Effect of magnesium on FOX-7 and its tautomers-A DFT treatment
2018-07-06Lemirker
Lemi Türker
Middle East Technical University,Department of Chemistry, Üniversiteler,Eskis¸ehir Yolu No:1,06800 Çankaya,Ankara,Turkey
1.Introduction
FOX-7 is structurally 1,1-diamino-2,2-dinitroethylene(DADE,DADNE)[1].It was synthesized in 1998 by members of the Swedish Defense Research Agency(FOI)[2,3]and its explosive potential was thoroughly investigated[4-18].It was also synthesized by the nitration of 4,6-dihydroxy-2-methylpyrimidine followed by hydrolysis[19].
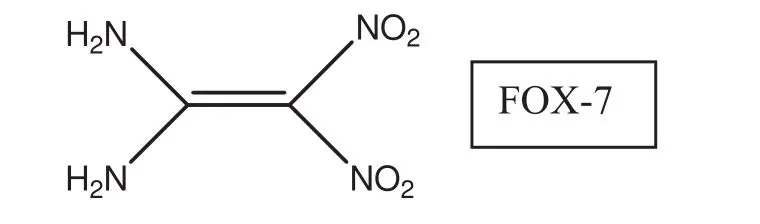
It is a novel high-energy insensitive material having good thermal stability and low sensitivity.It exhibits excellent application performance among the insensitive ammunitions and solid propellants.Although FOX-7 is simple in molecular composition and structure,its chemical reactivity is abundant and surprising,including salification reaction,coordination reactions,nucleophilic substitutions,acetylate reactions,oxidation and reduction reactions,electrophilic addition reactions[20,21]etc.
Interestingly enough,FOX-7 has the same C/H/N/O ratio as RDX or HMX but no structural resemblance exists among them.FOX-7 is much less sensitive than RDX(in terms of impact,friction,and electrostatic discharge sensitivities)[22].
FOX-7 possesses many polymorphic forms that theα-form reversibly turns intoβ-form by heat treatment[23,24].At higher temperature,β-polymorph undergoes an irreversible conversion to γ-phase which decomposes at 504 K[23].Its decomposition has been extensively searched[25].The effect of high pressure on the crystal structure of FOX-7 was studied[26].
FOX-7 is an attractive ingredient for application in high performance insensitive munition(IM)compliant explosive recipes.FOX-7 also possesses the ability of increasing the burning rate in propellants that is why it is of interest for high performance propellants[1].
Several FOX-7 based propellant formulations have been studied in order to obtain a minimum or reduced smoke composite propellant with inherent IM-properties[27].
Thermo chemical calculations show that PBX's based on FOX-7 and energetic binders could serve as a replacement of Comp-B even at rather low solid loadings.A plastic bound explosive based on FOX-7 and an energetic binder have been prepared,[28].
Effects of epoxidation and nitration on ballistic properties of FOX-7 were investigated within the realm of density functional theory(DFT)[29].Various ground state properties of FOX-7 were calculated based on B3LYP/aug-cc-pVDZ predictions[30].
Fang and McLuckie investigated Laser ignitibility of FOX-7 in order to achieve the direct optical ignition of an insensitive explosive.Such a process will obviously add more safety features to insensitive munitions(IM)or explosive devices[31].
On the other hand,metalized explosives have been used in various formulations since the beginning of the last century.Aluminum powders are added to explosives in order to enhance their blast and heat effects,as well as to increase the bubble energies in underwater explosions[32-35].Magnesium can also exhibit effects in formulations of composite explosives similar to aluminum.
Recently,some novel derivatives of FOX-7 and their properties as energetic materials have been reported[36,37].In the present study,the effect of magnesium atom on FOX-7 and its tautomers has been studied within the framework of density functional theory(DFT).
2.Method of calculation
Geometry optimizations of all the presently considered structures leading to energy minima were initially achieved by using MM2 method followed by semi-empirical PM3 self-consistent fields molecular orbital(SCF MO)method[38,39]at the restricted level[40,41].Subsequent optimizations were achieved at Hartree-Fock level using various basis sets.Then,geometry optimizations were managed within the framework of density functional theory(DFT)using B3LYP functional[42,43]at the level of 6-311++G(d,p).The exchange term of B3LYP consists of hybrid Hartree-Fock and local spin density(LSD)exchange functions with Becke's gradient correlation to LSD exchange[43,44].Note that the correlation term of B3LYP consists of the Vosko,Wilk,Nusair(VWN3)local correlation functional[45]and Lee,Yang,Parr(LYP)correlation correction functional[46].Presently,the vibrational analyses have been also done at the same level of calculations which had been performed for the optimizations.The total electronic energies(E)are corrected for the zero point vibrational energy(ZPE)to yield Ecvalues.The normal mode analysis for each structure yielded no imaginary frequencies for the 3N-6 vibrational degrees of freedom,whereNis the number of atoms in the system.This indicates that the structure of each molecule corresponds to at least a local minimum on the potential energy surface.All these calculations were done by using the Spartan 06 package program[47].
3.Results and discussion
Geminally linked two nitro and two geminal amino groups embedded into the structure of FOX-7(of which the former and later ones are electron attractors and electron donors,respectively)should generate an effective pull-push type(interacting resonance)system in theory[48].This property of the molecule not only dictates the chemical and physical properties of FOX-7(such as tautomerism)but also implicitly is responsible for its ballistic behavior.Tautomers in explosive material may act as additives(depending on their percentages)and influences the ballistic properties,stability,aging etc.
3.1.FOX-7 tautomers
The mesomeric structures of FOX-7 shown below enable one to predict the possible existence of 1,5-proton tautomerism[49-51]which should depend on various factors(temperature,solvent etc.).Note that FOX-7 does not have perfectly coplanar nitro and amino substituents,however they are in partial conjugation with each other as shown below.
