补食增加达乌尔黄鼠(Spermophilus dauricus)越冬存活率并减少冬眠表达
2018-07-12周颖慧孙明月宋士一
杨 明, 周颖慧, 邢 昕, 孙明月, 彭 霞, 宋士一
(1. 沈阳师范大学 生命科学学院, 沈阳 110034;2. 沈阳师范大学 生物系统进化与农业生态重点实验室, 沈阳 110034)
0 Introduction
Hibernation is the most effective way to conserve energy in endotherms. During winter,mammalian hibernators reduce metabolic rate substantially. The minimum metabolic rate is near constant at about 0.03 mL O2g-1h-1during deep hibernation under 2~7 ℃, which may be about 1% of the euthermic value in small hibernators. The hibernation season was interrupted by several spontaneous arousals periodically, the so-called inter-bout arousal[1]. The 70%~80% or even more than 85% of the energy expended over the winter are consumed during inter-bout arousals[1-4]. Thus, the hibernation pattern, including the duration of the hibernating season, the total time spent in deep torpor, and the frequency and duration of inter-bout arousals should affect the amount of energy required during hibernation, and vise versa[5-7].
The hibernation pattern is flexible. It varies not only among species, populations, and even individuals, but also from one season to another in the same individual[8]. Intraspecificly,that was mainly due to the differences on energy storage. Food availability reduced hibernation in Japanese dormouse (Glirulusjaponicus)[5]and estern chipmunks (Tamiasstriatus)[6]. Body fat reserve also affect hibernation pattern in grey mouse lemur (Microcebusmurinus)[9]and edible dormice (Glisglis)[10]. Humphriesetal. hypothesized that the hibernation pattern is the result of the trade-off between energy saving benefit and physiological cost during metabolic depression. Hibernators may benefit from using available energy reserves to minimize their hibernation[11].
According to the energy-saving strategies during hibernation, mammalian hibernators can be distinguished into food-storing hibernators mainly live on stored food, such as eastern chipmunks[6]and some hamsters (Cricetus,Mesocricetus)[11]; fat-storing hibernators mainly utilize the stored body fat to survive and generally cease feeding during hibernation[4,11-12]. However, some fat-storing species also caching food, such as arctic ground squirrels (Urocitellusparryii)[13], Richardson’s ground squirrels (Urocitellusrichardsoni)[14]and Columbian ground squirrels (Urocitelluscolumbianus)[8].
Daurian ground squirrel (Spermophilusdauricus) is a fat-storing hibernator mainly distributed in the Inner Mongolia/Xinjiang Region Palaearctic Realm[15]. They hibernate for about six months from late autumn to early spring of the next year in the field[15-16]. They store substantial body fat and double the body mass by increasing food intake before hibernation[17]. Captive individuals showed variation on hibernation patterns. The hibernation season differ among individuals from 20 days to 210 days, the depth and length of torpor bouts expressed also differ[18]. Our previous results showed that the body fat reserve plays an important role on hibernation expression. However, we have no idea if food availability would affect the hibernation pattern as a surplus energy. In this study, we investigated the hibernation expression and survival rates of captive Daurian ground squirrels. The squirrels were divided into 2 groups to test the dependency of hibernation pattern on food availability.
1 Materials and methods
1.1 Animals and housing
All animal procedures were approved by the Animal Care and Use Committee of Shenyang Normal University. 38 adult squirrels (26 females, 12 males) were captured in May from Gaolintun Pedigree Station (Livestock Breeding Station) (122.5°N, 43.9°E), Tongliao, Inner Mongolia, China, and raised in laboratory of Shenyang Normal University. They were housed individually in plastic cages (48×35×20 cm3) with wood shaving as bedding. Prior to hibernation, the squirrels were maintained under natural photoperiod and temperature, and provided with standard rodent pellets (maintenance diet) and wateradlibitum. Fresh carrots and Chinese cabbages were added during the first month of captivity. Body mass was measured and recorded weekly. The squirrels were transferred to a dark room (0L: 24D) with constant temperature (5±1)℃ when the body mass decreased and they showed torpor characteristics, such as body curving, reduced coordination and heterothermia. Cotton was added for keeping warm. The body mass was measured and recorded biweekly during hibernation.
