Identification of commonly regulated genes in HPV18- and HPV16-infected cervical cancer cells treated with the curcumin analogue 1,5-bis(2-hydroxyphenyl)-1,4-pentadiene-3-one
2018-06-30FeliciaPaulrajFaridahAbasNordinLajisIekhsanOthmanSharifahSyedHassanRakeshNaidu
Felicia Paulraj, Faridah Abas, Nordin H. Lajis, Iekhsan Othman, Sharifah Syed Hassan, Rakesh Naidu✉
1Jeffrey Cheah School of Medicine & Health Sciences, Monash University Malaysia, Jalan Lagoon Selatan, Bandar Sunway 47500, Selangor,Malaysia
2Laboratory of Natural Products, Faculty of Science, Universiti Putra Malaysia, UPM Serdang 43400, Selangor, Malaysia
3Department of Food Science, Faculty of Food Science and Technology, Universiti Putra Malaysia, UPM Serdang 43400, Selangor, Malaysia
1. Introduction
worldwide[1] and ranks as the second most frequent cancer affecting Malaysian women. Curcumin is a widely studied phytochemical.
Cervical cancer is the fourth most common cancer affecting women The treatment of cervical cancer cells with curcumin activates caspase-3-mediated apoptosis, restores p53 and pRb expression and inhibits AP-1 and NF-κB activity[2-4]. Notably, curcumin demonstrates potential antiviral activity due to its ability to inhibit the expression of E6 and E7 viral oncogenes[5,6] which significantly increases the cytotoxic and apoptotic effects of curcumin treatment on cervical cancer cells[7]. Synthetic 1,5-diaryl-3-oxo-1,4-pentadiene curcumin analogues known as diarylpentanoids reported greater growth suppressive effects in cancer cells over curcumin or other 7-carbon curcumin analogues[8]. Similarly with curcumin, these diarylpentanoids mediated programmed cell death, mitotic arrest and anti-proliferative effects in numerous cancer cell lines via a wide variety of molecular targets including p53, NF-κB, STAT3, c-Myc and PTEN[9-11]. EF24 is the most studied DAP to date due to its ability to collectively target molecules that induce, among others,anti-proliferative, apoptotic, anti-angiogenesis and anti-oxidative effects in cancer cells[12-14]. The treatment of HeLa cervical cancer cells by EF24 demonstrated the inhibition of cell viability via the suppression of NF-κB signaling[14], and studies by two other groups reported the potential anti-proliferative and apoptotic effects of treatment on cervical cancer cells[15,16]. Recently, a PGA-based curcumin nanoparticle formulation has been shown to effectively inhibit growth, induce apoptosis and cause cell cycle arrest in CaSki and SiHa cervical cancer cell lines[17]. Beyond these studies however, the anticancer effect of diarylpentanoids on cervical cancer cell lines have not been extensively studied.
We have previously established[18] that treatment of 1,5-bis(2-hydroxyphenyl)-1,4-pentadiene-3-one, known as MS17, on CaSki and HeLa cervical cancer cells demonstrated significant cytotoxic and anti-proliferative activity compared to curcumin.MS17 also demonstrated significant down-regulation of HPV18-and HPV16-associated E6 and E7 oncogene expression following treatment. Furthermore, MS17-exposed cells exhibited significant morphological changes consistent with apoptosis at their respective 2×EC50(HeLa; 6 μM, CaSki; 4 μM) and 3×EC50(HeLa; 9 μM,CaSki; 6 μM) dosages at 24 h of treatment. This suggested that an increase in treatment dose by 1.5-fold was able to effect apoptotic changes within the cells. To the best of our knowledge, this is the first report of its kind on HPV-positive cervical cancer cells.To investigate the mechanisms of action of the MS17 curcumin analogue on cervical cancer cells at a molecular level, microarraybased gene expression profiling of MS17-treated HPV-18 infected HeLa and HPV-16 infected CaSki cells was performed. Dosedependent changes in gene regulation that occurred in response to treatment were analysed and the regulation of 17 genes that were mutual to both cervical cancer cell lines were identified as potential common targets of MS17. The molecular changes in the regulation of these genes that potentially justifies the cytotoxic, antiproliferative and apoptotic activity of MS17 on cervical cancer cells have been presented here and discussed.