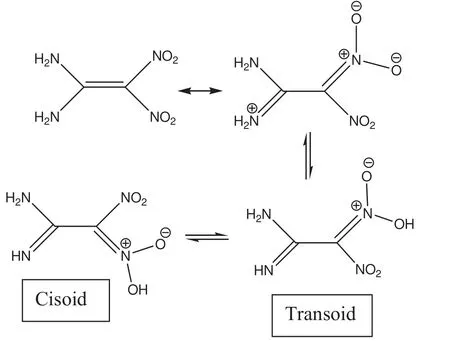
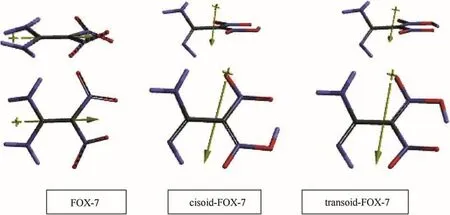
Fig.1.Optimized structures of FOX-7 and its tautomers(side and top views).
Possible free rotation about the C-C bond in the tautomers of FOX-7 results incisoidandtransoidconformers(namelys-cisands-trans)of the tautomeric form.Fig.1 shows the optimized structures of thecisoidandtransoidforms of 1,5-proton tautomers of FOX-7.The parent compound and the tautomers are not planar structures.FOX-7 has of f-plane nitro groups.Whereas the tautomers have highly oblique amino groups with respect to the planar NO2moieties.Note that the proton shifted to the nitro group prefers to be nearby the other nitro group in both thecisoidandtransoidforms(Fig.1).Also,it is worth mentioning that in both cases the remaining hydrogen atom on the imino nitrogen(originally amino group)is not face-to-face with the other amino group but oriented next to the other NO2in thetransoidform and in thecisoidcase it is towards the nitrogen of the nitro group(isonitro group)where the migrated hydrogen has been accommodated(see Fig.1).Note that the direction of dipole moments of the tautomers are drastically different from that of FOX-7.
Fig.2 displays the bond lengths of FOX-7 and itscisoidandtransoidtautomers.As expected C=C bond of the parent compounds gets longer in the tautomers(turns into C-C bond)and C-N bonds affected by tautomeric shift become shorter(forms C=N bond).
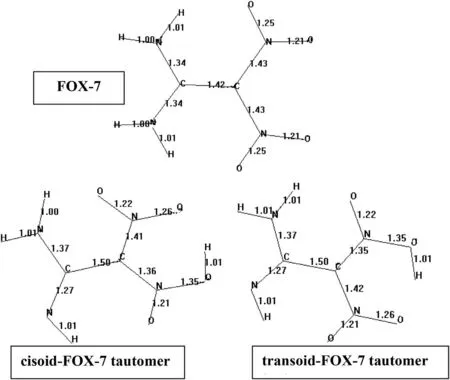
Fig.2.Bond lengths(Å)ofFOX-7 and its tautomers(σ-skeletons considered).
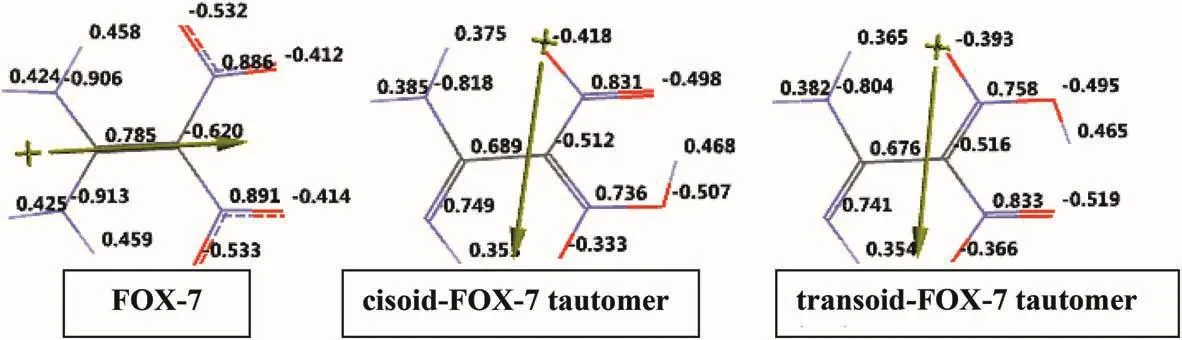
Fig.3.Electrostatic charges(ESP)on the atoms(in esu).
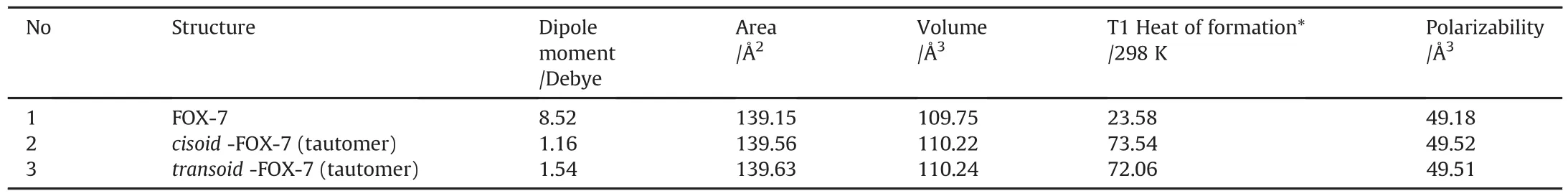
Table 1Some properties of FOX-7 and its tautomers.
Fig.3 shows the electrostatic charges(ESP)of the tautomeric systems considered.The ESP charges are obtained by the program based on a numerical method that generates charges that reproduce the electrostatic potential field from the entire wave function[47].Note that the amino group which is not involved in the tautomerism also lost some electron population as compared to the respective values in FOX-7.The carbon atoms in all these structures have opposite but unequal charges engendered by the substituents(the amino and nitro groups)which have adverse donor-acceptor characters.Note thatcisoidandtransoidnature of the structures greatly affects the charge distribution,even the same types of atoms are considered.
