Distinct synergetic effects in the ozone enhanced photocatalytic degradation of phenol and oxalic acid with Fe3+/TiO2catalyst☆
2018-08-18YongbingXieYingyingChenJinYangChenmingLiuHeZhaoHongbinCao
Yongbing Xie,Yingying Chen,Jin Yang,Chenming Liu,He Zhao,Hongbin Cao*
Beijing Engineering Research Center of Process Pollution Control,Division of Environment Technology and Engineering,Institute of Process Engineering,Chinese Academy of Sciences,Beijing 100190,China
Keywords:Catalysis Environment Waste water Fe3+/TiO2 Synergistic effect Photocatalytic ozonation
ABSTRACT In this work,phenol and oxalic acid(OA)degradation in an ozone and photocatalysis integrated process was intensively conducted with Fe3+/TiO2catalyst.The ferrioxalate complex formed between Fe3+and oxalate accelerated the removal of OA in the ozonation,photolysis and photocatalytic ozonation process,for its high reactivity with ozone and UV.Phenol was degraded in ozonation and photolysis with limited TOC removal rates,but much higher TOC removal was achieved in photocatalytic ozonation due to the generation of•OH.The sequence of UV light and ozone in the sequential process also Influences the TOC removal,and ozone is very powerful to oxidize intermediates catechol and hydroquinone to maleic acid.Fenton or photo-Fenton reactions only played a small part in Fe3+/TiO2catalyzed processes,because Fe3+was greatly reduced but not regenerated in many cases.The synergetic effect was found to be highly related with the property of the target pollutants.Fe3+/TiO2catalyzed system showed the highest ability to destroy organics,but the TiO2catalyzed system showed little higher synergy.
1.Introduction
Photocatalysis can transfer the inexhaustible solar energy into chemical energy,and it is a clean technology for organic pollutant degradation in wastewater treatment[1–4].However,low efficiency of organics removal in photocatalysis limits its practical application.This phenomenon normally derives from relatively low absorbance of light,severe recombination of electron–hole pairs and low oxidation ability of the hole.To improve the photocatalytic activity,many efforts have been devoted on element doping,dye sensitization and structure engineering on TiO2[5,6],graphitic carbon nitride(g-C3N4)[7,8],WO3[9],etc.Normally,two strategies have been intensively applied to inhibit the hole–electron recombination.The first is to dope an optimal concentration of noble metal or metal ions on TiO2[10–12],which can effectively trap the photogenerated electrons.The second strategy is to add some sacrificial agents in solution which can trap the photo-generated hole sorelectrons.For example,oxygen is often used in photocatalytic processes and reduced by photo generated electron to superoxide radical,which is a very important oxidizing species in photocatalysis.Similarly,adding metal ions into solution can also achieve the same goal[13,14].
Ozonation is another attractive advanced oxidation technology and has been widely applied in water treatment[15,16].Molecular ozone is a robust oxidant,and it can destroy many varieties of organic pollutants with unsaturated C=C bond.However,a complete mineralization can hardly be achieved in ozonation because many intermediates are produced which are resistant to ozone attack.Forit shighoxidizing ability,ozone can easily capture electron and thus be used as a sacrificial agent in the photocatalysis.This can enhance the separation of photo generated carriers,and reactive hydroxyl radicals are generated from ozone reduction by photo generated electrons[17].As a result,the combination of ozonation and photocatalysis can achieve a higher mineralization rate of organic contaminants.Many efforts have been paid to investigate these processes,focusing on the reaction kinetics[18,19]and the synthesis of highly active catalysts,such as g-C3N4[20–22],TiO2[23],WO3[24]and Bi2O3[25].In these studies,photocatalytic ozonation always showed higher efficiencies than the sum of ozonation and photocatalysis.The positive synergies are quantitatively calculated in somecases[20,22],and this cangreatlyvary,dependingon the different properties of the solid catalysts.However,very few concerns were paid on the Influence of the organics structure and adjustable operating modes in photocatalytic ozonation,which are also very crucial for the process design and evaluation.
In this paper,Fe3+/TiO2photocatalytic ozonation of two organics with distinct structures(phenol and OA)was intensively conducted.Fe3+was added in the system because it was widely used in photocatalysis to quench photo generated electrons.This can enhance the oxidation efficiency of the process,but this may need further removal if the residual concentration was too high.Phenol and OA have quite different reaction rates with ozone,and they are often served as simulated pollutants and degradation intermediates,respectively.The synergetic effects in these integrated processes are quantitatively analyzed,as well as the analysis of Fe3+transformation and active species in different systems.The result indicates that the synergy is greatly determined by the structure of organic pollutants,which will be of great bene fits to the evaluation of photocatalytic ozonation and optimal process design for removal of different contaminants.
