Cortical Thinning Pattern of Bulbar- and Spinal-onset Amyotrophic Lateral Sclerosis: a Surface-based Morphometry Study
2018-07-16ZhiyeChenMengqiLiuLinMa
Zhiye Chen, Mengqi Liu, Lin Ma*
1Department of Radiology, Hainan Branch of Chinese PLA General Hospital,Sanya, hainan 572013, China
2Department of Radiology, Chinese PLA General Hospital, Beijing 100853, China
Key words: amyotrophic lateral sclerosis; cortical thickness; magnetic resonance imaging;surface-based morphometry
AMYOTROPHIC lateral sclerosis (ALS) is a progressive neurodegenerative disease with selected upper and lower motor neuron involvement.1,2Pathological changes in cerebral cortical regions in ALS have been demonstrated as the selective involvement of the motor cortex in a postmortem study.3However, motor neuron density measurement in cortex and spinal cord suggested that corticomotoneuron loss was unlikely to be a primary event in ALS.4More and more researchers have recognized that ALS may be a multisystem disease.2,5
Voxel-based morphometry (VBM)6is widely used to investigate brain structure changes at voxel-level over the whole brain, and it has been widely used in evaluation of ALS patients. VBM studies demonstrated that the volume of grey matter decreased in ALS,7and primary sensorimotor cortex atrophy was regarded as a prominent feature of the disease.8A meta-analysis9,10demonstrated that the asymmetric motor cortex atrophy was the main cerebral changes in ALS, but the pathophysiological mechanism has not been completely elucidated by far.
Cortical thickness measurement is an important research project for neuroimaging, and it can provide more valuable information than volume measurement in evaluation of neurodegenerative and psychological diseases. Like VBM technique, surface-based morphometry(SBM)11,12is also a whole brain structure analysis technique, and it can provide objective and accurate information in cortical thickness changes.SBM has been widely used in clinical researches on Huntington disease,13Parkinson’s disease,14aging brain,15etc. Previous studies16-18demonstrated that motor cortex thinning was associated with ALS, and a SBM study19suggested that the motor cortex thinning might reflect upper motor neuron impairment, whereas the extra-motor involvement may be related to the disability, progression, and duration of the disease. It has gradually come into a consensus that motor cortex atrophy is a common finding for the central nervous system damage in ALS.
Our previous voxel-wise meta-analysis of VBM study suggested that right precentral gray matter atrophy was a common finding and prominent feature of brain structural changes in ALS.12Further VBM study in ALS subtypes demonstrated that the pattern of gray matter damage is likely to distribute wider in spinal-onset ALS than in bulbar-onset ALS.20However, the precise cortical thinning pattern has not been elucidated in different onset subtypes of ALS. Herein, we hypothesized that some brain regions would intrinsically be suffered from cortical thinning in ALS, and different ALS subtypes (ALS-bulbar and ALS-spinal) present different cortical thinning pattern.
MATERIALS AND METHODS
Participants and study design
The study was approved by the institutional ethics committee, and written informed consents were obtained from all participants. Sixty-five patients (28 females and 37 males) were recruited from the outpatient clinic for motor neuron disease (MND) in our hospital from 2007 to 2010, including 34 diagnosed ALS cases and 31 probable ALS cases according to the revised El Escorial.21All the subjects were right handed and had no history of cerebrovascular disease, long-standing hypertension,diabetes mellitus, inflammatory diseases of the central nervous system and cranium trauma. None of the patients were taking psychoactive drugs and hormone. Patients with brain tumors, demyelination and other brain disorders were also excluded by conventional MRI examinations. Sixty-five normal controls (NCs) were recruited from volunteers of hospital staff and local community in the same time period with the same exclusion criteria. ALS functional rating scale-revise (ALSFRS-R)22was administered to all the patients for clinical rating of ALS symptoms,and the Mini Mental State Examination23was applied by a dedicated neurologist with 15-years’ experience to evaluate the cognitive function of all subjects.