Some properties of FOX-7 and its tautomers are presented in Table 1.Rather high dipole moment of the parent molecule has been highly diminished in the tautomers.The heat of formation(ΔHf)values calculated by T1 method[52,53]reveal that structures 1-3 are all endothermic structures following the order of 1< 3<2.Whereas,PM3//B3LYP/6-311++G(d,p)type calculations yieldΔHfvalues as-27.71,49.41,and 76.31 kJ/mol for structures 1-3,respectively(the same order as given above).The experimental value obtained by bomb calorimetry has been reported to be-32 kcal/mol(-133.88 kJ/mol)[8]which is highly different from the theoretical predictions.
Table 2 includes the total electronic energy(E),zero point vibrational energy(ZPE)and the corrected total electronic energy of the tautomers.The data in the table reveal that the stability order is 1> 3>2 which is opposite to the order of endothermicity.As expected from Fig.2,possibility of hydrogen bonding exists in the tautomers.The distance between the NO2oxygen and the hydrogenof isonitro group is 1.50 Å in both thecisoidandtransoidtautomers.
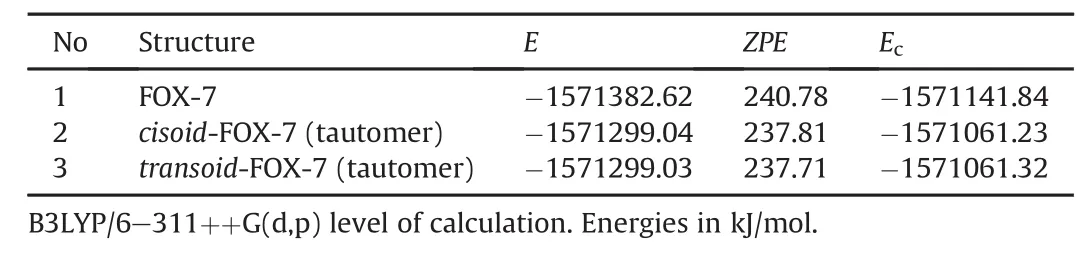
Table 2Some energies of FOX-7 and its tautomers.
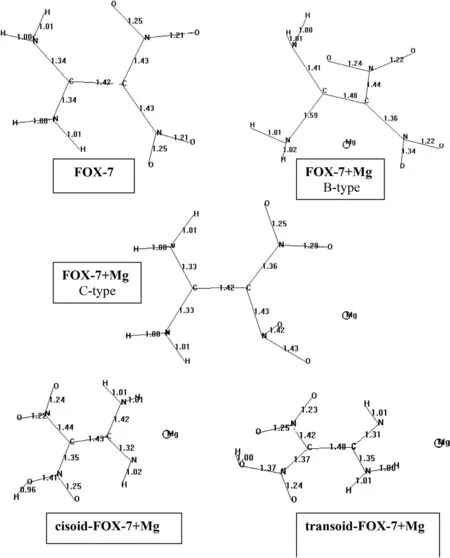
Fig.5.Bond lengths(Å)of the structures(σ-skeletons considered).
3.2.FOX-7 magnesium composites
Mg,B and Al like elements are common ingredients of some composite energetic materials[22].They contribute the heat evolved while they are oxidized.However,most of the explosives contain nitro group(s)which is/are rather strong oxidizing moiety in general.On the other hand,those elements,especially Mg is an easily oxidizable metal.Therefore,some interaction of Mg with NO2having molecules are expected which may affect the ballistic properties as well as the shelf-life of those composite type explosives.It is to be mentioned that the oxidation power of NO2group depends on its location in a molecule where it is attached.Any conjugative effects present and involving the nitro group(s)may highly alter that power.On the other hand,FOX-7,an explosive having both electron donor and acceptor groups is an interesting molecule.The electron demand of nitro groups in FOX-7 structure should,up to a certain extent,be satisfied by the amino groups present.Hence,the consequent interaction of Mg atom with FOX-7 molecule should be worth investigating.
It is known that 1,5-tautomers are less likely compared to 1,3-tautomers.In the case of FOX-7,1,3-tautomers are structurally impossible and contribution of 1,5-tautomers should be then less important.Due to that fact,in the present treatment the interaction of them with Mg has not been considered.
Mg atom may approach FOX-7 molecule roughly resulting mainly three types of topology in space(shown below).Presently only B-and C-type optimized composites computationally have been obtained.A-type composite is unlikely because no stabilizing interaction develops with NH2groups which are electron deficient in A-type due to the electron attracting type mesomeric effects of NO2groups.Indeed,all the attempts of optimization of the structure,initially constructed as A-type,turns to B-or C-type.In B-and C-type composites both the Mg atom and NH2groups all have electron donor type character.

Fig.4 shows the optimized structures of FOX-7 and its magnesium composites.Note that they are all isomeric.Presently,C2H4N4O4+Mg composites involving FOX-7 and its tautomers are considered.They contain 13.9%Mg by weight.Inspection of fig.4 reveals that in B-type composite,FOX-7 structure becomes disturbed and C-NH2bond is elongated(1.59 Å,see Fig.5).The distance between Mg and the nearby NH2nitrogen is 2.07 Å whereas the distances of Mg to oxygen of the nearby NO2groupand to nitrogen of that group are 1.93 Å and 2.73 Å,respectively.The charges on the nitrogen atoms of the considered amino and nitro groups are-0.906 esu and+0.886 esu,respectively in FOX-7.Whereas,in the Mg composite the charges change to-0.925 esu and+0.503 esu,respectively.Note that Mg atom acquires+1.3 esu of charge which is indicative of transfer of some electron population from the magnesium atom.On the other hand,Mg nearby the nitro groups(C-type composite)cannot perturb the FOX-7 structure to a great extent,the molecule is nearly planar but some distortion of the NO2substituents occurs.Interesting point is that C=C bond which is 1.42 Å in FOX-7 elongates to 1.48 Å in B-type composite.Also C-NH2bonds are elongated from 1.34 Å to 1.41 Å and 1.59 Å.It means that parallel to decrease of the electron demand of NO2group(affected by Mg atom in the composite)no longer electrons of the amino groups are needed as compared to FOX-7.Whereas,in C-type composite,C=C bond length retains its value as it is in FOX-7 but one of C-NO2bond shrinks from 1.43 Å to 1.36 Å.In the case of composites of the tautomers,Mg atom prefers to be on the side of amino/imino nitrogens and the organic moiety is not completely planar.In thetransoidtype composite C-C bond is 1.48 Å which is longer than the respective bond in thecisoidform.