2. Materials and methods
2.1. Cell culture and preparation of MS17
HPV-infected HeLa and CaSki cervical cancer cell lines were obtained from American Type Culture Collection (ATCC, Rockville,MD, USA) and maintained using MEM and RPMI 1640 (Gibco)culture media respectively and supplemented with 10% Fetal Bovine Serum (FBS, Gibco) and penicillin (100 U/mL)/streptomycin(100 μg/mL) (Gibco). Cells were cultured at 37 ℃ in a humidified atmosphere with 5% CO2. The curcumin analogue 1,5-bis(2-hydroxyphenyl)-1,4-pentadiene-3-one (MS17) was prepared as previously described[18].
2.2. Treatment of cervical cancer cells and total RNA extraction
Cancer cells were plated at 4×105cells per mL in 1 mL culture medium per well as described earlier[18], exposed to MS17 at their respective doses for 24 h and prepared for RNA extraction.Experiments were performed in triplicate and included a set of untreated control cells (media with DMSO alone). Total RNA was extracted using the Qiagen RNeasy® Mini Kit (Qiagen, Valencia,CA., USA) according to the manufacturer’s instructions. RNA samples with an A260/A280and A260/A230ratio of ≥1.9 and with a RNA integrity number score of approximately ≥8 were selected for gene expression profiling.
2.3. Microarray analysis
Agilent oligonucleotide microarrays were used for one-colour gene expression analysis and samples were prepared according to the protocols provided by One-Color Low Input Quick Amp labeling kit(Agilent, Valencia, CA, USA). Information from features on the array was extracted using Agilent’s Feature Extraction Software 11.5.1(Agilent Technologies, 2004, USA) and analysed (GeneSpring GX version 13.0 software, Agilent, USA). Normalisation was performed using Agilent Single Color 28004 Technology. Raw signals were log transformed, normalized and baseline transformed to the median of all samples. Values were normalised by shifting to the 75th percentile. Probes were subjected to additional steps of filtering on detected flags and expression value. Filtering on expression removes probes with low raw signal intensities (<20) and entities where at least 100 percent of samples in any 1 out of 4 conditions have values exceeding 20 qualified to proceed to the next filtering criteria.
2.4. Statistical analysis
Statistical analysis was performed using analysis of variance with Benjamini-Hochberg false discovery rate multiple testing correction,followed by apost hoctesting using Tukey’s Honestly Significance Difference to identify pair-wise significant genes. Genes that passed the significance threshold with a false discovery rate-corrected adjustedP-value ofP<0.05 was analysed for its fold change (FC)compared to control samples. FC measures the relative change in expression between treatment (MS17-treated samples) and control(DMSO-treated) conditions for a particular gene. Differentially expressed genes (DEGs) with FC≥2.0 (up-regulated; FC≥2.0 and down-regulated; FC≤-2.0) compared to the control samples were filtered through and analysed to create a global gene expression profile. Hierarchical cluster analysis was performed on this DEG list using software-recommended parameters.
2.5. Data analysis
For an analysis on the dose-dependent regulation of genes by MS17, downstream analysis focused on selected mutually regulated DEGs in MS17-treated HeLa (6 μM and 9 μM) and CaSki (4 μM and 6 μM) cells. The top twenty DEGs that were mutually upregulated and down-regulated each in both cell lines were selected to obtain a list of top ranked genes. Ontological analysis of mutually regulated genes was performed using the Database for Annotation,Visualisation and Integrated Discovery (DAVID)[19] which is freely accessed at http://david.abcc.ncifcrf.gov. Gene symbols of mutually expressed genes from each cell line were submitted to DAVID.Annotations were limited to ‘Homo sapiens’ and gene ontological(GO) analysis was performed using the functional annotation tool and further analysed using the GO functional categories which provide a description of associated biological processes and molecular functions for the selected genes. Genes were grouped into functional categories based on the exported GO terms from DAVID.