2.Experimental
2.1.Materials and reagents
Phenol,OA and KI were purchased from Xilong Chemical Corporation.Fe(NO3)3,tert-butanol(t-BA)and benzoquinone were obtained from National Chemical Corporation.Horseradish peroxidase was provided by Sigma Aldrich.A commercial TiO2(Degussa P25)was used as catalyst or catalyst support without further treatment.All the solutions were prepared with ultrapure water(Millipore Milli-Q).
2.2.Phenol and OA degradation
The degradation of phenol and OA was performed in 500 ml of unbuffered solution at 25°C.The initial concentrations of the contaminants were both 100 mg·L−1and the dosage of catalyst was 0.2 g·L−1.The solution was magnetically stirred in dark condition for 30 min to establish an adsorption/desorption equilibrium before reactions.In the photocatalytic ozonation process,the solution was irradiated under a Xenon lamp(CEL-HXUV300,Aulight Corporation)with UV light(200–400 nm).The light intensity on the solution surface was 320 mW·cm−2measured by a photometer(CEL-NP2000,Aulight Corporation).Ozone was produced through an ozone generator(COMAD-01,Anseros Corporation)from pure oxygen,and was bubbled into the bottom of the solution.The flowrate of the mixed gas was 115 ml·min−1and the ozone concentration was 27 mg·L−1.For the comparison with photocatalytic ozonation,sequential photocatalysis and ozonation(SPO)and sequential ozonation and photocatalysis(SOP)were also designed for phenol degradation.
The concentrations of phenol and organic intermediates were analyzedbyhigh-performanceliquidchromatography(HPLC,Agilentseries 1260)withaCEcolumnC18andaUV–Visdetectorqualifiedat215,245 and 278 nm,respectively.The concentration of OA was detected by ion chromatography(IC,Dionex DX500)usinga NaOHsolution(1.1g·L−1)as the eluent.The total organic carbon(TOC)and pH of the solution were analyzed by a TOC analyzer(TOC-V CPH,Shimadzu)and pH meter(Five Easy,Mettler-Toledo),respectively.The concentrations of ozone in the gas phase and liquid solution were monitored by an ozone analyzer(Anserors ozomat GM,Germany)and the indigo method[26],respectively.The concentration of H2O2in the solution was detected with the photometric method[27],and the concentration of Fe2+in the solution was measured by the 1,10-phenanthroline method[28].
3.Results and Discussion
3.1.Degradation of phenol

Fig.1.Photocatalytic degradation of phenol with Fe3+/TiO2.
Phenol degradation was firstly carried out in the photocatalytic processwithFe3+/TiO2andtheconcentrationofFe3+was optimized.Fig.1 showed that TiO2greatly enhanced the phenol removal rate from 14.5%in photolysis to 38.5%in photocatalysis.When different amounts of Fe3+were added into the solution,the degradation rate of phenol was further accelerated.The degradation rate of phenol firstly increased with the concentration of Fe3+,but higher concentration than 75 μmol·L−1had no promoting effect.This was possibly because photo generated electrons were limited and 75 μmol·L−1of Fe3+wasenough to quench them.This optimized concentration was thus used in the following experiments.The highest removalrate of phenol wasonly 53.6%after a 120 min photocatalytic reaction,but in the TiO2catalytic ozonation,phenol can be totally decomposed within 60 min.Further adding Fe3+into the TiO2catalyzed system did not show an improvement in phenol degradation,because ozone can directly attack phenol.
In general,photo and ozone were simultaneously introduced into the photocatalytic ozonation system.Here we varied the order of them and designed three processes:sequential photocatalysis and ozonation(SPO),sequential ozonation and photocatalysis(SOP)and photocatalytic ozonation.The phenol degradation and TOC removal in the three processes were investigated and the result was shown in Fig.2.Fig.2A showed that ozone was much more powerful than UV light in phenol degradation,as phenol was degraded much faster in the first 60 min of the SOP process than that of the SPO process.In both sequential processes,phenol was completely removed in 120 min,whereas the TOC removal rates in the SOP processes were about 10%higher(Fig.2B).