The animals were divided into two groups after balancing sex and body mass: 1) food provided group (FP, 11 females, 6 males), the animals were provided with food and water ad libitum, and 2) food deprived group (FD, 15 females, 6 males) in which the animals were only provided with water.
1.2 Body temperature and definitions of hibernation events
Core body termperature (Tb) of the animals were recorded every 60 min by implanted data loggers Thermochron®iButton (Maxim/Dallas, DS1921L, range -40 ℃ to 85 ℃, 0.5 ℃ accuracy). The iButtons were sealed in a coating of Ethylene-Vinyl Acetate copolymer (EVA) and paraffin (v∶v=1∶5) and sterilized using 75% alcohol and iodophor prior to implantation[18-19].
We defined torpor as when theTbdecreased to or below 30 ℃ in Daurain ground squirrels[1,18-20]. Onset of hibernation season was defined as when the first timeTbfell below 30 ℃; the offsett of hibernation season was defined as the animals shown the final arousal and stay constant euthermic[18]. The torpid or euthermic states were observed and recorded twice a day with a infrared thermometer (TES-1327, accuracy of ±1 ℃) in combination with a small piece of paper and a little sawdust which were put on the body of each hibernating animal to avoid missing the arousal between the consecutiveTbmeasurements in order to test the food intake in the inter bout arousal. Euthermia was characterized by temperature readings 0~8 ℃ below 37 ℃, by call and by moving, and the paper dropped or sawdust lost means the animals had aroused after the last inspection.
1.3 Body mass and food intake
Both body mass and food were weighed by using an electronic balance(±0.01 g). Body mass was measured weekly for euthermic animals, and biweekly for torpid animals. Food intake was measured by weighing the pellets in the food box every day for euthermic squirrels. During the hibernation, the food intake was measured on the offset time of arousal. Three food control samples were weighted every day to calculate the water absorbed.
1.4 Energy availability estimation
The available energy during hibernation (Eh) only comes from the body fat (Efat) for the food deprived animals. The energy of consumed food (Efood) was added for the food provided to animals. The decrease of body mass was assumed to be lipids (body fat) consumption[4]. Both the consumed body fat and the food intake were calculated as energy (kJ). The caloric value of body fat was calculated as 39.356 kJ per gram fat as previously reported[4]. Caloric value of the rodent pellets was 17.82 kJ/g as measured by Parr 1 281 oxygen bomb calorimeter[21].The digestibility of food (Dfood) was 80.66% for Daurian ground squirrels according to our previous results[22]. Therefore, the energy can be calculated by using the following equations:
1.5 Statistical analysis
Data were analyzed using SPSS 13.0 software (SPSS Inc., Chicago, IL, USA). Prior to all statistical analyses, data were examined for assumptions of normal distribution using the Kolmogorov-Smirnov test and all distributions of the data were shown to be normal. Effect of food providing on over winter survival rate were analyzed by Crosstabs with Chi-Square tests. Differences in duration of hibernation season, total heterothermy and daily energy cost between the food provided and deprived animals were assessed by independent-samplet-test. Differences in body mass and total energy cost among the surviving and dead squirrels in the food provided and deprived groups were assessed by one-way ANOVA, followed by LSD post hoc tests. Pearson correlation analysis was used to detect the possible associations of hibernation duration with body mass. Data were expressed as the mean ±SEM.P<0.05 was taken as significant difference.
2 Results
2.1 Over winter survival rate
The survival rate of FP squirrels was 82.4% (14 of 17 squirrels), 39.5% higher than that of FD squirrels (42.9%; 12 of 21 squirrels;P<0.05)(Fig.1). In the FD group, 28.6% animals died during the early period of hibernation (18 days after transferred to cold room and deprived food), died in the late period of hibernation and had hibernated for at least 3 months.
