Inhibitory activities of plumbagin on cell migration and invasion and inducing activity on cholangiocarcinoma cell apoptosis
2018-07-26LuxsanaPanritTullayakornPlengsuriyakarnPongsakornMartvisetKesaraNaBangchang
Luxsana Panrit, Tullayakorn Plengsuriyakarn, Pongsakorn Martviset, Kesara Na-Bangchang✉
1Drug Discovery and Development Center, Thammasat University, Pathumthani 12121, Thailand
2Center of Excellence in Molecular Biology and Pharmacology of Malaria and Cholangiocarcinoma, Chulabhorn International College of Medicine,Thammasat University, Pathumthani 12121, Thailand
3Graduate Program in Bioclinical Sciences, Chulabhorn International College of Medicine, Thammasat University, Pathumthani 12121, Thailand
4Faculty of Medical Technology, Rangsit University, Pathumthani 12000, Thailand
Keywords:Cholangiocarcinoma Plumbagin Cancer migration Cancer invasion Apoptosis
ABSTRACT Objective: To investigate the cytotoxic, apoptotic and inhibitory activities on cell migration and invasion of plumbagin in the human cholangiocarcinoma (CCA) cell line (CL-6) in comparison with human embryonic fibroblast cell line (OUMS). Methods: Cytotoxicity activity was evaluated using MTT assay. Inhibitory effect on cell migration and invasion were investigated using label-free real-time cell analysis and QCM ECMatrix cell invasion chamber,respectively. Apoptotic activity was evaluated using flow cytometry and CellEvent™ Caspase 3/7 assay. Results: Based on results of the cytotoxicity test in CL-6 cells, 50% inhibitory concentration (IC50, Mean±SD) values of plumbagin and the standard drug 5-fluorouracil were (24.00±3.33) and (1 036.00±137.77) μmol/L, respectively. The corresponding values for OUMS cells were (57.00±5.23) and (2 147.00±209.98) μmol/L, respectively. The selectivity index was 2.28. The inhibitory activities of plumbagin on cell migration and invasion were potent and concentration-dependent with IC50 of 25.0 μmol/L and complete inhibition at 25.0 μmol/L. Flow cytometry analysis showed that plumbagin at 12.5 μmol/L (half IC50) induced CL-6 cell apoptosis (43.24% of control) through stimulation of caspase 3/7 activities. Complete cell apoptosis was observed at 12.5 μmol/L. Conclusions: The cytotoxic activity and inhibition of migration and invasion including apoptosis induction in the human CCA cell line (CL-6)suggest that plumbagin could be a promising candidate for CCA chemotherapeutics. However,its relatively low selective cytotoxic effect on CCA cells is a major concern.
1. Introduction
Cholangiocarcinioma (CCA) is the malignancy of epithelial cells of the biliary tract that occurs anywhere along the intra- and extrahepatic biliary tree[1,2]. The highest incidence of this type of cancer is reported from the northeastern region of Thailand. Current treatment with standard chemotherapeutics is unsatisfactory with approximately 5-year survival rate of 5%–10%[3]. Surgery remains the only possible intervention, which offers the chance of cure,but only few patients can be rendered. Radiation coupled with chemotherapy is an alternative choice of treatment after resection,but local recurrence of cancer is problematic[3,4]. Research and development of new alternative chemotherapeutics is urgently needed, including those derived from natural products.
Plumbagin (5-hydroxy-2-methyl-1,4-naphthoquinone, PL) is a napthoquinone which is the major constituent isolated from the root of Plumbago indica Linn. (fire plant leadwort, Indian leadwort, or rose-colored leadwort). It is crystalized into amber sharp needle shape crystal and vaporized when heating at 78–79 ℃. Suitable solvents for PL solubilization are polar solvents, i.e., methanol,ethanol, chloroform, benzene, and acetic acid[5]. PL has been shown to exert potential health benefits due to its anti-oxidant, antiin flammatory, and anticancer properties. In addition, it also exhibits inhibitory activities against bacteria (Staphylococcus aureus and Pseudomonas aeruginosa), fungi (e.g., Candida albicans), malaria(Plasmodium falciparum) and helminthes (e.g., Schistosoma mansoni)[6–11]. With regard to activity against cancers, PL as well as the root extract of Plumbago indica Linn. has been reported to inhibit the growth of various types of cancer including leukemia, gastric cancer, breast cancer, ovarian cancer, prostate cancer, melanoma,HEPA-3B hepatocellular, and CCA cell lines[4]. For CCA, promising cytotoxic activity of the crude ethanolic extract of Plumbago indica Linn. root has been demonstrated with IC50(concentration that inhibits cell growth by 50%) of (77.79±14.31) μmol/L(mean±SD)[11]. Nevertheless, the underlying molecular mechanism associated with such inhibitory activity in CCA has not been investigated. In the present study, the effects of PL on cell migration,cell invasion, as well as cell apoptosis induction were investigated in the human CCA cell line CL-6.