2.6. Validation of microarray results by quantitative realtime PCR
To assess the robustness of the microarray analysis, quantitative real-time PCR analysis was performed to validate a selected panel of DEGs (CCL26,MMP10,MMP3,CTDSP1,IGFBPandMXD3)and normalised against the endogenous gene, human glyceraldehyde 3-phosphate dehydrogenase (GAPDH). Complementary DNA was generated from the RNA isolated from MS17-treated HeLa and CaSki cells that were used for microarray profiling following the previously described protocol[18]. Transcript levels of the selected DEGs andGAPDHwere measured by TaqMan® Gene Expression Assays specific to each gene. The primers and TaqMan probe sequences used for the selected genes corresponded to assay IDs (CCL26, Hs00171146_m1; MMP10, Hs00233987_m1, MMP3, Hs00968308_m1; CTDSP1, Hs01105503_m1;IGFBP5, Hs01052295_m1; MXD3, Hs00361007_m1; GAPDH,Hs99999905_m1) acquired from the TaqMan Gene Expression Assay database (Applied Biosystems, USA). Samples were prepared according to manufacturer’s instructions and loaded into the StepOnePlus™ real-time PCR system (Applied Biosystems), cycled using the Fast run method and relative mRNA expression levels of the target genes were calculated using the comparative Ct method.Each reaction was run in duplicates and fold change expression of mRNA results from quantitative real-time PCR was converted to log2 fold change and represented in bar graphs alongside log2 fold change data from microarray analyses. The bar graph representing the results was constructed using GraphPad Prism version 6.00 for Windows, GraphPad Software (San Diego, CA., USA).
3. Results
Based on dosages that were previously established to induce apoptosis, HeLa was exposed to 6 μM and 9 μM, while CaSki was exposed to 4 μM and 6 μM of MS17 for 24 h. Only significant entities that demonstrated aP-value ofP<0.05 and were differentially expressed compared to the control by a fold change greater than or equal to 2.0 (FC≥2.0) were considered for analysis. Amongst these genes, 1 167 and 1 836 genes in HeLa were differentially expressed by 6 μM and 9 μM treatment respectively while in CaSki, 1 069 and 1 091 genes were differentially expressed by 4 μM and 6 μM treatment. DEGs were subjected to a hierarchical cluster analysis from which a dose-dependent effect of MS17 treatment on both HeLa and CaSki gene regulation was suggested as 826 genes in HeLa and 491 genes in CaSki composed DEGs that were mutually expressed by both treatment doses. Of these DEGs,we have identified top 20 mutually up- and down-regulated genes each in HeLa and CaSki cells.
Ontological classification was performed on the top 20 ranking genes that were differentially regulated by MS17 treatment and mutually expressed by both treatment doses in HeLa (Table 1) and CaSki (Table 2) cells. These genes were subsequently grouped according to their respective biological functions. Amongst the top 20 genes, 17 genes were commonly regulated by MS17 in both cell lines (denoted as asterisks in Table 1 and Table 2). These included the up-regulation ofCCL26,DEFB103B,IL1RL1,LY96,GCNT3,MMP10,MMP3,GADD45GandHSPA6which were associated with immune response, metabolic processes, proteolysis, programmed cell death and unfolded protein response, and the down-regulation ofTENM2,NEBL,KIFC1,CTDSP1,IGFBP5,LTBP1,NREPandMXD3which were associated with cell adhesion, cytoskeletal organisation,phosphatase activity, signal transduction and transcription regulator activity. Of these 17 genes, 3 up- and 3 down-regulated genes were selected and validated, and the expression patterns determined from real-time PCR were consistent with the microarray results (Figure 1).