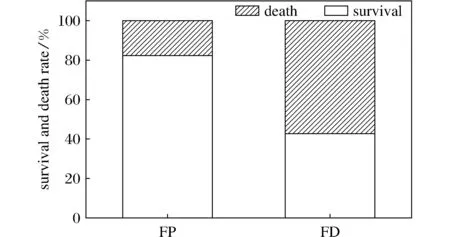
Fig.1 Survival and death rate of food-provided (FP) and food-deprived(FD) Daurian ground squirrels during the hibernation season
2.2 Food intake in the hibernation season
In the FP squirrels, 17.6% ingested more than 800 g(809.14~1 170.43 g) pellets during the hibernation season, 17.6%ingested more than 38.17~54.75 g, 1♀ ingested 13.63 g and 1♂ ingested 12.44 g and died during hibernation, the rest 52.9%(6♀, 3♂) including 2 died during hibernation ingested less than 10 g(0~5.38 g) pellets. The squirrels ingested food mainly in the preparation period prior to hibernation when the body temperature “test drop” and daily torpor were expressed, or in several inter-bout euthermia during the first or the last period of hibernation, which lasted longer than the middle period of hibernation(Fig.2). The squirrels did not ingested food in the middle period of hibernation when they expressed deep hibernation bouts. Except three animals, who expressed mainly daily torpor and fewer hibernation bouts, spent a smaller proportion of time in heterothermy, ingested food all the time during hibernation, the remaining 54.5%(6 of 11 survival individuals) of FP squirrels ingested 0.37 g to 10.43 g food in the first inter-bout arousal period after entering the deep hibernation, and 72.7%(8 of 11) ingested 0.27 g to 8.20 g food in the last inter-bout arousal period before emergence.
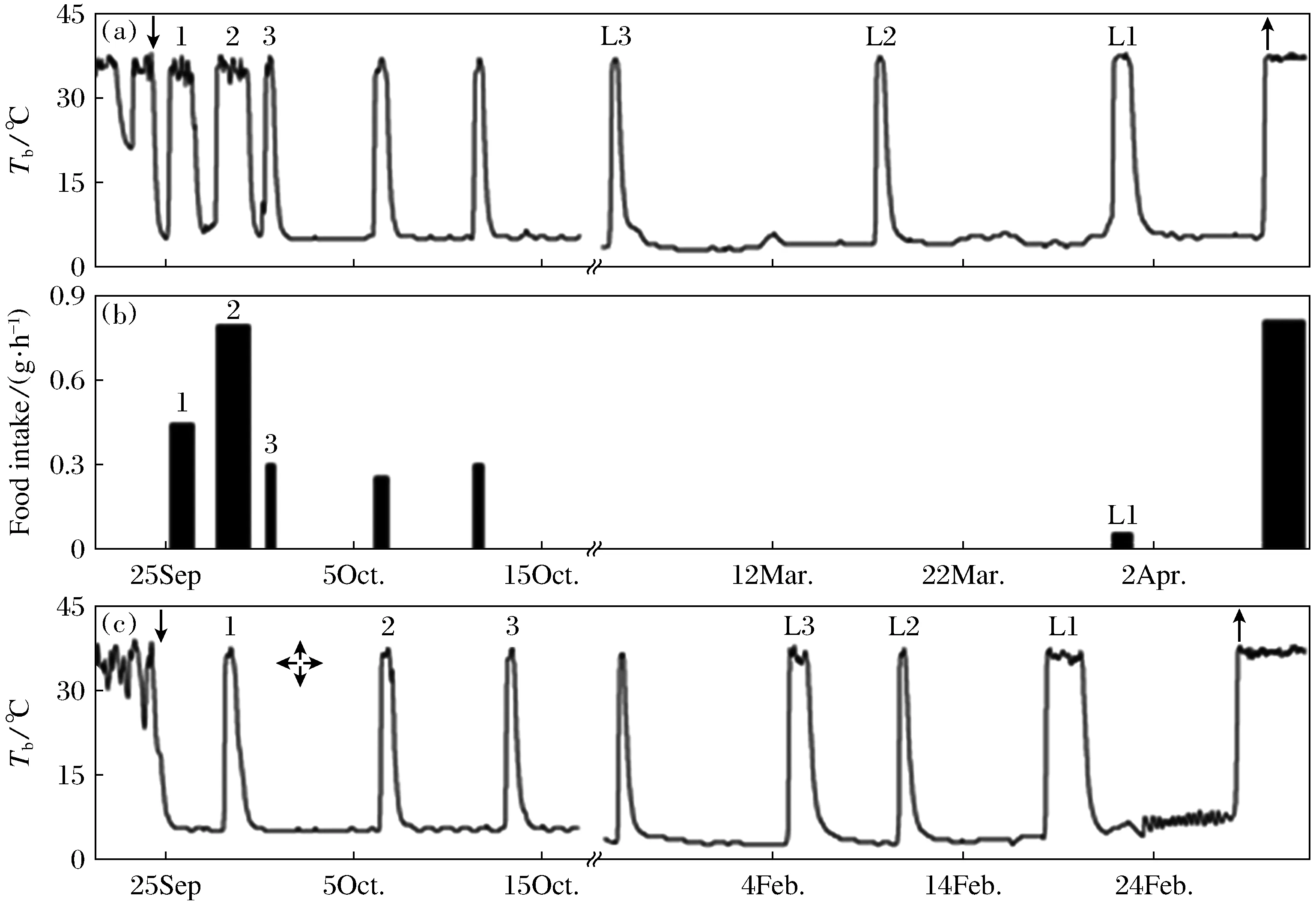
