wastewater after the aerobic treatment indicated that the organic compounds mainly included phenolic substances,quinoline substances,1-hydroxy-3,3′-dimethylbutane,and octanal[15].Results are shown in Table 4.

Table 4 Analytic results of cyclic and intermediate organics in aerobic ef fluent
From the comparison bet ween Tables4and3,itcanbeseenthat50%of aromatic substances are degraded during the aerobic treatment through comparing to the effluent from the anoxic treatment.The COD value in the coking wastewater is 354 mg·L−1,85%lower than in the original water.
From Table 4,it can be seen that cyclic substances are degraded greatly but not sufficiently during the A2/O treatment,the COD is still very high,so an advanced deep chemical treatment is needed further.
3.5.Experimental analysis of AgNO3+K2FeO4catalytic oxidation
This research showed that an advanced oxidation treatment by way of using the strong oxidant,potassium ferrate(K2FeO4),was an effective method to eliminate most cyclic organics following the biochemical treatment.The main disadvantage of potassium ferrate is that it isn't very stable in water,especially in weak acid solutions,and easily decomposes itself[16,17].Normally its decomposition in water occurs after 5 min,but such a time isn't sufficient to achieve a high oxidation efficiency.This research showed that the oxidation of K2FeO4was accelerated by adding a small amount of AgNO3as a catalyst and the efficiency of the process,in terms of COD reduction,could be improved sufficiently to reach the target value of this parameter.
Two samples of 100 ml solution resulting from the biochemical treatment were placed in two separate beakers,a 0.5 wt%K2FeO4solution and a 0.5 wt%K2FeO4plus 0.01 wt%AgNO3solution were mixed,stirred,and left standing respectively,their values of COD were measured to be 205 mg·L−1and 43 mg·L−1.After adding the AgNO3catalyst,the COD degradation rate was 98%lower than the one of the untreated water and the TOC value decreased by 96.5%.This indicated that the catalyst was able to accelerate the oxidation reaction suf ficiently to reach the aim value before the complete decomposition of K2FeO4.This finding is the main innovation of the present work.Table 5 reports the composition circumstances of the wastewater after the catalytic oxidation.

Table 5 Analytic results of cyclic organic components in coking wastewater after the catalytic oxidation
Fig.3 shows the degradation trend of COD and TOC at each stage.From the figure,it can be seen that cyclic organics and total organic compounds underwent basic complete degradation after the A2/O biochemical treatment and AgNO3+K2FeO4catalytic oxidation treatment.
Fig.4shows the chromatogram of the waste water resulting from the comp letetreatment.Thes pectra are compared to the NIST(National Institute of Standards and Technology of American)database,and peaks having an area higher than 5%of the maximum peak are selected and normalized.

Fig.3.COD and TOC variations over time at each stage.
The first peakshown in Fig.4 is due to the extract ant,dichloro methane,which appeared in large quantities and thus leads to a high peak.The low height of the remaining peaks makes known that the degradation of cyclic compounds has been carried out thoroughly.Compared with the A2/O treatment,the quantity of all cyclic substances present in the wastewater is much lower after the AgNO3+K2FeO4advanced deep oxidation,justifying the small COD value.
4.Mechanism Analysis of Catalytic Oxidation
The role of Ag+is embodiedintwoaspects,the first promotes there duction ofand the production of hydroxyl free radicals(•OH);the second is its combination with organics to generate intermediate products and to reduce the reaction activated energy.
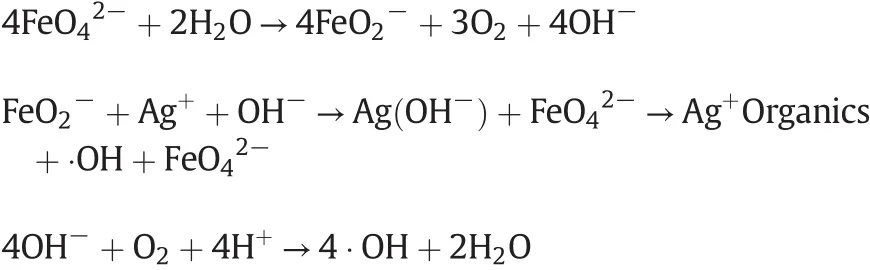

Fig.4.Final peak order of all components after AgNO3+K2FeO4treatment.
(2)Ag+ions react with cyclic substances to generate an additional intra molecular reaction,then leading to intra molecular nucleophilic reaction under the action of hydroxyl free radical and cracking the ring.After a series of electron transfers and further oxidation have happened,CO2and H2O are finally gotten out.

5.Conclusions
The coking wastewater was treated by designing the device of A2/O and fur the rtreated by the catalyticoxidation of Ag NO3+K2FeO4,a very ob viousdegradation effect was obtained,and the expected goals of several technological parameters had been achieved.Moreover,the degradation mechanism of each stage in coking wastewater was clarified,especially that of aromatics and heterocyclicorganics which are difficult to degrade.Furthermore,by way of the above deep catalytic oxidation,the contents of thet wokinds of organics were greatly reduced,an effective method for treating coking wastewater was found out.
(1)The analysis of the coking wastewater showed that it contained cyclic organics including phenol,quinoline,pyridine,in dole and carbazole.After the A2/O treatment,cyclic organic's contents decreased greatly and over85%of the COD was degraded;however,the final COD value was still high,exceeding 300 mg·L−1.
(2)GC–MS was used to perform characteristic analysis onthedegradation products of each step.There sults were in agreement with the trends tested for the COD and TOC,thereby con firming the overall analytic results.
(3)Taking K2FeO4as an oxidant and AgNO3as a catalyst to perform an advanced deep oxidation treatment on the wastewater after A2/O treatment,the residual cyclic organics can undergo basic complete degradation,the absolute COD value fell below 50 mg·L−1.It was also shown that the catalyst of AgNO3could greatly improve the performance of K2FeO4in the oxidation treatment,and Ag+is a very effective catalyst of K2FeO4.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- High efficiency production of ginsenoside compound K by catalyzing ginsenoside Rb1 using snailase☆
- Understanding the Influence of microwave on the relative volatility used in the pyrolysis of Indonesia oil sands☆
- Degradation and mineralization of aniline by O3/Fenton process enhanced using high-gravity technology☆
- Three-liquid-phase extraction and separation of V(V)and Cr(VI)from acidic leach solutions of high-chromium vanadium–titanium magnetite☆
- Insight into the degradation mechanism of cefixime under crystallization condition☆
- Experimental and simulation of the reactive dividing wall column based on ethyl acetate synthesis☆