Figure 1. Validation of mRNA expression of selected genes from microarray data by quantitative real time PCR.
4. Discussion
Treatment of HeLa and CaSki cells with MS17 reveals an expression profile that contained common gene targets. This is interesting because although HeLa and CaSki are both infected with high risk subtypes that cause cervical cancer, both cell lines are derived from different sources such that CaSki is of squamous origin, while HeLa is an adenocarcinoma. Our results suggest that the 17 commonly regulated genes are important to specific biological functions and may be implicated in the anticancer activity of this curcumin analogue. Identification of common gene targets in cervical cancer cells despite being infected with differentHPV subtypes may suggest that targeting these genes could have therapeutic implications.

Table 1 Selected genes mutually induced and repressed in HeLa cells following 6 μM and 9 μM treatment of MS17 at 24 h.
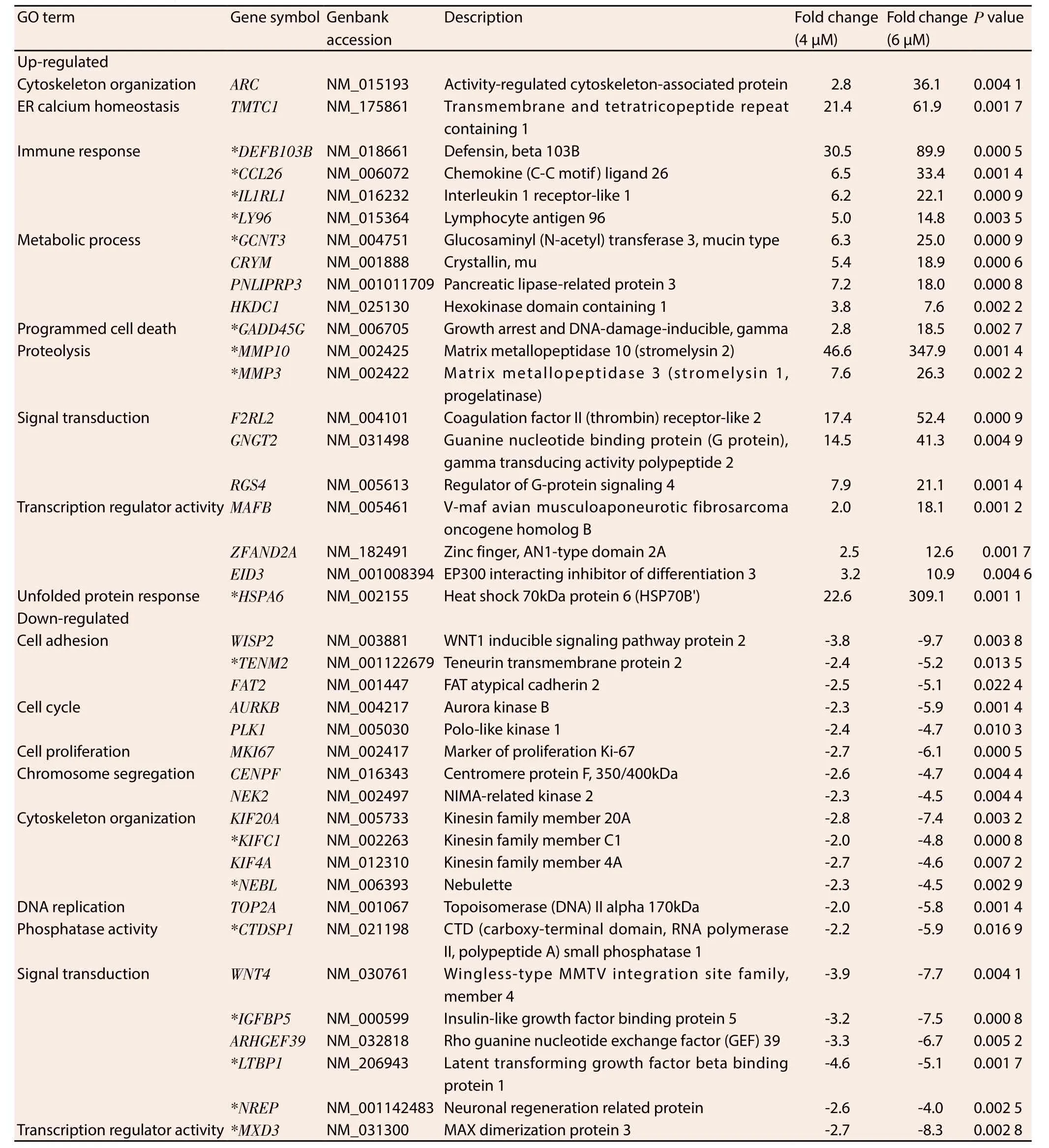
Table 2 Selected genes mutually induced and repressed in CaSki cells following 4 μM and 6 μM treatment of MS17 at 24 h.