Fig.2 Body temperature of a food-provided (a) and a food-deprived (c) Daurian ground squirrels, and food intake (b) of the food-provided Daurian ground squirrels during the early and end periods of hibernation. The downward arrow (↓) indicates immergence, upward arrows (↑) indicate emergence, numbers 1,2 and 3 indicate the first,second and third inter-bout arousal after entering deep hibernation, respectively. L1, L2 and L3 indicate the first, second and third inter-bout arousal before emergence, respectively
The amount of food intake was not related to their body mass at immergence (P> 0.05,n=14) (Fig.3a), but positively correlated with the body mass at emergence (BMe) (r2=0.299,<0.05,n=14). The regression equation is BMe=0.062 2FI+169.65(Fig.3b).
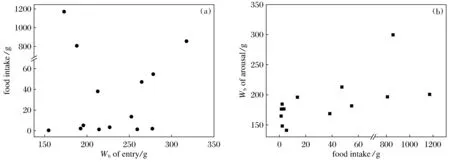
Fig.3 The relationship between food intake and body mass of entry and arousal
2.3 Body mass loss during hibernation
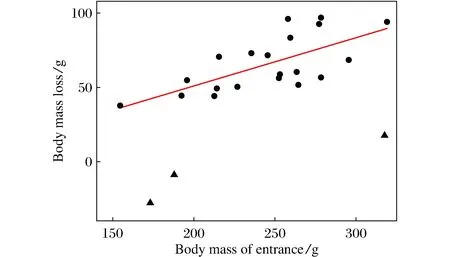
Fig.4 Effect of body mass at entry on the body fat consumption during hibernation
When initially transferred into the cold room, thebody mass of FP squirrels was 232.1±10.4 g(n=17), which was similar to that (230.0±10.7 g,n=21) of FD squirrels (t=0.139,df=36,P>0.05). However, when excluding the dead animals in the course of the experiment, the body mass at immergence (BMi) of FD squirrels (268.6±9.8 g,n=8) was significantly higher than that (229.0±12.6g,n=14) of FP squirrels (t=2.482,df= 20,P<0.05). However, there was no difference in the body mass at emergence between the two groups (183.0±11.3 in FP,196.1±9.7 in FD,t=0.782, df=20,P>0.05). The body mass loss (BMloss) during hibernation was 46.0±9.3g(n=14) in FP group and 72.5±5.4g(n=8) in FD group (t=2.028,df=20,P=0.056) (Tab.1). It positively correlated with the body mass BMi(except the three individuals that ingested a large amount of food during hibernation) with a regression equation of BMloss=0.324BMi-13.68(r2=0.43,P=0.001) (Fig.4).