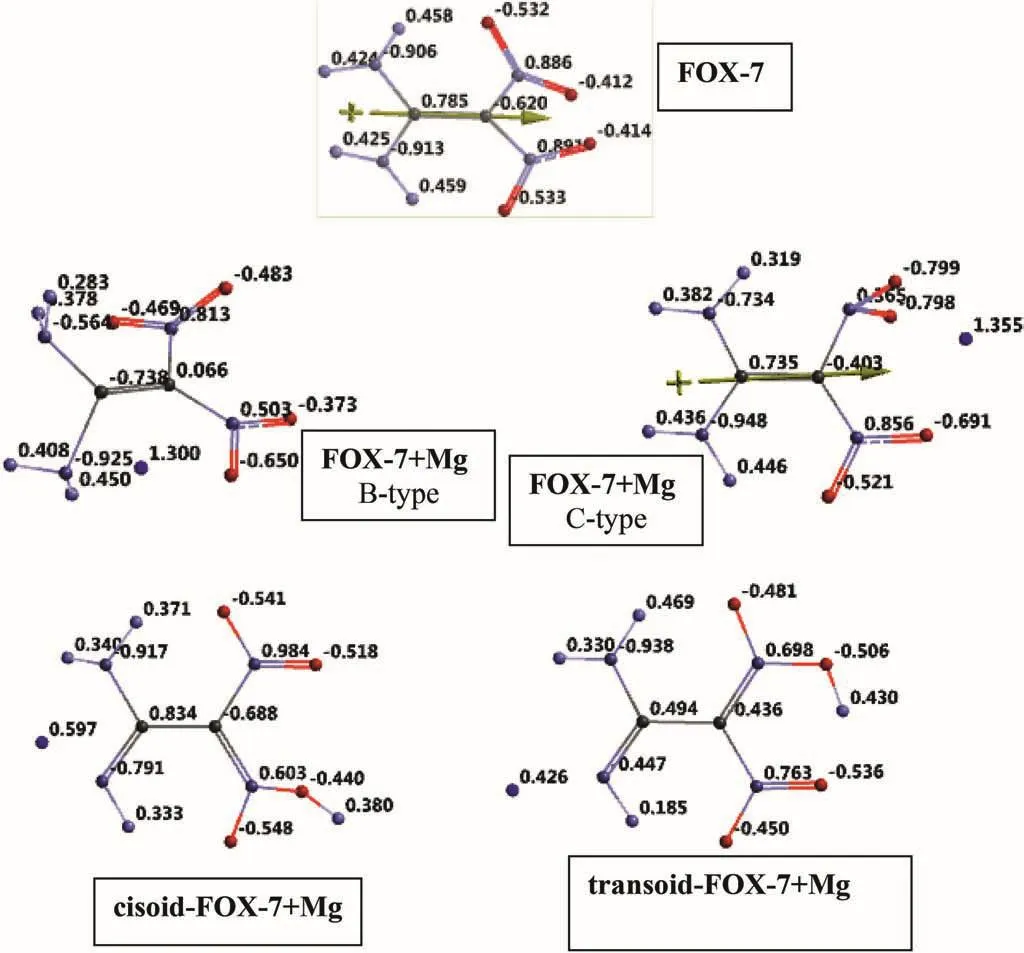
Fig.6.Electrostatic charges(esu)on the atoms of FOX-7 and the composite systems considered.
Fig.6 shows the electrostatic charges(EPS)on the atoms of FOX-7 and its composites considered.In FOX-7 structure,the carbon atom linked to amino group has positive charge as expected.In every composite case the Mg atom is positively charged.Inspection of the charges of atoms of nitro groups and the Mg atom indicates that in the FOX-7+Mg composites(B-and C-types)some electron population has been transferred to one of the NO2moieties so partial reduction of nitro group is to be mentioned.The charge of Mg in each case is about 1.3 esu.In B-and C-types the carbon atom linked to amino group have negative and positive charges,respectively.When the over all charges of the nitro groups are considered,they are positive(0.058 and 0.056 esu)for FOX-7 whereas they are negative for B-type(-0.139 and-0.520 esu)and C-type(-1.032 and-0.356 esu)composites,namely certain amount of electron population has been transferred to nitro groups in the composites.This effect seems to be less in the composites of the tautomers that is why Mg atom has been charged less than unit of charge.Note that in the tautomers one of the nitro groups is in theaci-form and less electron demanding.Of course this type of reduction of NO2group(s)affects the ballistic properties of the composites compared to FOX-7 although some desirable heat contribution occurs by the presence of Mg atom.However,one might conjecture that the heat effect of already partially oxidized Mg should not be as high as the neutral Mg atom.
Table 3 shows some properties of the Mg composites of FOX-7.B-type composite is characterized with comparatively high dipole moment than the other isomeric composites.
Table 4 includes some energies of the composites.The stability orderis 5>4>7>6.The sequence indicates that Mg atom prefers to C-type orientation where it interacts with geminal nitro groups.Magnesium atom transfers some electron population to NO2group(s)whereas such a transfer process is unlikely for NH2groups.Note that amino nitrogen has negative oxidative valance[54].Note that magnesium atom can form complexes with oxygens of alcohols and ethers(e.g.,Grignards).Complexation with oxygen(s)of the nitro groups might arise due to the pull-push character of FOX-7 molecule.As a result of this effect oxygen atoms of the nitro group should have rather high electron density to act as ligands.As for the composites of the tautomers,Mg atom prefers to be nearby the imino nitrogen atom.