The largest proportion of differentially up-regulated genes is associated with immune response. Many members in this cluster encode cytokines which are involved in immune system regulation and inflammation processes. Four highly up-regulated immune response-associated transcripts that were common to both cell lines includedCCL26, followed byDEFB103B,IL1RL1andLY96.CCL26encodes dendritic-cell specific chemokines that attract naive lymphocytes and neutrophils;DEFB103Bcodifies human β-defensin-3;IL1RL1expresses the receptor for interleukin-1 andLY96responds to toll-like receptor 4 on dendritic cells. They are respectively involved in the repression of cancer cell migration[20],growth[21,22] and the mediation of anticancer immunity[23]. The findings suggest that induction of an immune response, inhibition of cell migration and growth could be potential mechanisms by which MS17 exhibits anticancer activity on HeLa and CaSki cells.
The metabolism-associatedGCNT3encodes a beta-6-N-acetylglucosamine-transferase which catalyses O-glycan branching on mucin-type glycoproteins. Alterations in the O-glycosylation of mucins often accompany cancer development and the overexpression ofGCNT3reportedly suppresses cell adhesion, motility and invasion of colorectal cancer cells[24] and may contribute towards anti-tumorigenic activity in treated HeLa and CaSki cells.MS17 treatment positively modulated the increased expression of proteolytic matrix metalloproteinases, specifically ofMMP10andMMP3which degrade the extracellular matrix[25].MMP10is commonly implicated in inflammatory diseases and may be secreted by T-lymphocytes to assist in extracellular matrix degradation[26]during inflammation or an immune response. Cross-talk between matrix metalloproteinases suggests that they may possess proand anti-tumorigenic effects. Squamous cell carcinoma tumours in MMP3-null mice exhibited enhanced tumour progression and were inversely correlated with leukocyte infiltration[27]. Therefore the up-regulation of matrix metalloproteinases by MS17 indicates the potential modulation over the proteolytic extracellular matrix degradation of cervical cancer cells to exert its anti-tumorigenic effects. The exact mechanism by which this occurs however needs to be investigated further.
The up-regulation of programmed cell death associated geneGADD45Gdemonstrated that increasing the treatment dose by 1.5-fold showed almost 3.6-fold up-regulation in MS17 treated HeLa cells and 6.6-fold up-regulation in CaSki cells.GADD45Gcodifies the expression of DNA damage inducible proteins which is activated by heat shock or UV irradiation stimuli, a response which is abolished in approximately 50% of cervical cancer tumours[28,29].Notably, its expression is up-regulated following exposure to curcumin treatment, DNA damage and genotoxic stress and induces cell cycle arrest, apoptosis and inhibits cell growth[30,31] and its induction could contribute to the apoptotic cell death mechanism of MS17 in treated HeLa and CaSki cells.