Photocatalytic ozonation only showed little promotion in phenol degradation(Fig.2C),but its magic power especially lied on the effective TOC removal(Fig.2D).In the photocatalytic ozonation,about 90%of TOC was removed at 90 min,while those in the sequential processes were no more than 32%.The TOC removal rate in Fe3+/TiO2catalyzed SOPwas44.8%at120min,whichwaslargerthanthatintheSPOprocess(32.2%).The same trend was also revealed in the sequential processes with TiO2or without catalyst.
We analyzed the evolution of three typical intermediate products during phenol degradation(Fig.3).Pyrocatechol and hydroquinone were slowly generated and no maleic acid was detected in the first stage of SPO.Whenozonewass witchedon,pyrocatecholandhydroquinone were quickly oxidized to maleic acid,but maleic acid was not totally decomposed at the end of the reaction.That is why the mineralization of phenol was low in SPO.While in the first stage of SOP,pyrocatechol,hydroquinone and maleic acid were simultaneously produced in catalytic ozonation.Pyrocatechol and hydroquinone were almost completely decomposed after 45 min,the concentration of maleic acid reached the maximum and then decreased with reaction time.Its concentration at 120 min was lower than that in SPO,which was consistent with Fig.2B that SOP was a little more efficient than SPO in mineralization.Very low concentration of resorcinol(<2 mg·L−1)was detected only in ozone related processes,and thus was not quantitatively compared here.At 60 min of SOP,low concentration of ozone might still retain in the solution and H2O2canbe produced from direct reaction between ozone and phenol,and they both can contribute to the phenol and intermediates degradation in the second stage.This well explained the higher TOC removal rate in SOP than SPO.The results also revealed that though sequential processes were powerful for the target pollutant removal in some cases,it was essential to combine the two processes together at the same time.

Fig.2.Phenol degradation(A)and TOC removal(B)in the sequential processes and phenol degradation(C)and TOC removal(D)in the integrated process.Solid symbol:SPO,hollow symbol:SOP.
3.2.Degradation of oxalic acid
Fig.4 shows OA degradation in the single and integrated processes.UV and O3were both unable to degrade OA without catalyst,whereas TiO2greatly promoted OA removal and the addition of Fe3+further increased its removal(Fig.2A and B).OA degradation is greatly accelerated in the integrated system even without catalyst(Fig.2C).Undoubtedly,Fe3+/TiO2greatly accelerated OA degradation and it was nearly completely removed in 10 min.Different from that in phenol degradation,the promoting effect of Fe3+was much more obvious in the photocatalytic ozonation of OA because Fe3+can react with oxalate to produce ferrioxalate complex,which decomposed much faster than Fe(OH)2+under UV and produced more•OH[29,30].Ferrioxal ate comp lexanal soquicklyreactwi tho zone molecular and thus promoted the degradation of OA[31,32].For the setworeasons,there movalrate of OA was dramatically accelerated in the single or integrated systems with Fe3+/TiO2.
3.3.Reaction mechanism and transformation of Fe3+
The ozone concentrations in the solution and outlet gas during photo catalytic ozonation of phenol and OA were detected and shown in Fig.5Aand B.During phenol degradation,the ozone concentration in the effluent gas gradually increased and kept stable after 60 min.The trends were the same in photolytic ozonation or TiO2and Fe3+/TiO2catalyzed photo cat alyticozonation.While in the OA degradation process,the concentration in the outlet gas quickly reached the maximum(around 5 mg·L−1)because ozone can hardly directly react with OA molecule,and it was only partly consumed by UV light or the photo generated electrons.

Fig.4.OA degradation in photocatalysis(A),catalytic ozonation(B)and photocatalytic ozonation(C).
The con centration of ozone in the solution in creased in the firststage of phenol degradation,and it decreased after reaching the maximum concentration at 45 min(Fig.5A).This was highly related with the degradation extent of phenol,as phenol was nearly completely removed at 45min in the integrated process(Fig.2C).A fterthat,the concentrations of ozone in the solution all decreased in the systems with or without catalysts.The ozone concentrations in the solution during OA degradation were much lower than those in phenol degradation,and the highest concentration was even lower than 0.5 mg·L−1.This was probably caused by the different properties of target compounds and the pH of the solution(Fig.5B).