MRI acquisition
Image data of all patients and controls were acquired on a GE 3.0T MRI system (SIGNA EXCITE, GE Healthcare, Milwaukee, WI, USA). and a conventional eight channel quadrature head coil was used. A 3-dimensional T1-weighted fast spoiled gradient recalled echo(3D T1-FSPGR) sequence generating 118 contiguous axial slices [TR (repetition time)=6.3 ms, TE (echo time)=2.8 ms, flip angle=15°, FOV (field of view)=24 cm×24 cm, Matrix=256×256, voxel size=0.9375×0.9375×1 mm3, NEX (number of acquisition)=1] was used for structural images. Conventional T2-weighted image(TR=5000 ms, TE=113.4 ms, FOV=24 cm×24 cm, Matrix=384×384) and T1-FLAIR (TR=2040 ms, TE=6.9 ms,FOV=24 cm×24 cm, Matrix=384×192) were also acquired.The image protocol was identical for each subject.
Image data processing
All MR structural image data were processed on workstation of MATLAB 7.6 (The Mathworks, Natick, MA,USA) for VBM using Statistical Parametric Mapping 12(SPM 12) and CAT12 tools (http://www.fil.ion.ucl.ac.uk/spm/). The following processing steps were carried out(Fig. 1): (1) The artifacts of raw data for each subject were inspected and image origin was set at the anterior commissure (AC); (2) structural images of each subject were normalized to the DARTEL templates space to improve inter-subject registration of structural images, and were segmented into gray matter (GM), white matter (WM) and cerebrospinal fluid (CSF); (4) projection-based thickness (PBT) was used to estimate cortical thickness and to create the central cortical surface for the left and right hemisphere;24(5) the mean cortical thickness of 152 brain regions were computed based on FreeSurfer atlas aparc.a2009s;25(6) after the preprocessing was finished, quality check was performed to evaluate the sample homogeneity; (7) before the statistical analysis, all the surface data was smoothed using a kernel with 15 mm full width at half maximum (FWHM).
Statistical analysis
Comparison of surface-based morphometric data between two groups was performed using two-sample t-test with age and sex as covariates. Significance was set at a P value without correction (Puncorr) of <0.001. The minimal number of contiguous voxels was set based on the expected voxels per cluster. The quantitative data were presented as mean±standard deviation. The correlation analysis was applied between the clinical variables and the mean cortical thickness of the abnormal brain regions with age and sex as covariates.Significant difference was set at a P value of <0.05. The statistical analysis was performed using SPSS 19.0.
RESULTS
The clinical characteristics of ALS and NCs
There was no significant difference in the gender (χ2=0.03, P=0.86), but a significant difference in the age(t=1.98, P=0.002) and for MMSE (t=1.98, P=1.43) between ALS and NC group were found between the ALS patients and normal controls (Table 1). The average disease duration and ALSFRS scores for ALS patients were 17.74±21.46 days and 29.43±5.23, respectively.There was no significant difference in MMSE (t=2.00,P=0.09), diseased duration (t=2.00, P=0.50) and ALSFRS score (t=2.00, P=0.76) between ALS-bulbar and ALS-spinal patients.
Brain regions with altered cortical thickness in ALS, ALS-spinal, ALS-bulbar compared with NCs
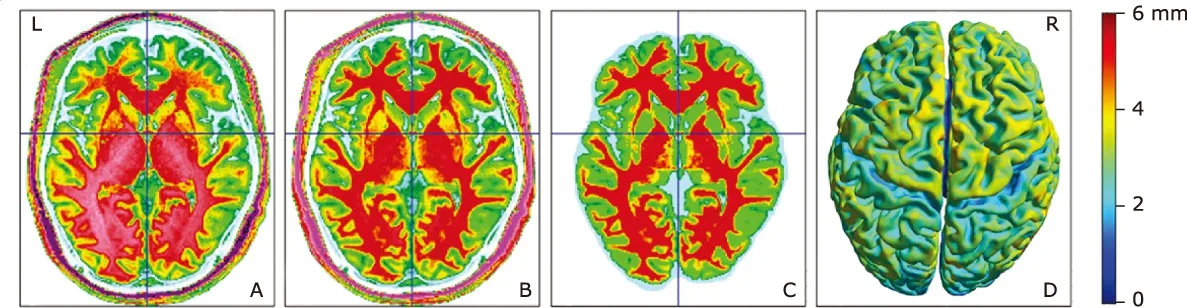
Figure 1. The flow graph of image processing. A, original T1 image; B, normalized T1 image; C, gray and white matter segmentation; D, cortical surface creation. Color bar represents the cortical thickness.