In the FD group, the body mass loss in thedead squirrels (73.2±6.2 g;n=6) was similar to that in the surviving animals (72.5±5.4 g,n=8)(P>0.05). In addition, most animals died at late phase of hibernation had significent lower body mass (123.8±1.7,n=6) than survived individuals (196.1±9.7 g,n=8;P<0.05) (Table 1). It can be speculated that the critical body mass of over winter survival without supplemental food should be the minimal survival body mass at immergence and the body mass loss during the winter in FD animals.
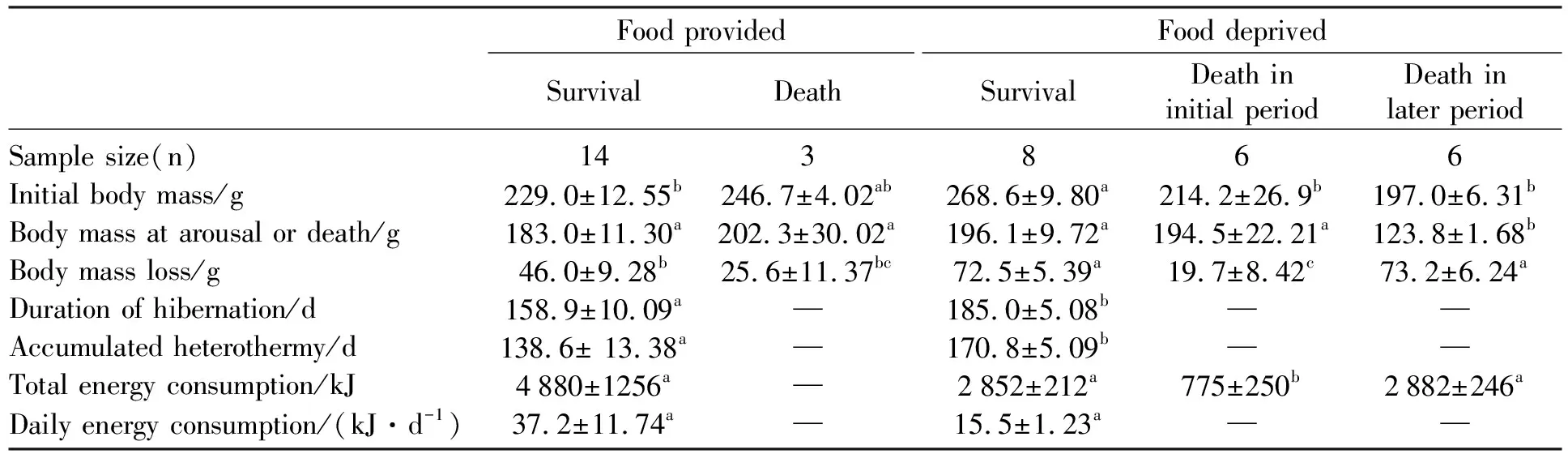
Tab.1 Energy reserves and hibernation expression in food-provided and -deprived Daurian ground squirrels
Data are expressed as the mean±SEM. The same letters in a row indicate no significant differences between the two groups assessed by independent-sample t-test, or among the four or five groups assessed by one-way ANOVA, followed by LSD post hoc tests. Data did not labeled with same letters in a row indicate significant difference.