2. Materials and methods
2.1. Cell culture and reagents
The human CCA cell line CL-6 was kindly provided by associate professor Dr. Adisak Wongkajornsilp, Department of Pharmacology,Faculty of Medicine (Siriraj Hospital), Mahidol University. Normal human embryonic fibroblast cell line (OUMS) was purchased from Japanese Collection of Research Bioresources cell bank(Osaka, Japan). CL-6 cells were cultured in complete RPMI media supplemented with 10% FBS, 12.5 mmol/L, HEPES (pH 7.3),and 1× antibiotic-antimycotic. The OUMS cells were cultured in complete DMEM media consisting of 10% FBS, 12.5 mmol/L HEPES (pH 7.3), and 1× antibiotic-antimycotic (Thermo Fisher Scientific, NY, USA)[11]. Both cells were maintained under an atmosphere of 5% CO2at 37 ℃. The cells were subcultured every 3–4 d using 0.25% trypsin-EDTA.
PL (97% purity) was obtained from Wako (Osaka, Japan) and the stock solution (5 mmol/L) was prepared in 50% ethanol (Labscan,Bangkok, Thailand). The reference control drug 5-fluorouracil(5-FU) was purchased from Sigma-Aldrich Inc. (St. Louis, MO,USA) and the stock solution (5 mmol/L) was prepared freshly before use in 50% ethanol. The MTT reagent [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] was purchased from Sigma-Aldrich Inc. (St. Louis, MO, USA). Dimethylsulfoxide was obtained from Amresco LLC (Solon, OH, USA).
2.2. Cell cytotoxicity assay
CL-6 and OUMS cells (1×104cells/well) were seeded onto a 96-well microtiter plate and incubated at 37 ℃ under 5% CO2atmosphere for 24 h. PL (150.0, 100.0, 50.0, 25.0, and 12.5 μmol/L) or 5-FU (5 000.0, 2 500.0, 1 250.0, 625.0, and 312.5 μmol/L) was added into each well. The cells were further incubated for 48 h and cell viability was evaluated using MTT assay[12].The IC50was calculated using the concentration-response analysis software CalcuSynTM[11]. The selectivity index was determined as the ratio of IC50of OUMS or 5-FU in OUMS to CL-6 cells. Cell morphology was examined under inverted light microscope. The experiment was repeated three times, triplicate each.
2.3. Investigation of inhibitory effect on CL-6 cell migration
The inhibitory effect of PL on CL-6 cell migration was investigated using modified CIM-16 plates and analysis was performed by Real-Time Cell Analyzer (RTCA; xCelligence, ACEA Biosciences Inc.,Boston, MA, USA). RTCA migration assay measures the effect of PL in a real-time setting. The cells migrated from the upper chamber through a membrane (8 μm pores) into the bottom chamber in response to a chemo-attractant (FBS) and adhered to the electronic sensors on the underside of the membrane. The CL-6 cells were resuspended in serum-free medium and seeded in the upper chamber(3×104) cells in 100 μL in CIM-16 plates. The plates were incubated at 25 ℃ for 30 min. The lower chamber contained medium containing 10% fetal bovine serum (positive control), serum-free medium (negative control) or PL (12.5, 25.0, and 50.0 μmol/L). The chamber was incubated at 37 ℃ under 5% CO2for 24 h, in the Real-Time Cell Analyzer Dual Purpose (xCelligence RTCA DP) and cell migration was monitored real-time. Inhibitory effect of PL on cell migration was evaluated by determination of the resultant change in impedance signal correlated with the number of cells attached to these electrodes. The experiment was repeated three times.