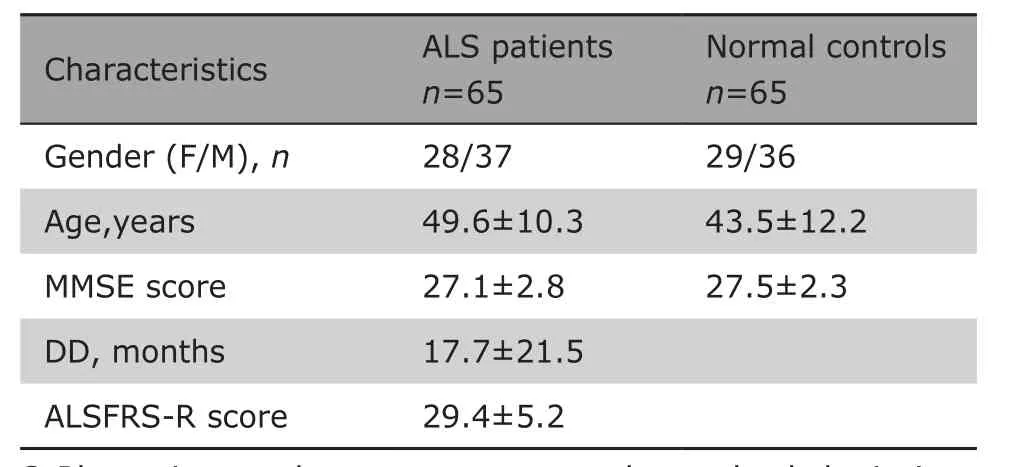
Table 1. Demographic and clinical characteristics of patients and controls§
Compared with that in NCs, the brain regions with decreased cortical thickness in ALS patients were located in the left precentral gyrus and postcentral gyrus, right gyrus rectus and medial precentral gyrus (Table 2)(Fig. 2). There was no thickening brain regions detected in ALS patients compared with NCs.
Comparing with the NC group, ALS-bulbar group presented regional thinning in the left precentral gyrus and right supplementary motor cortex (SMC), and ALS-spinal group presented regional thinning brain regions in the left posterior insular and right gyrus rectus. There was no significant difference in the cortical thickness over the whole brain between ALS-bulbar and ALS-spinal patients (Fig. 3).
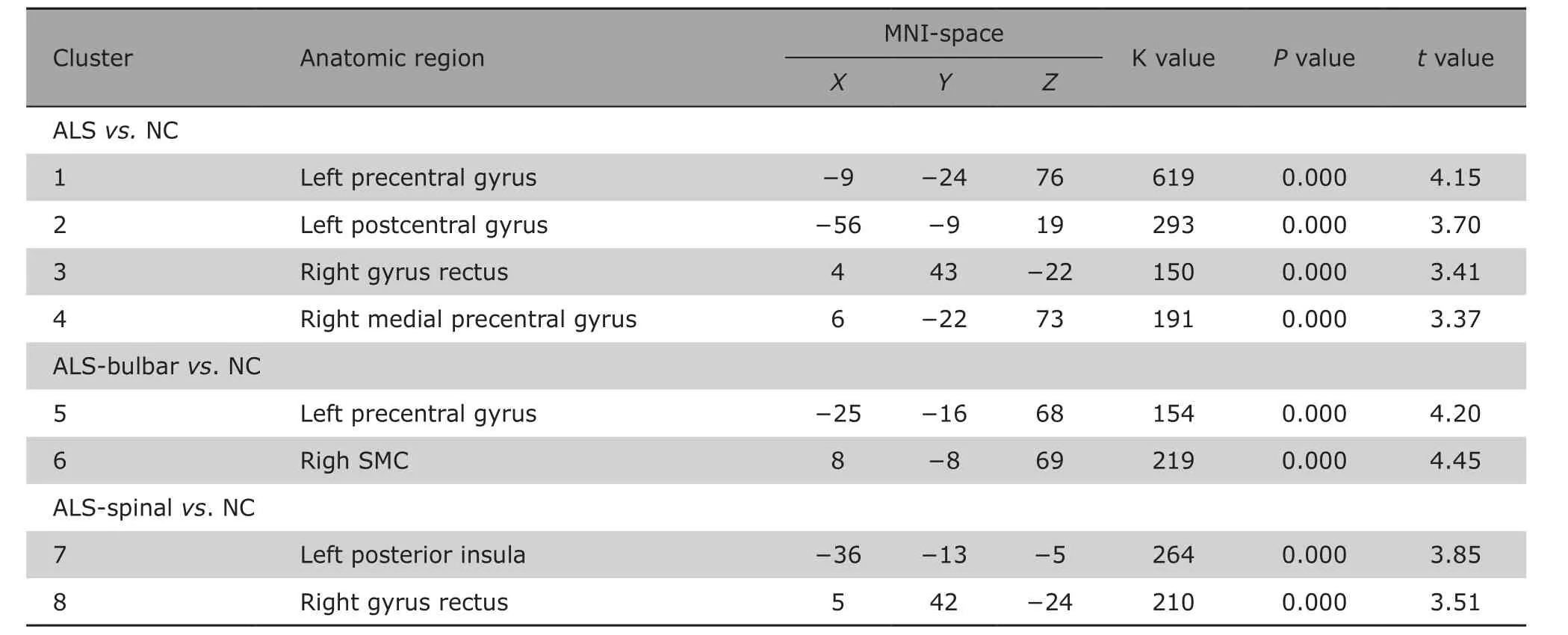
Table 2. Brain regions with cortical thinning in ALS compared with controls
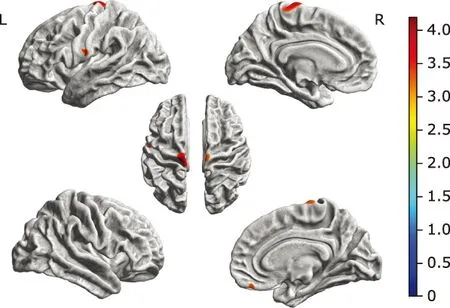
Figure 2. Brain regions with decreased cortical thickness in ALS patients were located in the left precentral gyrus, postcentral gyrus, right gyrus rectus and medial precentral gyrus compared with NC group. Color bar represents t value.
Relationship of cortical thickness changes with clinical characteristics in ALS patients