2.4 Relationship between energy availability and the hibernation pattern
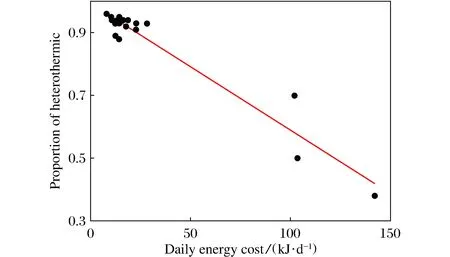
Fig.5 Relationship between daily energy consumption and proportion of heterothermy
The duration of hibernation season in FD squirrels was 185.0±5.08 days, which was significantly longer than 158.9±10.09 days in FP squirrels (t=2.314,df=18.256,P<0.05). Accumulated heterothermy in FD animals was 170.8±5.09 days, which was also significantly longer than that of 138.6±13.4 days in FP animals (t=2.253, df=16.399,P<0.05). In FD squirrels, the total energy consumption during hibernation was 2 852±212 J, which was similar with 4 880±1 256 J in FP animals (t=1.592,df=13.731,P> 0.05). The daily energy expenditure in FD animals was 15.5±1.23 J/day, which was only 41.7% of that in FP animals (37.2±11.74 J/day;t=1.838,df=13.284,P>0.05) (Table 1). The daily energy consumption in two groups was negatively correlated with the total heterothermic time (Tb< 30 ℃) with the regression equation ofy=-0.004x+0.994 (r2=0.929,F=277.304,P<0.001)(Fig.5). The three animals that ingested food continually spent much less proportion of heterothermy (53±0.46%) during hibernation than the others (93±0.46%) (t=11.81,df=20,P<0.001).
When food was sufficient, both the duration of hibernation and the accumulated heterothermy were not correlated to thehad no significant relationship with BMi(Fig.6a,6c). By contrast, when food was deprived, the duration of hibernation was negatively correlated to the BMiwith the regression equation ofy=-0.41x+296.12 (r2=0.58,P<0.05,n=8) (Fig.6b). The accumulated heterothermic time during hibernation was also negatively correlated to the body mass at entrance with the regress equation ofy=-0.38x+273.53(r2=0.47,P<0.05,n=8) (Fig.6d).
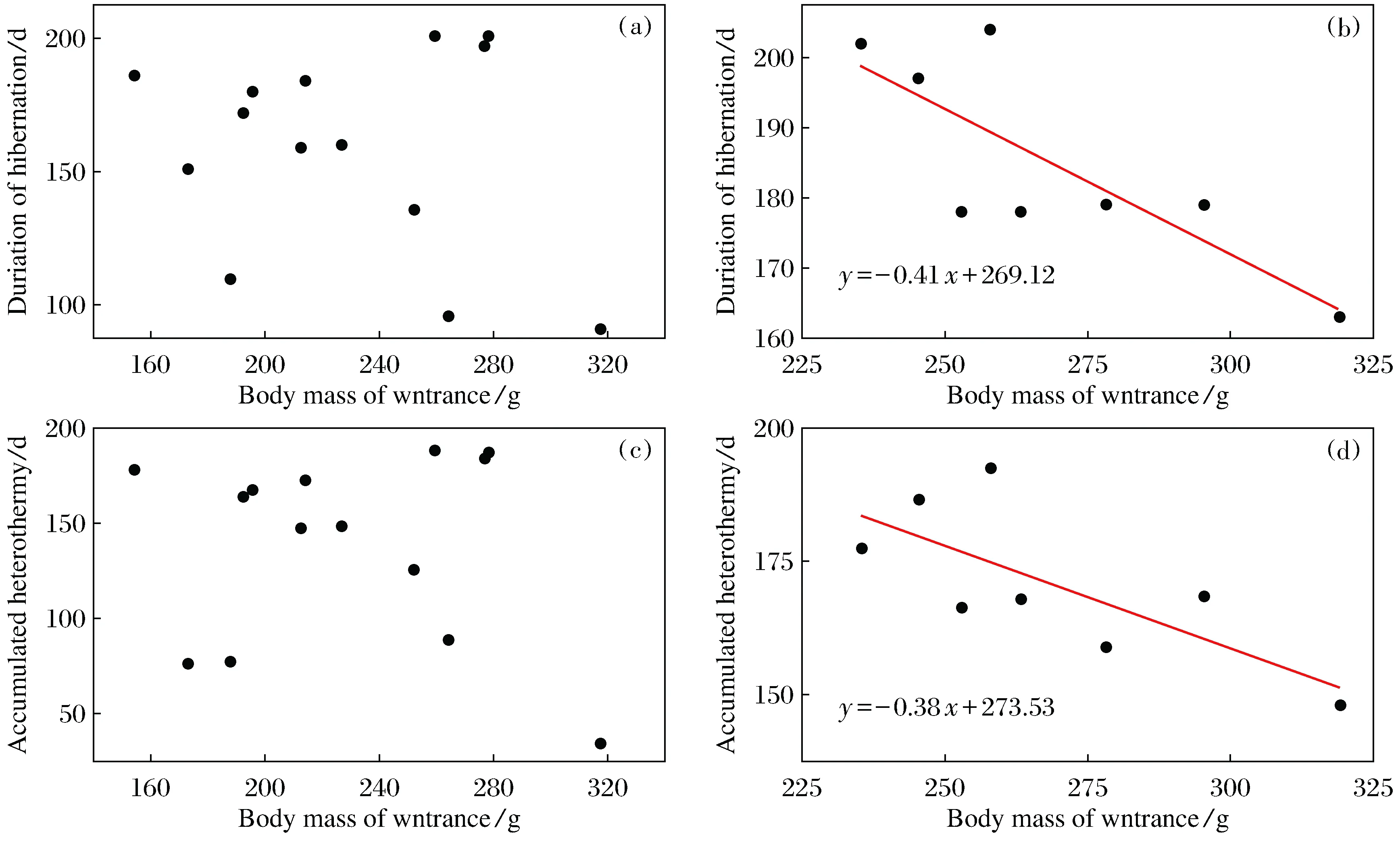
Fig.6 Relationships between hibernation patterns and body mass at entry in Daurian ground squirrels a and c indicate food provided animals, b and d indicate food deprived animals