Fig.7 shows the IR spectra of FOX-7 and its Mg composites.The asymmetric and symmetric N-H stretchings of FOX-7 occur at 3675 cm-1and 3468 cm-1,respectively which are almost disappeared(very weak)in B-type composite.Whereas in C-type,they appear at 3689 cm-1(medium,asym)and 3643 cm-1(medium,sym).In the region of 1500-1600 cm-1C=C and N=O stretchings coupled with some bendings occur for FOX-7 and its B-and C-type composites as well.
In thecisoidtype composite O-H stretching occurs at 3743 cm-1(weak)whereas in thetransoidtype it is at 3116 cm-1(weak).In both types composites(cisoidandtransoid)all sorts of N-H stretchings are weak and at 3400-3600 cm-1.Obviously in the case of tautomer composites C=C stretching disappeared.Instead various C=N stretchings should appear but they are superimposed with bendings occurring below 1600 cm-1.
Table 5 tabulates the highest occupied molecular orbital(HOMO),lowest unoccupied molecular orbital(LUMO)energies and the frontier molecular orbital(FMO)energy gaps(Δε)of the structures considered.The data reveal that the tautomers have lower HOMO and LUMO energies as compared to FOX-7.The unequal extent of energy lowering of HOMO and LUMO causesΔε value of tautomers to be less than the respective value of the parent compound,namely theΔε order is 1> 3>2.
As for the composites,the presence of Mg atom raises up the HOMO energy level compared to FOX-7 and its tautomers.The HOMO energy order is 3< 2<1< 4<5< 6<7,whereas the LUMO energy order is 2< 3<7< 4<6< 1<5.Consequently,the overallΔεsequence is 1 > 3>2 > 5>4> 6>7.The data also reveal that the tautomers have much narrow HOMO-LUMO energy gap as compared to FOX-7.Moreover,Mg in the tautomer composites is more influential on narrowing of the FMO energy gap as compared to B-and C-type FOX-7 composites.It is known that as the HOMOLUMO energy separation decreases,the sensitivity to impact increases[55-59].Hence,composite 7(transoid-FOX-7+Mg)in Table 5 should have higher impact sensitivity than the others.
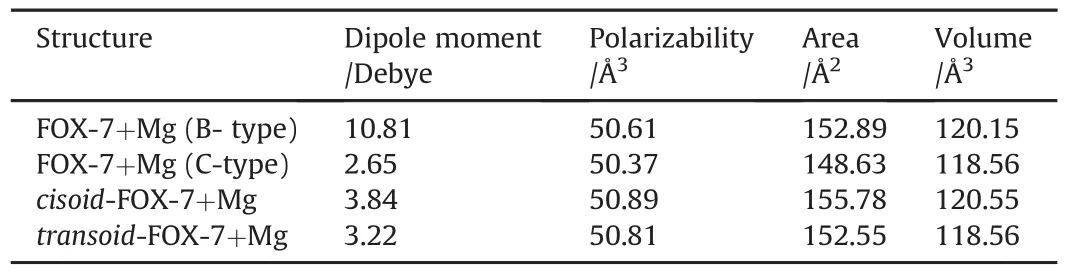
Table 3Some properties of FOX-7+Mg composites.
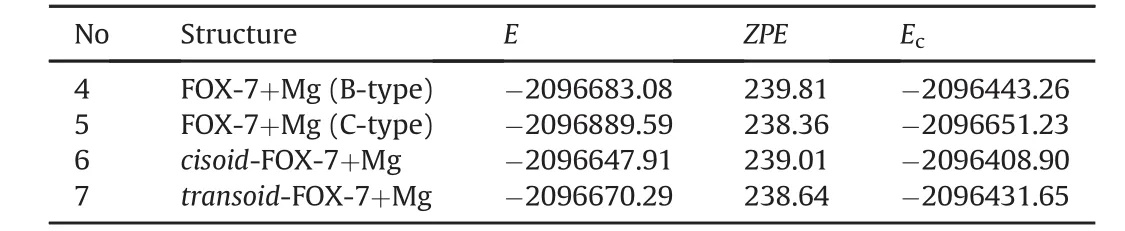
Table 4Some energies of FOX-7+Mg composites.
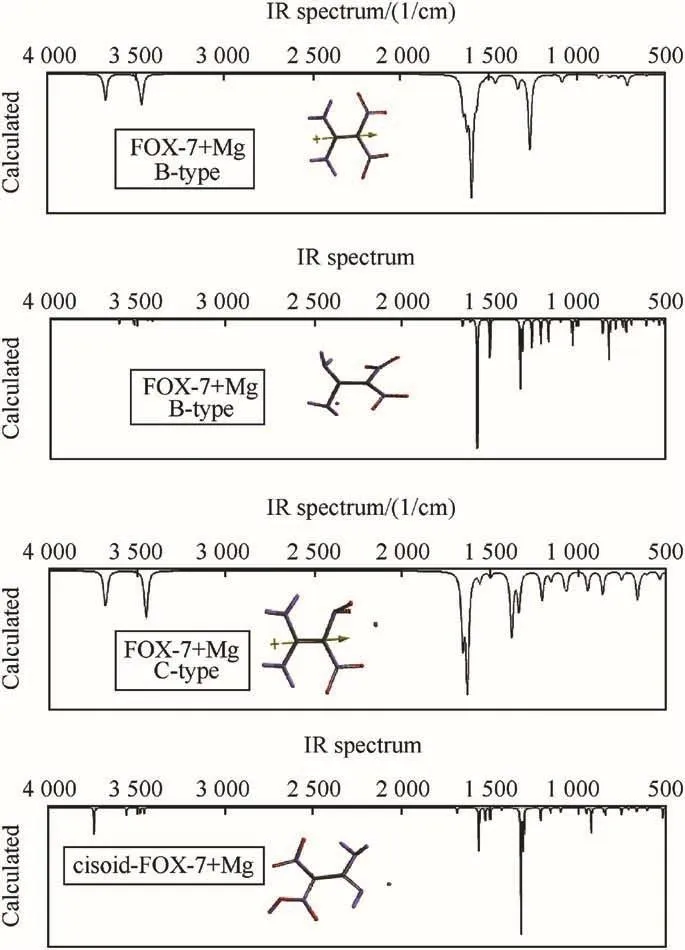
Fig.7.Calculated IR spectra of the composite structures considered.