There were mainly two reaction pathways to produce H2O2in photolytic ozonation in acid or neutral solution.The first pathway was ozone decomposition under UV irradiation to produce atom oxygen and it reacted with water molecule to produce H2O2[33].The second was the cycloaddition of ozone to phenolic ring and produce dicarboxylic acids and H2O2as by-product[34].This is the main reason for the quite different concentrations of H2O2in solution during phenol and OA(Fig.5C).In photolytic ozonation of phenol,H2O2was continuously accumulated in solution from direct reaction between UV light and O3.But the generat ionwaslimited in the first45minbecauseozonemainly reacted with phenol.After phenol was almost totally removed,the concentration of H2O2increased much faster withreactiontime.When TiO2was added,the concentration of H2O2increased initially and gradually decreasedafterreaching a peakvalueat45min.During OA degradation,the concentrations of H2O2were much lower than those in phenol degradation.
In the Fe3+/TiO2catalyzed degradation of phenol and OA,the transformation of Fe3+in different processes was monitored and shown in Fig.6.In the photocatalytic degradation of phenol,the concentration of Fe2+quickly increased to about 4 mg·L−1within 10 min.This meant Fe3+was almost completely reduced,possibly due to its role of electron scavenger and the start of photo-Fenton reaction(Eqs.(1)and(2))[28].But the function of•OH was very limited in this process(Fig.7),and we deduced that Fe3+was mainly reduced by the UV generatedelectrons.Fe2+wasnotre-oxidizedintherestoftime,thismeant that photo-Fenton reaction was unable to continue and its role in phenol degradation was negligible.

In the photocatalytic ozonation of phenol,Fe3+was also reduced very quickly and kept a stable concentration in the first stage.It was oxidized again after 45 min and was almost completely oxidized at 90 min.The reduced Fe2+can be re-oxidized by H2O2to produce Fe3+and•OH in Fenton reaction(Eqs.(3)and(4)),oritreactswithozonetoproduceFeO2+(Eq.(5)):


Fig.5.Ozone concentration in the outlet gas(A)and solution(B),and H2O2concentration in the solution during photocatalytic ozonation of phenol(solid symbols)and OA(hollow symbols).
The intermediate FeO2+can further evolve tobe•OHand Fe3+(Eqs.(6)and(7)),and also was able to oxidize Fe2+at a slow rate(Eq.(8))[32]:


Fig.6.The concentration of Fe2+in solution during Fe3+/TiO2photocatalysis and photocatalytic ozonation of phenol(solid symbols)and OA(hollow symbols).

The regeneration of Fe3+after phenol removal indicated that in the presence of phenol and Fe2+,O3preferentially reacted with phenol other than Fe2+.After phenol was to tally removed,O3startedtooxidize Fe2+and other organic intermediates.
In the photocatalytic oxidation of OA,Fe3+was also greatly reduced within10min.Photo-Fentonre actionhad little effect to OA degradation as Fe2+was not regenerated anymore in the solution.Fe3+was only slightly reduced in the first few minutes during photocatalytic ozonation of OA,and the concentration of Fe2+decreased later.This showed a quite different role from that in phenol removal.It was reported that fer rioxalate complex is easily formed,and it can quickly decompose under UV irradiation to generate oxidative•OH[30,35,36].It is also very reactive with ozone[31],which results in a higher removal rate of OA and high consumption of ozone in solution.
In the TiO2photo catalytic degradation of phenol,threekinds of scavengers were added in the solution respectively to reveal the reactive species.Fig.7A showed that benzoquinone and t-BA had no effect in phenol degradation,this indicated that function of •OH and O2−•was negligible to phenol degradation.I−partially inhibited the phenol degradation from 39.5%to 19.0%at 120 min,which meant photo generated holes played an important role in phenol degradation.In the photocatalytic degradation of OA,the addition of t-BA also partially reduced the removal rates of OA,from 98.2%and 69.8%to 78.5%and 59.6%with Fe3+/TiO2and TiO2,respectively(Fig.7B).The degradation of OA was dramatically inhibited by the addition of t-BA in the Fe3+/TiO2catalyzed ozonation(Fig.7C).This indicated that•OH was the key oxidant in the catalytic ozonation of OA,while it only partly contributed to OA removal in the photocatalytic process.We did not quench•OH in the photocatalytic ozonation,as it was widely regarded as the oxidative species in many literatures[17,37].