Table 3 shows that for patients with ALS, the cortical thickness of right gyrus rectus was negatively associated with disease duration (r=−0.311, P=0.013).The positive correlation did also present between the cortical thickness of right precentral gyrus and ALSFRS-R score (r=0.271, P=0.032). For patients with ALS-bulbar, no correlation was shown between the cortical thickness of positive brain regions with either disease duration or ALSFRS-R score (both P>0.05).For patients with ALS-spinal, the partial correlation analysis demonstrated the cortical thickness of left insula (r=−0.409, P=0.004) and right gyrus rectus(r=−0.351, P=0.014) were negatively associated with the disease duration. No correlation was shown between the cortical thickness of these regions with ALSFRS-R score.
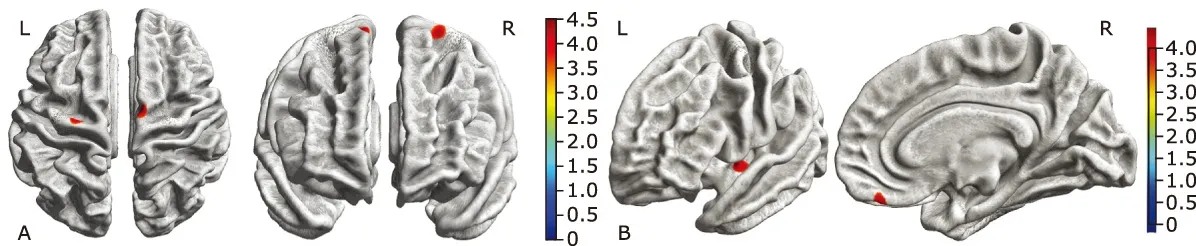
Figure 3. Brain regions with decreased cortical thickness in the ALS-bulbar patients and in the ALS-spinal patients compared with the NC group. A. Decreased cortical thickness in ALS-bulbar located in the left precentral gyrus and the right supplementary motor cortex. B. Decreased cortical thickness in the ALS-spinal located in the left posterior insula and the right gyrus rectus. Color bar represent t value.