Table 5The HOMO,LUMO energies and FMO energy gaps(Δε)of the structures considered.
Fig.8 shows the calculated UV-VIS spectra(time-dependent DFT)of the systems considered.The presence of Mg causes not only a bathochromic effect compared to FOX-7 spectrum but also appearance of new peaks in the visible region.It is to be noticed that some absorptions,especially in the case of composites of the tautomers,shift beyond the visible region towards the near IR.All these effects indicate that the HOMO-LUMO energy gap is narrowed by the presence of Mg atom.It is known that narrowing of the FMO energy gap acts as a positive contributor to increase the impact and shock sensitivity[56-59].
Fig.9 shows the HOMO and LUMO patterns of FOX-7 and the composites presently considered.Theπ-symmetry of FOX-7 is disturbed in the composites up to a certain extent.Although,in B-type composite the NH2groups contribute to the HOMO there is almost no contribution to the HOMO and LUMO in C-type.In the case of composites of the tautomers the amino groups again have some contribution to both the HOMO and LUMO.Generally,the HOMOs and LUMOs are characterized with a large contributions located around Mg atom.All is indicative of the electron donor role of Mg in the system(see Fig.6 for the electrostatic charges).
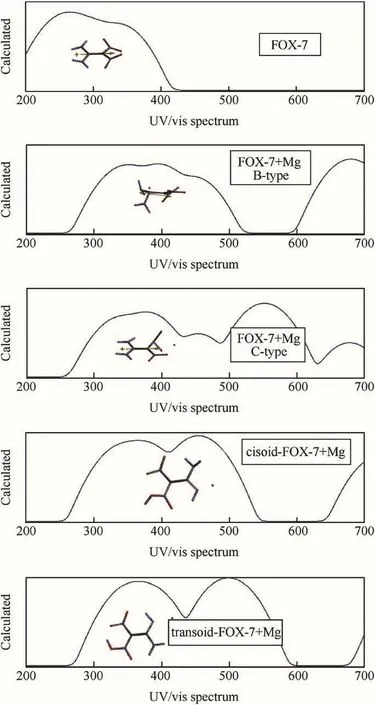
Fig.8.Calculated UV-VIS spectra of the structures considered.
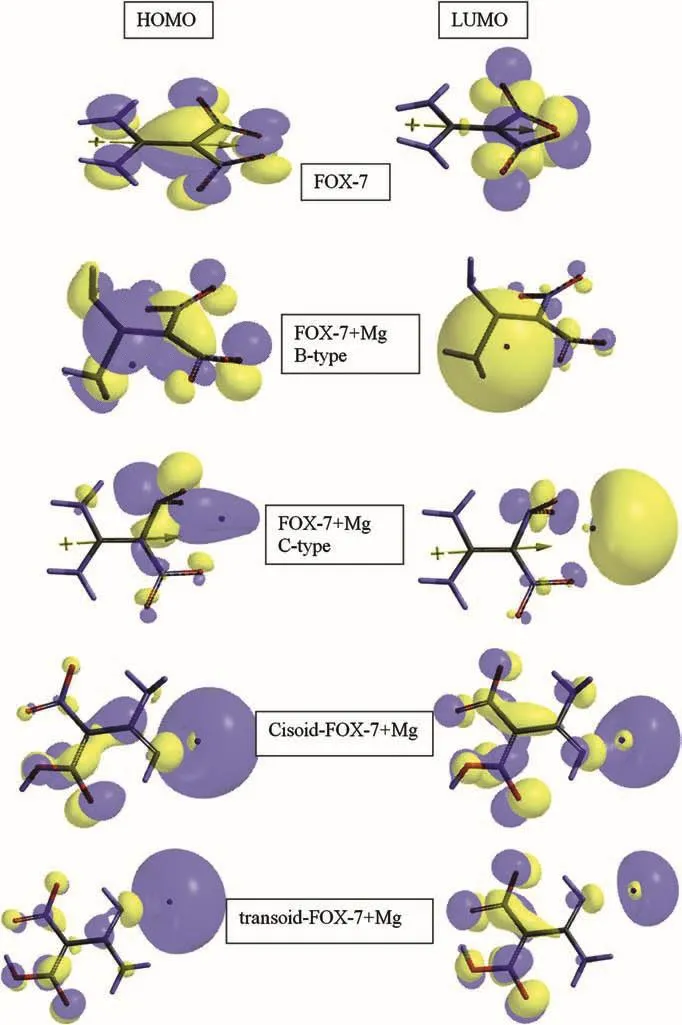
Fig.9.The HOMO and LUMO patterns of the composite structures considered.
4.Conclusion
Presently,within the limitations of DFT,FOX-7 and itscisoidandtransoid1,5-tautomers and all their Mg composites have been investigated.The tautomers have comparable energies but less stable than FOX-7.FOX-7 and its tautomers are endothermic structures but the parent compound is less endothermic than its tautomers.Of the Mg composites,C-type is the most andcisoidtype tautomer composite is the least stable ones.FMO energygaps of the tautomers are less than FOX-7.As for the composites,thetransoidtautomer composite has the smallest and C-type FOX-7 composite has the greatest FMO energy gap.The presence of Mg affects the IR and UV-VIS spectra.Thus,Mg(1:1 mol ratio)composite should be highly different than FOX-7 alone in terms of various properties.
[1]Agrawal JP.High energy materials.Weinheim:Wiley-VCH;2010.
[2]Politzer P,Murray JS.Energetic materials,Part 1.Amsterdam:Elsevier;2003.
[3]Lochert IJ.FOX-7-A new ınsensitive explosive.FOX-7.DSTO aeronautical and maritime Research laboratory 506 lorimer st, fishermans bend,Victoria 3207 Australia 2001,AR-012-065.November 2001.