2.4. Investigation of inhibitory effect on CL-6 cell invasion
The inhibitory effect of PL on CL-6 cell invasion was investigated using QCM ECMatrix cell invasion chamber 96-wells (Millipore, MA,USA) according to the manufacturer’s protocol with modification. In brief, the cells were pre-treated with PL (12.5, 25.0, and 50.0 μmol/L)for 48 h before the experiment. The pre-warmed serum-free medium(100 μL) was added to the inserts of the invasion assay plate coated with extracellular matrix layer and rehydrated at 25 ℃ for 1–2 h.The medium from the inserts was carefully removed and 150 μL of medium containing 10% fetal bovine serum was added to each well of the feeder tray. Cells at the density of 2×105cells per 100 μL were added into the invasion chamber and incubated at 37 ℃ under 5%CO2atmosphere for 24 h. At the end of the incubation period, the cells and culture medium were gently discarded from the top side of the inserts. The inserts were rinsed by placing the chamber plate onto the new 96-well feeder tray containing 150 μL of PBS (Ca2+and Mg2+free) and incubated at 25 ℃ for 1 min. The invasion chamber plate was placed back into the 96-well feeder tray containing 150 μL of pre-warmed cell detachment solution and incubated at 37 ℃under 5% CO2atmosphere for 30 min. Lysis buffer/dye solution(50 μL) was added to each well of the feeder tray containing cell detachment solution (150 μL). Following incubation at 25 ℃ for 15 min, the mixture (150 μL) was transferred to a new 96-well plate,and the absorbance was measured at 480/520 nm. The experiment was repeated three times.
2.5. Analysis of cell apoptosis by flow cytometry
The flow cytometry analysis was performed to confirm the inducing effect of PL on CL-6 and OUMS cell apoptosis using the Annexin V FITC Apoptosis Detection Kit (BD Pharmingen, USA). The cells were treated with PL (12.5 and 25.0 μmol/L for CL-6 cells, and 57.0 and 28.5 μmol/L for OUMS cells) for 24 h. The untreated cells served as control. Cells were trypsinized and washed twice with PBS(pH 7.4) and stained with FITC Annexin-Ⅴ and PI. The stained cells(10 000 cells) including apoptotic cells were examined using flow cytometry (BD Pharmingen, NJ, USA).
2.6. Investigation of mechanism of cell apoptosis
The inducing effect of PL on CL-6 and OUMS cell apoptosis(inducing effects on caspase 3/7 apoptosis pathway) was investigated using CellEvent™ Caspase-3/7 Green detection assay (Thermo Fisher Scientific, NY, USA). The CL-6 or OUMS cells (1×104cells)were seeded onto each well of a 96-well and incubated at 37 ℃under 5% CO2atmosphere for 24 h. PL (12.5 and 25.0 μmol/L for CL-6 cells, and 57.0 and 28.5 μmol/L for OUMS cells) was added into each well. The cells were further incubated for 24 h and washed with PBS (pH 7.4). Fluorescence solution was added and cells were further incubated at 37 ℃ for 30 min. The inducing effect of PL on cell apoptosis was evaluated under the ZOE fluorescent microscope(Bio-rad, CA, USA) observing the green fluorescent signals in apoptotic cells compared to control (no green fluorescence color).
2.7. Statistical analysis
Qualitative and quantitative variables are summarized as number(percentage) and mean±SD, respectively.
3. Results
3.1. Cell cytotoxicity
PL potently exhibited inhibitory effect on CL-6 cell growth compared with 5-FU-treated cells. The IC50(mean±SD) of PL and 5-FU were (24.00±3.33) μmol/L and (1 036.00±137.77) μmol/L,respectively. The corresponding IC50values of PL and 5-FU for OUMS cells were (57.00±5.23) μmol/L and (2 147.00±209.98) μmol/L,respectively. The selectivity index of PL on CL-6 cells was 2.28.
3.2. Inhibitory effect of PL on CL-6 cell migration and invasion
The inhibitory effects of PL on the CL-6 cell migration and invasion were investigated at the exposure time of 6, 12, 18, and 24 h. The effects were concentration-dependent at the concentrations ranging from 12.5 to 50.0 μmol/L. At all exposure time, PL at 25.0 μmol/L inhibited cell migration and invasion by 50% and at higher concentrations (50.0 μmol/L) complete inhibitory effect (100%)was observed (Figure 1). Complete inhibition of cell migration and invasion was also observed in the untreated control cell, while no migration (0%) was observed in the negative control cell.

Figure 1. Suppression of human cholangiocarcinoma cell line (CL-6)migration (A) and invasion (B) following exposure to plumbagin(PL) (12.5,25.0, and 50.0 μmol/L) for 6, 12, 18, and 24 h, compared with untreated control cell (no plumbagin).