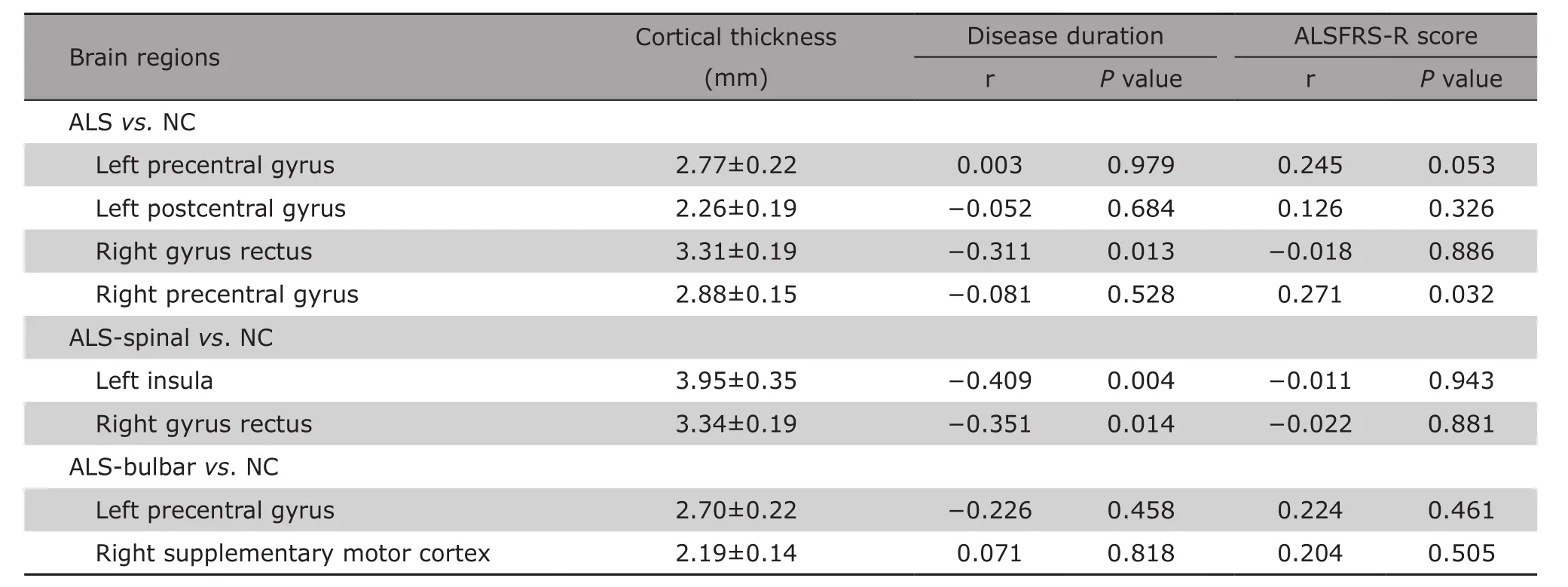
Table 3. The correlation analysis between the cortical thickness of thinning brain regions and clinical variables in ALS§
DISCUSSION
The results in this study demonstrated that selective motor cortex and extra-motor cortex were involved in patients with ALS. The thinning of bilateral motor cortices was consistent with previous studies,16,17and could underlie the relation between pathological characteristics in ALS and MR spectroscopy findings.26However,meta-analysis of VBM studies9,10,27demonstrated that asymmetric motor cortex volume loss commonly present in ALS patients. The inconsistence of this result with our findings may be associated with the different image processing methods. SBM method we used in this study reflected the altered cortical thickness, while VBM method presented the altered cortical volume. In the current study, the thinning of bilateral motor cortices may be the main neuromechanism and served as the direct evidence for upper motor neurons damage,and it may be associated with the decreased number and shape of cortical neurons.28,29
In this study, the extra-motor cortices thinning was also observed in left postcentral gyrus and right gyrus rectus in ALS patients, which suggested the ALS involves the motor cortex but may not confine to the motor cortex. On contrast, in the previous document, motor cortex involvement was a common finding, while extra-motor cortex involvement was not commonly reported. Herein,the extra-motor cortices involvement should be carefully interpreted. It may be associated with the disease duration, cognitive state, or other clinical variables. From the pathological viewpoints, it may be related to the widespread reactive astrocytes in the cortex of ALS.30
The partial correlation analysis revealed that only right motor cortex thinning was associated to disease disability. Considering the symmetrical thinning pattern we found in this study, the asymmetrical correlation with disease disability further indicated that the right motor cortex thinning may play a key roles in ALS development and could be considered as a imaging biomarker for ALS.12This study also demonstrated that extra-motor (right gyrus rectus) thinning was related to disease duration, which suggested that ALS was a complex degenerative disease involving multisystem beyond the motor system.9
Further clinical subtypes analysis in this study demonstrated that bulbar-onset and spinal-onset ALS had different cortical thinning patterns. For the bulbar-onset ALS, motor cortex was involved and the extra-motor cortex was spared, whereas in spinal-onset ALS, extra-motor cortex was involved, and the motor cortex was spared. This phenomenon of cortical thinning indicated that bulbar-onset ALS may only involve the upper motor neuron (UMN) and might be considered as UMN disorder with sparing of extra-motor. Meanwhile, spinal-onset ALS could be regarded as lower motor neurons (LMN) disorder with sparing of extra-motor cortex and motor cortex involvement. The findings support the viewpoint that bulbar-onset ALS is likely a simple motor neuron disorder, which is inconsistent with the conventional understanding that it is a multi-systematic disorder.2,5