[4]Latypov NV,Bergman J,Langlet A,Wellmar U,Bemm U.Synthesis and reactions of 1,1-diamino-2,2-dinitroethylene.Tetrahedron 1998;54:11525-36.
[5]Bemm U,Ostmark H.1,1-Diamino-2,2-dinitroethylene:a novel energetic material with ınfinite layers in two dimensions.Acta Crystallogr 1998;C54:1997-9.
[6]Latypov NV,Langlet A,Wellmar U.New chemical compound suitable for use as an explosive,intermediate and method for preparing the compound.Patent WO99/03818.1999.
[7]Ostmark H,Bergman H,Bemm U,Goede P,Holmgren E,Johansson M,et al.2,2-Dinitro-ethene-1,1-diamine(FOX-7)-properties,analysis and scale-up.In:32nd international annual conference of ICT on energetic materialsignition,combustion and detonation,karlsruhe,Germany;2001.
[8]Ostmark H,Langlet A,Bergman H,Wingborg N,Wellmar U,Bemm U.FOX-7-A New explosive with low sensitivity and high performance.In:The 11th international detonation symposium,Colorado,USA;1998.
[9]Bergman H,Ostmark H,Pettersson A,Petterson ML,Bemm U,Hihkio M.Some ınitial properties and thermal stability of FOX-7.In:Insensitive munitions and energetic materials symposium(NDIA).Tampa,Florida,USA;1999.
[10]Trzci′nski WA,Belaada A.1,1-Diamino-2,2-dinitroethene(DADNE,FOX-7)-properties and formulations(a review).Central Eur J Energetic Mater 2016;13(2):527-44.
[11]Janzon B,Bergman H,Eldsater C,Lamnevik C,Ostmark H.FOX-7-a novel,high performance,low vulnerability high explosive for warhead applications.In:20th int symp ballistics.Orlando,Florida,USA:september 23-27;2002.
[12]Matyushin YN,Afanas’ev GT,Lebedev VP,Mahov MN,Pepekin VI.TATB and FOX-7:thermochemistry,performance,detonability,sensitivity.In:34th int annu conf ICT,karlsruhe,Germany;June 24-27,2003.
[13]Bellamy AJ,Latypov NV,Goede P.Studies on the nitration of new potential precursors for FOX-7.New Trends Res Energ Mater Proc Semin 2004:74-81.7th,Pardubice,Czech Republic:April 20-22.
[14]Cudziło S,Chyłek Z,Diduszko R.Crystallization and characterization of 1,1-diamino-2,2-dinitroethene(DADNE).In:36th int annu conf ICT.Karlsruhe,Germany.June 28-july 1;2005.
[15]Trzci′nski WA,Cudziło S,Chyłek Z,Szyma′nczyk L.Investigation of sensitivity and detonation properties of FOX-7.In:37th int annu conf ICT.Karlsruhe,Germany:june 27-30;2006.
[16]Anniyappan M,Talawar MB,Gore GM,Venugopalan S,Ganghe BR.Synthesis,characterization and thermolysis of 1,1-diamino-2,2-dinitroethylene(FOX-7)and its salts.J Hazard Mater 2006;B137:812-9.
[17]Trzci′nski WA,Cudziło S,Chyłek Z,Szyma′nczyk L.Detonation properties of 1,1-diamino-2,2-dinitroethene(DADNE).J Hazard Mater 2008;157:605-12.
[18]Mishra VS,Vadali SR,Garg RK,Joshi VS,Wasnik RD,Asthana S.Studies on FOX-7 based melt cast high explosive formulations.Cent Eur J Energ Mater 2013;10(4):569-80.
[19]Latypov NV,Johansson M,Holmgren E,Sizova EV,Sizov VV,Bellamy AJ.On the synthesis of 1,1-diamino-2,2-dinitroethene(FOX-7)by nitration of 4,6-dihydroxy-2-methylpyrimidine.Org Process Res Dev 2007;11(1):56-9.https://doi.org/10.1021/op068010t.
[20]Zhang Y,Sun Q,Xu K,Song J,Zhao F.Review on the reactivity of 1,1-diamino-2,2-dinitroethylene(FOX-7).Propellants Explos Pyrotech 2016;41:35-52.
[21]Baum K,Nguyen NV,Gilardi R,Flippen-anderson JL,George C.Nitration of 1,1-diamino-2,2-dinitroethylenes.J Org Chem 1992;57:3026-30.
[22]KlapOtke TM.Chemistry of high-energy materials.Berlin:De Gruyter;2011.
[23]Evers J,KlapOtke TM,Mayer F,Oehlinger G,Welch J.α-and γ-FOX-7 polymorphs of a high energy density material,studied by X-ray single crystal and powder investigations in the temperature range from 200 to 423 K.Inorg Chem 2006;45:4996-5007.
[24]Crawford MJ,Evers J,GObel M,KlapOtke TM,Mayer P,Oehlinger G,et al.γ-FOX-7:structure of a high energy density material immediately prior to decomposition.Propellants Explos Pyrotech 2007;32:478-95.
[25]Gindulyte A,Massa L,Huang L,Karle J.Proposed mechanism of 1,1-diaminodinitroethylene decomposition:a density functional theory study.J Phys Chem 1999;103:11045-51.
[26]Dreger ZA,Stash AI,Yu ZG,Chen YS,Tao Y,Gupta YM.High-pressure crystal structures of an insensitive energetic crystal:1,1-diamino-2,2-dinitroethene.J Phys Chem C 2016;120(2):1218-24. https://doi.org/10.1021/acs.jpcc.5b10644.
[27]Lips H,Menke K.FOX-7/GAP rocket propellants for a shoulder launched projectıle.In:27th ınternatıonal symposıum on ballıstıcs.Freıburg,Germany:aprıl 22-26;2013.