3 Discussion
In the present study, the Daurian ground squirrels with food deprivation had higher morbidity during hibernation season. It suggests that, in lab condition, the Daurian ground squirrels need some supplemental food to survive the hibernation. The feeding behavior mainly occurs in early and late period of hibernation indicated the food intake might due to the adaptation of digestive tract. Besides, the early and late periods of hibernation may also concern with the transition of physiological status between hibernating and active seasons.
The Daurian ground squirrel is an omnivorous species living mainly on plant roots, stems, leaves, flowers and seeds, and occasionally on animals such as insects[15]. The food-storing or caching behavior has not been reported in this animal species previously. In this study, about half of the animals took food in FP group ingested food during the inter-bout arousal in early and late period euthermia in course of hibernation, but mainly in the first several and the last inter-bout arousal periods. We did not observe the feeding behavior in the middle period of the hibernation.
In addition, corn seeds were found in a male Daurian ground squirrel’s burrow in the field in May 2006 (Fig.7). We suppose that these seeds may be stored in the previous autumn. Through we need more evidence on the feeding behavior of Daurian ground squirrels in the field, the feeding behavior form the other species of ground squirrels[8,13,23]indicated that the Daurian ground squirrels who did not fattening enough may also need some food as supplemental energy. The cached food will be consumed before emergence to replenish their body energy reserves and to meet the reproductive requirements such as finding spouses and avoid predation in the coming summer[14,23-24].