Further correlation analysis revealed that bilateral motor cortex thinning was related to neither the disease duration nor the disease disability in bulbar-onset ALS,which suggested that motor cortex thinning may be the intrinsic pathophysiological changes in bulbar-onset ALS.This finding in bulbar-onset ALS was not consistent with the results in overall ALS patients, where the right motor cortex thinning was positively associated with ALS functional rating score. The small sample size and heterogeneity in the severity of the disease in this study might contribute to this inconsistence. This study also showed that in spinal-onset ALS, extra-motor cortex thinning was associated to the disease duration but not to the disease disability, which indicated that it may be the secondary pathophysiological change, and the underlying mechanism need to be further elucidated in future.
This study did not find significant difference in cortical thinning between bulbar-onset and spinal-onset ALS, which was different from our previous study where we found altered volumes of gray matter between ALS-bulbar and ALS-spinal group.20The reason may be associated with the different imaging processing methods in these two studies. In the previous study,20VBM method was used and the result indicated the volume changes, while in the current study, SBM method was used and the result represent the cortical thickness changes.
The limits of this study included: 1. the relatively small sample size of bulbar-onset ALS patients compared to the spinal-onset ALS in this study may cause sampling errors; 2 although MMSE was performed to exclude dementia, the mild cognitive impairment was not excluded,which could be a confounder factor besides age and sex;3. this study only observed bulbar-onset ALS and spinal-onset ALS, and for those of bulbar-spinal-onset ALS,further studies are needed in the future.
In summary, bilateral motor cortex thinning was the MRI signature of bulbar-onset ALS patients, and extra-motor cortex thinning was the MRI signature of spinal-onset ALS patients. Bulbar- and spinal-onset ALS may be a simple MND instead of multisystem disorder. The motor cortex thinning may be the intrinsic pathophysiological change that is associated to disease disability and play a key role in brain damage for upper motor neuron disorder. Extra-motor cortex thinning may be a secondary pathophysiological change related to disease duration and act as a pivotal in brain damage of lower motor neuron disorder.