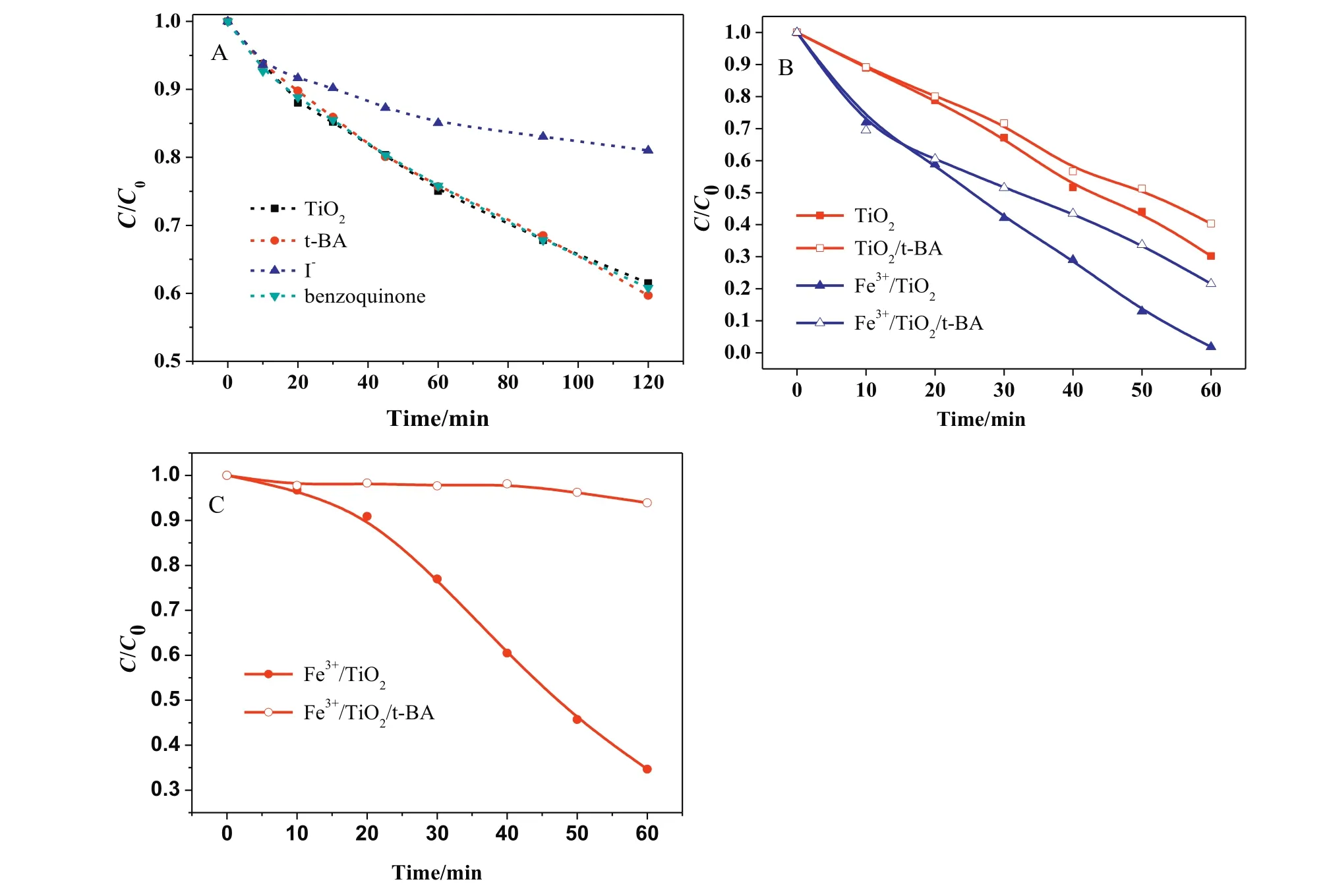
Fig.7.The Influence of different scavengers in TiO2photocatalysis of phenol(A),and the Influence of t-BA in the photocatalysis(B)and ozonation(C)of OA.