3.3. Inducing effect of PL on cell apoptosis

Figure 2. Human cholangiocarcinoma cell line (CL-6) apoptosis investigated by flow cytometry in untreated control cells [A, no plumbagin(PL)] and in cells treated with PL respectively at 12.5 (B) and 25.0 μmol/L (C) for 24 h.
The effects of PL on CL-6 and OUMS cell apoptosis were investigated by flow cytometry. Results showed that PL induced CL-6 apoptosis at 24 h of exposure (Figure 2). The mechanism of cell apoptosis was investigated by fluorescence staining of caspase 3/7 activities involved in cell apoptotic pathway. The green signal was observed following exposure to PL at all concentrations (Figure 3). The apoptotic cells with green fluorescence staining of caspase 3/7 activities of PL confirmed the inducing effect of PL on CL-6 cells. For OUMS cells on the other hand, no cell apoptosis but cell necrosis occurred at both concentrations following 24 h exposure to PL.
4. Discussion
An ideal cancer therapeutic agent is the molecule that attributes anticancer activity by inhibition of cancer cell metastasis/angiogenesis and destroying the cells through apoptotic mechanism.PL has been shown to suppress the growth of several types of cancer through several mechanisms including inhibition of angiogenesis and disruption of cell growth by induction of cell apoptosis, and stimulation of generation of reactive oxygen species in cancer cells through NF-κB and other kinases[13–15]. In hepatocellular carcinoma, PL was shown to inhibit the migration and invasion of tumor derived endothelial cells leading to inhibition of tumor angiogenesis through abrogation of PI3K/AKT pathway[16]. In HepG2 (hepatocellular carcinoma cell line), it was shown to inhibit cell migration and invasion by interference with the production of methalloprotease-2 and urokinase-plasminogen activators[17].Inhibitory activity against cell viability was concentrationdependent[16,17]. In lung cancer, PL stimulated caspase 3/7 activities and enhanced Bax/Bcl-2 ratio, leading to cell apoptosis[18–20].
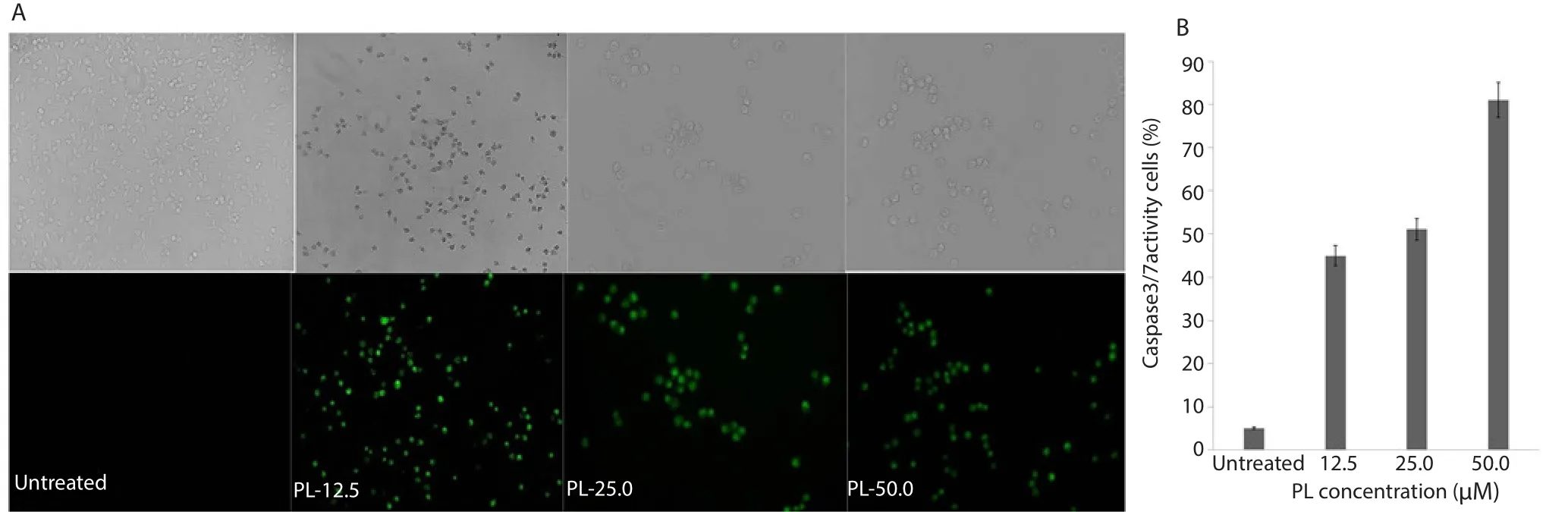
Figure 3. Inhibitory effect of PL on CL-6 cell apoptosis.