REFERENCES
1. Chou SM, Norris FH. Amyotrophic lateral sclerosis:lower motor neuron disease spreading to upper motor neurons. Muscle Nerve 1993;16(8):864-9. doi:10.1002/mus.880160810.
2. van der Graaff MM, de Jong JM, Baas F, et al. Upper motor neuron and extra-motor neuron involvement in amyotrophic lateral sclerosis: a clinical and brain imaging review. Neuromuscul Disord 2009;19(1):53-8.doi: 10.1016/j.nmd.2008.10.002.
3. Pringle CE, Hudson AJ, Munoz DG, et al. Primary lateral sclerosis. Clinical features, neuropathology and diagnostic criteria. Brain 1992;115(Pt2):495-520. doi:10.1093/brain/115.2.495.
4. Pamphlett R, Kril J, Hng TM. Motor neuron disease: a primary disorder of corticomotoneurons? Muscle Nerve 1995;18(3):314-8. doi: 10.1002/mus.880180308.
5. Abrahams S, Goldstein LH, Suckling J, et al. Frontotemporal white matter changes in amyotrophic lateral sclerosis. J Neurol 2005;252(3):321-31. doi:10.1007/s00415-005-0646-x.
6. Ashburner J, Friston KJ. Voxel-based morphometry—the methods. Neuroimage 2000;11(6 Pt 1):805-21.doi: 10.1006/nimg.2000.0582.
7. Thivard L, Pradat PF, Lehéricy S, et al. Diffusion tensor imaging and voxel based morphometry study in amyotrophic lateral sclerosis: relationships with motor disability. J Neurol Neurosurg Psychiatry 2007;78(8):889-92. doi: 10.1136/jnnp.2006.101758.
8. Grosskreutz J, Kaufmann J, Frädrich J, et al. Widespread sensorimotor and frontal cortical atrophy in Amyotrophic Lateral Sclerosis. BMC Neurol 2006;6(17):1-10. doi: 10.1186/1471-2377-6-17.
9. Shen D, Cui L, Fang J, et al. Voxel-wise meta-analysis of gray matter changes in amyotrophic lateral sclerosis. Front Aging Neurosci 2016;8(64):1-12. doi:10.3389/fnagi.2016.00064.
10. Sheng L, Ma H, Zhong J, et al. Motor and extra-motor gray matter atrophy in amyotrophic lateral sclerosis:quantitative meta-analyses of voxel-based morphometry studies. Neurobiol Aging 2015;36(12):3288-99.doi: 10.1016/j.neurobiolaging.2015.08.018.
11. Fornito A, Yücel M, Wood SJ, et al. Surface-based morphometry of the anterior cingulate cortex in first episode schizophrenia. Hum Brain Mapp 2008;29(4):478-89. doi: 10.1002/hbm.20412.
12. Chen Z, Ma L. Grey matter volume changes over the whole brain in amyotrophic lateral sclerosis: A voxel-wise meta-analysis of voxel based morphometry studies. Amyotroph Lateral Scler 2010;11(6):549-54.doi: 10.3109/17482968.2010.516265.
13. Kim H, Kim JH, Possin KL, et al. Surface-based morphometry reveals caudate subnuclear structural damage in patients with premotor Huntington disease. Brain Imaging Behav 2016;11(5):1365-72. doi:10.1007/s11682-016-9616-4.
14. Huang P, Lou Y, Xuan M, et al. Cortical abnormalities in Parkinson’s disease patients and relationship to depression: A surface-based morphometry study.Psychiatry Res Neuroimaging 2016;250: 24-8. doi:10.1016/j.pscychresns.2016.03.002.
15. Lu H, Ma SL, Chan SS, et al. The effects of apolipoprotein epsilon 4 on aging brain in cognitively normal Chinese elderly: a surface-based morphometry study.Int Psychogeriatr 2016;28(9):1503-11. doi: 10.1017/S1041610216000624.
16. Butman JA, Floeter MK. Decreased thickness of primary motor cortex in primary lateral sclerosis. Am J Neuroradiol 2007;28(1):87-91.