3.4.Synergetic effects in photocatalytic ozonation
The synergetic effects in the integrated processes can be quantitatively calculated by the coupling coefficiency(CC)by Eqs.(9)and(10):

whereDUV+O3-catrepresented the TOC removal rate of phenol or OA removal rate(%)in photocatalytic ozonation,DUV-cat,DO3-cat,DUVand DO3meant the TOC removal rates of phenol or OA removal rates(%)in the photocatalysis,catalytic ozonation,photolysis and ozonation,respectively.This meant the synergetic effect can be evaluated by two ways:the combination of UV light to catalytic ozonation processes,or ozonation to photocatalysis.A CC value higher than 1 means synergistic,while a CC value lower than 1 means antagonistic[38].We did not calculate the synergy regarding phenol degradation,because it was easily degraded in many reaction systems with ozone.
The results in Fig.8A were calculated based on the combination of UV light and catalytic ozonation.It showed that the synergy in the photolytic ozonation was much higher than the photocatalytic ozonation processes,because OA was very stable in ozonation or under UV irradiation.As TiO2or Fe3+/TiO2catalyzed ozonation can quickly degrade OA(Fig.4A),the addition of UV light was not so impactful.The average CC was lower than 3 and it decreased with time.On the contrary,Fig.8B showed the synergy on the combination of ozone with photocatalysis,and the CC values were normally higher than those in Fig.8A.This should be due to thelower activity of catalytic ozonation in OA removal than photocatalysis.Especially in the first 10 min,the CC was as high as 34 in the combination of Fe3+/TiO2photocatalysis with ozonation.As we mentioned above,the formed ferrioxalate complex was very reactive to ozone molecule and UV light,and photocatalytic ozonation was efficientin•OHgenerationtooxidizeOA.Thesethreereactionpathways jointly contributed to the quick removal of OA in 10 min.
The synergies in phenol mineralization were all lower than those in OA removal(Fig.8C and D).The CC values in the photolytic ozonation were around 1 or even lower,which meant direct combination of UV and ozone was ineffective in phenol mineralization.In the combination of ozone with TiO2or Fe3+/TiO2photocatalysis,the CC values were among 1.5 to 2.5,which meant slightly positive synergy in the simultaneous process.While in the combination of catalytic ozonation with UV light,the CC values were among 2.3 to 4.3.The difference originated from the partial mineralization of phenol in ozonation(36.0%at 120 min),while that under UV irradiation was only 3.0%at 120 min.
Comparing Fig.8A–B and C–D,it was concluded that the structure greatly Influenced the synergetic effect in photocatalytic ozonation.During phenol mineralization,the synergy effect should be Influenced by the intermediates,such as pyrocatechol,hydroquinone and maleic acid.As there are two different ways to quantitatively evaluate the synergy,the same equation should be used when comparing the systems in different works.Meanwhile,it should be mentioned that the catalysts were very importantinac celerating OA removal and phenol mineralization,though the synergy was not so obvious in some cases.The CC values canhelptound erst and there action mechanism,but it cannot directly guide the catalyst selection.
4.Conclusions
In this paper,the sequence of UV light and ozone,the role of Fe3+and the synergy in photocatalytic ozonation of phenol and OA were intensively studied.The order in phenol degradation and TOC removal was photocatalytic ozonation>SPO>SOP>photocatalysis,which proved the importance of the combination of UV and ozone.Fe3+bene fited to phenol degradation in all photocatalysis,catalytic ozonation and photocatalytic ozonation.It played an important role as electron scavenger in photocatalysis,but this function was concealed in photocatalytic ozonation as ozone was even more ef ficient in capturing photo generated electrons.Fe3+was quickly reduced under UV irradiation in photocatalytic ozonation,and gradually oxidized by ozone again only after phenol was completely degraded.Photolytic ozonation showed great synergetic effect in OA degradation due to the reaction between ozone and UV to produce H2O2,which further reacted with ozone to produce•OH,while ozone or UV light alone can hardly degrade OA.Ferrioxalate complex can be easily oxidized both by UV light and ozone,and consequently promoted the removal of OA.The results indicated that the synergetic effects in the photocatalytic ozonation were highly related with the properties of the organics.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- High efficiency production of ginsenoside compound K by catalyzing ginsenoside Rb1 using snailase☆
- Understanding the Influence of microwave on the relative volatility used in the pyrolysis of Indonesia oil sands☆
- Degradation and mineralization of aniline by O3/Fenton process enhanced using high-gravity technology☆
- Three-liquid-phase extraction and separation of V(V)and Cr(VI)from acidic leach solutions of high-chromium vanadium–titanium magnetite☆
- Insight into the degradation mechanism of cefixime under crystallization condition☆
- Experimental and simulation of the reactive dividing wall column based on ethyl acetate synthesis☆