[28]Karlsson S,Ostmark H,Eldsater C,Carlsson T,Bergman H,Wallin S,et al.Detonatıon and sensıtıvıty propertıes of fox-7 and formulatıons contaınıng FOX-7.FOI,Swedish Defence Research Agency GrindsjOns Research Center SE-147 25.Tumba,SWEDEN.2002.
[29]Türker L,Varıs S.Effects of epoxidation and nitration on ballistic properties of FOX-7.Z Anorg Allg Chem(ZAAC)2013;639:982-7.
[30]Dorsett H.Computational studies of FOX-7,a new insensitive explosive.Published by DSTO Aeronautical and Maritime Research Laboratory PO Box 1500 Salisbury South Australia 5108 Australia,Commonwealth of Australia 2000 AR-011-596.September 2000.
[31]Fang X,McLuckie WG.Laser ignitibility of insensitive secondary explosive 1,1-diamino-2,2-dinitroethene(FOX-7).J Hazard Mater 2015;285(21):375-82.https://doi.org/10.1016/j.jhazmat.2014.12.006.Epub 2014 Dec 15.
[32]Vadhe PP,Pawar RB,Sinha RK,Asthana SN,Rao SA.Cast aluminized explosives(review).Combust Explos Shock Waves 2008;44(4):461-77.
[33]Wildegger-Gaissmaier AE.Aspects of thermobaric weaponry.Mil Technol 2004;28(6):125-6.
[34]Yen NH,Wang LY.Reactive metals in explosives.Propellants Explos Pyrotech 2012;37(2):143-55.
[35]Cook MA,Filler AS,Keyes RT,Partridge WS,Ursenbach W.Aluminized explosives.J Phys Chem 1957;61(2):189-96.
[36]Zhou T,Li Y,Xu K,Song J,Zhao F.The new role of 1,1-diamino-2,2-dinitroethylene(FOX-7):two unexpected reactions.New J Chem 2017:1.https://doi.org/10.1039/c6nj03370a.
[37]Gao H,Shreeve JM.Recent progress in taming FOX-7(1,1-diamino-2,2-dinitroethene).RSC Adv 2016;1.https://doi.org/10.1039/c6ra12412g.
[38]Stewart JJP.Optimization of parameters for semiempirical methods I.Method J Comput Chem 1989;10:209-20.
[39]Stewart JJP.Optimization of parameters for semi empirical methods II.Appl J Comput Chem 1989;10:221-64.
[40]Leach AR.Molecular modeling.Essex.Longman;1997.
[41]Fletcher P.Practical methods of optimization.New York:Wiley;1990.
[42]Kohn W,Sham L.Self-consistent equations including exchange and correlation effects.J Phys Rev 1965;140:1133-8.
[43]Parr RG,Yang W.Density functional theory of atoms and molecules.London:Oxford University Press;1989.
[44]Becke AD.Density-functional exchange-energy approximation with correct asymptotic behavior.Phys Rev A 1988;38:3098-100.
[45]Vosko SH,Vilk L,Nusair M.Accurate spin-dependent electron liquid correlation energies for local spin density calculations:a critical analysis.Can J Phys 1980;58:1200-11.
[46]Lee C,Yang W,Parr RG.Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density.Phys Rev B 1988;37:785-9.
[47]SPARTAN 06.Irvine CA,USA:Wavefunction Inc.;2006.
[48]Ferguson LN.Themodern structural theory of organic chemistry.New Delhi:Prentice-Hall;1969.
[49]Reutov O.Theoretical principles of organic chemistry.Moscow:Mir Pub;1970.
[50]Anslyn EV,Dougherty DA.Modern physical organic chemistry.Sausalito,California:University Science Books;2006.
[51]Reichardt C.Solvents and solvent effects in organic chemistry.Weinheim:Wiley-VCH;2003.
[52]Ohlinger WS,Klunzinger PE,Deppmeier BJ,Hehre WJ.Efficient calculation of heats of formation.J Phys Chem A ACS Publ 2009;113:2165-75.
[53]Curtiss La,Raghavachari K,Redfern PC,Rassolov V,Pople JA.Gaussian-3(G3)Theory for molecules containing first and second-row atoms.J Chem Phys 1998;109:7764-76.
[54]Hendricson JB,Cram DJ,Hammond GS.Organic chemistry.Tokyo:McGraw-Hill-Kogakusha;1970.
[55]Luty T,Ordon P,Eckhardt CL.A model for mechanochemical transformations:applications to molecular hardness,instabilities,and shock initiation of reaction.J Chem Phys 2002;117:1775-85.
[56]Zhou H,Ma Z-l,Wang J-l,WANG D.Theoretical study of an energetic material di-1H-1,3,4-triazole derivatives.Def Technol 2014;10:384-92.
[57]Xu XJ,Zhu WH,Xiao HM.DFT studies on the four polymorphs of crystalline CL-20 and the influences of hydrostatic pressure on epsilon CL-20 crystal.J Phys Chem B 2007;111(8):2090-7.
[58]Ravi P,Shee SK,Gore GM,Tewari SP,Sikder AK.Quantum chemical investigation on the structure-property relationship of aminopolynitrotriazoles.Strct Chem 2011;22:661-9.
[59]Badders NR,Wei C,Aldeeb AA,Rogers WJ,Mannan MS.J Energetic Mater 2006;24(˙I):17-33.
杂志排行
Defence Technology的其它文章
- Effects of ply orientation and material on the ballistic impact behavior of multilayer plain-weave aramid fabric targets
- Influence of welding consumables on tensile and impact properties of multi-pass SMAW Armox 500T steel joints vis-a-vis base metal
- Effect of functional composite coating developed via sulphate and chloride process parameter on the UNS G10150 steel for structural and wear mitigation in defence application
- Optimizing submerged arc welding using response surface methodology,regression analysis,and genetic algorithm
- Virtual ballistic impact testing of Kevlar soft armor:Predictive and validated finite element modeling of the V0-V100probabilistic penetration response
- Pitting and stress corrosion cracking studies on AISI type 316N stainless steel weldments