With regard to CCA, previous studies have demonstrated antiproliferative activities of plant extracts or isolated compounds on CCA cell lines or animal models, or on their underlying mechanisms of action on cell apoptosis and cell cycle arrest. These include triptolide from Tripterygium wilfordii[21], ubiquitous tannic acid[22], Resina draconis[23], luteoli[24], Ganodermal ucidum[25],gallic acid from Caesalpiniam imosoides Lamk[26], scabraside D from Holothuria scaba[27], Cratoxylum formosum[28], matrine from Sophora flavescentsait[29], Derris indica[30], Andrographis paniculate[31], Kaempferia parviflora[32], Kaempferia galanga Linn[33],and epigallocatechin gallate (from green tea)[34]. Various molecular targets underlying the anti-proliferative activities of these plant extracts or isolated compounds have been demonstrated, i.e.,Bcl-2 and Bax[35,36], Bak[23], JAK/STAT3 (janus kinase/signal transducer and activator of transcription signaling pathway)[37],phosphatidylinositol 3-kinase/AKT mammalian target of rapamycin(PI3K/AKT/mTOR)[23], caspase 3[31], caspase 8[23] and poly(ADP-ribose) polymerase[23]. The present study is the first that report the effect of PL on CCA cell apoptosis. This apoptotic effect was not observed with normal human embryonicfibroblast cells where cell death involved cell necrosis. Results supported previous observation on anti-proliferative activity of the crude extract of Plumbago indica in CL-6 cells[11]. In addition, the migration and invasion of the CCA cell CL-6 were also interrupted by PL similarly to that observed in other types of cancer. These effects, however,occurred at relatively higher potency[13–20]. Complete inhibitory effects on cell migration and invasion were observed at IC50for its cytotoxic effect (12.5 μmol/L). Induction of apoptosis was shown to be mediated through stimulation of caspase 3/7, the intracellular cysteine protease enzymes in thefinal step in both the intrinsic or extrinsic pathways of apoptosis[38]. The NF-κB and Bcl-1 were shown to be inactivated in breast cancer following PL exposure[39].In melanoma, induction of cell apoptosis via reactive oxygen species/c-Jun N-terminal kinase pathway was reported[40] and the thioredoxin reductase was shown to be the target of apoptotic action of PL[41]. This preliminary information on PL as the potential candidate for CCA requires further detailed investigation of the underlying molecular mechanism through which PL exerts its antiproliferative and anti-metastasis (inhibition of cell invasion and migration) activities against CCA. Nevertheless, the relatively narrow selectivity index value of 2.0 together with the necrotic cell killing nature raise concern about its safety in cancer chemotherapy.Confirmation of the anticancer activity as well as its safety profile in other in vitro and animal models is essential for further development of PL as CCA chemotherapeutics. In addition, molecular targets of PL in cell apoptosis including cell migration and invasion need to be elucidated.
Conflict of interest statement
The authors declare they have no con flict of interest.
Acknowledgments
The study was supported by Thammasat University research grant(Grant No.20/2556: Ms. Luxana Panrit), Thammasat University Center of Excellence in Pharmacology and Molecular Biology of Malaria and Cholangiocarcinoma, The Commission of Higher Education, Ministry of Education of Thailand (National University Project) and National Research Council of Thailand.
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- A critical review on Nepal Dock (Rumex nepalensis): A tropical herb with immense medicinal importance
- Attenuation of oxidative stress-induced neuronal cell death by Hydnophytum formicar-um Jack.
- Phytochemical, antioxidant and hepatoprotective effects of different fractions of Moringa oleifera leaves methanol extract against liver injury in animal model
- Chemical analysis and antioxidant content of various propolis samples collected from different regions and their impact on antimicrobial activities
- Phytochemical analysis and antioxidant profile of methanolic extract of seed, pulp and peel of Baccaurea ramiflora Lour.
- Zika virus: Is Pakistan next?