Fig.7 Corn seeds in a male Daurian ground squirrels’ chamber in the field in May 2006 (Arrows show the corn seeds)
Compared to thesimilar sized fat-storing hibernator, euthermic intervals are four times greater and the duration of torpor bouts half short in food-storing rodents[25]. In fat-storing species, the digestion system undergoes profound atrophy during hibernation[26-29], and the intestinal absorption at torpor is negligible at 7 ℃[26]. Both the short euthermic intervals and the function degenerating are not favor of food consumption in fat-storing hibernators. The mass of digestive tract (fresh mass of intestine and stomach) during the mid period of hibernation is lower than that during the euthermic season (unpublished data). These changes may be correlated with the cease of ingestion and assimilation during hibernation in Daurian ground squirrels. However, the atrophy in digestive system and suppression in feeding may be gradually developed accompanying with the entry into hibernation and restored before emergence. In fact, the food intake of Daurian ground squirrels[30-31]and woodchucks[32]decreased before their fattening is over. Perhaps these progressive changes make it possible to ingest food in the first several and the last one inter-bout arousal periods. Obviously, feeding behavior and energy homeostasis are controlled by the central nerve system and multiple peripheral signals. However, the detailed mechanisms need to be further illustrated, especially in fat-storing hibernators that annually undergo fluctuation not only in food intake but also in metabolic transfer[33-35].
The absolute amount of available energy depends mainly on body mass in hedgehogs[4]. Evidences from bats and squirrels have shown that mass loss during hibernation is positively correlated with amount of fat reserves at the onset of hibernation[6,36-37]. Field data indicate that the maximum body fat reserve of fat-storing hibernators is about 40%~50% of total body mass[4,11], which is increased around 3-fold than the minimum energy (including that needed for periodic arousals) required for hibernation in these species[6,11]. Under natural condition, many individuals may store less energy than they needed[11]. In this study, body mass loss in the Daurian ground squirrels was (72.5±5.4) g, which was 27.1% of the body mass at immergency in food-deprived animals. The daily energy expenditure was (15.5±1.2) kJ/d, equivalent to (0.39±0.03) g fat per day. This amount is similar to the consumption of 6.5 mg fat/d for little brown bats (Myotis lucifugus) with body mass of 10 g, and 0.7 g fat/d for arctic ground squirrels with body mass of 1 000 g. Some of the food-deprived animals died during hibernation. The surviving squirrels were heavier than the dead squirrels when they enter into hibernation, while consumed the same amount of body mass as the survival animals (Table 1), implying the available energy deficiency might be the cause of death.
The calculation method of energy cost in this study was an estimated one.Besides fat consumption, change in body mass during hibernation also includes hydrolysis of a small portion of muscle protein and glycogen[38]. In addition, the digestibility during hibernation may different from that measured druing active phase. In this study, those differences were ignored to make the calculation possible.
There isindividual variation on the responses to food availability during hibernation season. Most of the animals did not ingest food at all and expressed typical deep hibernation bouts, while some (3/17) ingested food continually, lost less body fat, expressed shorter hibernation and daily torpor bouts, spend less time in heterothermy and more time in euthermia, and consumed more total energy than the non-feeding animals in hibernation. The species used both food-and fat-storage may change their characteristics of hibernation when food was continually supplied. For example, the captive Japanese dormice (Glirulus japonicus) would switch from long, multi-day torpor to daily torpor[5,8]and garden dormouse (Eliomys quercinus) would become free of torpid at all[8,39].
4 Acknowledgments
We would like to thank Dr. Qingsheng Chi at Institute of Zoology, Chinese Academic of Science and Dr. Quansheng Liu at Institute of Insect, Guangdong Province for their valuable comments on the manuscript. This study was financially supported by the National Natural Foundation of China(No. 31170380).
Our study shows thatthe Daurian ground squirrels with food deprivation had higher morbidity during hibernation season. The feeding behavior mainly occurs in early and late period of hibernation. The survival individuals in FD group were heavier than the dead individuals. The FD squirrels had longer duration and accumulated heterothermic time of hibernation than FP squirrels. These results indicated that, in lab condition, the hibernation pattern of the Daurian ground squirrels was affected by supplemental food.