17. Roccatagliata L, Bonzano L, Mancardi G, et al. Detection of motor cortex thinning and corticospinal tract involvement by quantitative MRI in amyotrophic lateral sclerosis. Amyotroph Lateral Scler 2009;10(1):47-52. doi: 10.1080/17482960802267530.
18. Cosottini M, Donatelli G, Costagli M, et al. High-resolution 7T MR imaging of the motor cortex in amyotrophic lateral sclerosis. AJNR Am J Neuroradiol 2016;37(3):455-61. doi: 10.3174/ajnr.A4562.
19. d’Ambrosio A, Gallo A, Trojsi F, et al. Frontotemporal cortical thinning in amyotrophic lateral sclerosis. AJNR Am J Neuroradiol 2014;35(2):304-10. doi: 10.3174/ajnr.A3753.
20. Chen Z, Liu M, Ma L. Gray matter volume changes over the whole brain in the bulbar- and spinal-onset amyotrophic lateral sclerosis: a voxel-based morphometry study. Chin Med Sci J 2018; 33(1):20-8. doi: 10.24920/11804.
21. Brooks BR, Miller RG, Swash M, et al. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord 2000;1(5):293-9. doi:10.1080/146608200300079536.
22. Ohashi Y, Tashiro K, Itoyama Y, et al. Study of functional rating scale for amyotrophic lateral sclerosis:revised ALSFRS(ALSFRS-R) Japanese version. Brain and nerve 2001;53(4):346-55. Japanese.
23. Galea M, Woodward M. Mini-Mental State Examination (MMSE). Aust J Physiother 2005;51(3):198. doi:10.1016/S0004-9514(05)70034-9.
24. Dahnke R, Yotter RA, Gaser C. Cortical thickness and central surface estimation. Neuroimage 2013;65:336-48. doi: 10.1016/j.neuroimage.2012.09.050.
25. Desikan RS, Ségonne F, Fischl B, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 2006;31(3):968-80. doi: 10.1016/j.neuroimage.2006.01.021.
26. Hamilton RL, Bowser R. Alzheimer disease pathology in amyotrophic lateral sclerosis. Acta Neuropathol 2004;107(6):515-22. doi: 10.1007/s00401-004-0843-1.
27. Chen Z, Ma L. Grey matter volume changes over the whole brain in amyotrophic lateral sclerosis: A voxel-wise meta-analysis of voxel based morphometry studies. Amyotroph Lateral Scler 2010; 11(6): 549-54. doi: 10.3109/17482968.2010.516265.
28. Gredal O, Pakkenberg H, Karlsborg M, et al. Unchanged total number of neurons in motor cortex and neocortex in amyotrophic lateral sclerosis: a stereological study. J Neurosci Methods 2000;95(2):171-6.doi: 10.1016/S0165-0270(99)00175-2.
29. Kiernan JA, Hudson AJ. Changes in shapes of surviving motor neurons in amyotrophic lateral sclerosis. Brain 1993;116 (Pt 1): 203-15. doi: 10.1093/brain/116.1.203.
30. Nagy D, Kato T, Kushner PD. Reactive astrocytes are widespread in the cortical gray matter of amyotrophic lateral sclerosis. J Neurosci Res 1994;38(3):336-47.doi: 10.1002/jnr.490380312.
杂志排行
Chinese Medical Sciences Journal的其它文章
- Early Diagnosis of Recurrent Optic Neuritis Using Contrast-Enhanced T2 Fluid-attenuated Inversion Recovery Imaging: a Case Report
- Effect of Red Ginseng Extract on the Pharmacokinetics of Aspirin Metabolite in Sprague Dawley Rats
- A Cohort Study of Incidences and Risk Factors for Thromboembolic Events in Patients with Idiopathic Membranous Nephropathy
- Effects of Short-term High Dose Atorvastatin on Left Ventricular Remodeling in Patients with First Time Attack of Anterior Acute Myocardial Infarction
- Irrationality of Allogeneic Red Blood Cell Transfusion in Intraoperative Cell Salvage Patients: a Retrospective Analysis
- Midterm Follow-up of Coronary Artery Bypass Grafting with 64-Slice Multi-detector Computed Tomography:Identification of Risk Factors Affecting Graft Patency