The heat shock geneHSPA6which encodes the stress-inducible Hsp70 protein, is the topmost mutually up-regulated transcript in HeLa cells and demonstrates 13.7-fold up-regulation in response to increasing treatment dose in CaSki cells. Heat shock proteins act as protein chaperones to prevent protein misfolding and Hsp70 expression is commonly up-regulated during cellular oxidative stress which promotes tumour cell growth and contributes to genomic instability and DNA damage in cancer cells. However,the overexpression of oxidative stress also induces cancer cell death[32] and the induction of heat shock protein expression in response to anticancer compounds is not uncommon. Treatment with elescomol triggered apoptosis in melanoma cancer cells by inducing a transcriptional gene profile characteristic of an oxidative stress response, which also includedHSPA6as its top most up-regulated transcript[32]. Notably treatment of HeLa and leukaemia cells with curcumin and exposure of melanoma cells with curcumin analogue D6 induced the up-regulation ofHSPA6, correlating strongly with cellular toxicity and was suggested to cause extreme endoplasmic reticulum stress resulting in apoptotic cell death[33-35]. This may thus reflect a similar mechanism by which MS17 up-regulatesHSPA6expression to modulate oxidative stress responses and trigger apoptosis induction in treated cells thus contributing to antitumorigenesis.
The expression of cell adhesion-associatedTENM2is downregulated in both cell lines following MS17 treatment.TENM2encodes a transmembrane protein that enhances proliferation,promotes cell adhesion, facilitates microtubule organization[36] and is up-regulated by more than 250-fold in vincristine-resistant ovarian cancer cells[37]. The suppression ofTENM2by MS17 could disrupt adhesive contact between cells and mediate anti-proliferative activity in treated HeLa and CaSki cells.
Cytoskeleton organizing genesNEBLandKIFC1are also downregulated by MS17 treatment in both cell lines. NEBL codifies nebulette, an actin protein involved in cytoskeletal organization.The up-regulation ofNEBLstimulates cell migration[38] while silencing of a related family member,Lasp1, in cell lines abrogates metastasis[39].KIFC1encodes microtubule motor proteins that function during spindle formation. The down-regulation ofKIFC1may destabilize spindle assembly, trigger cell cycle arrest and reduce cell viability[40]. Thus the repression of cytoskeletal organisation genes indicates the role of MS17 in modulating an anchorage network that is important for chromosome stability in mitosis.
The nuclear phosphatase expressed byCTDSP1regulates gene expression by selective dephosphorylation of phosphoserine 5 within RNA polymerase Ⅱ and is commonly associated with the silencing of neuronal genes[41]. Its down-regulation by MS17 in cervical cancer cells suggests the potential modulation of transcriptional regulation to inhibit cell survival.IGFBP5,LTBP1andNREPare grouped under signal transduction ontologies.IGFBP5encodes IGF-binding proteins which are highly expressed in CIN tissues[42] and regulates the survival of cervical cancer cells by conferring it with antiapoptotic properties[43]. The down-regulation ofIGFBP5expression by MS17 suggests the reactivation of apoptosis in HeLa and CaSki cells which potentially contributes to cell death.LTBP1encodes a latent TGFβ binding protein which contributes towards glioma cell malignancy while silencing is shown to reduce TGFβ activity[44]suggesting an important modulatory effect ofLTBP1over malignant tumour phenotypes. On the other hand,NREPencodes a neural regeneration protein but demonstrates RAC1-mediated involvement in glioma cell motility[45]. Hence the suppression of NREP by MS17 targets the disruption of actin cytoskeletal organization of HeLa and CaSki cells and impairs malignant cell proliferation and motility. Furthermore,MXD3encodes a MAD family transcription factor which contributes to cell proliferation upon up-regulation but demonstrates anti-proliferative activity following MDX3-specific silencing[46] which may result in the suppression of cervical cancer proliferation following MS17 treatment.
In conclusion, the dose-dependent modulation of high ranking genes demonstrates the notable regulation of 17 genes that are common to both cell lines, suggesting the possibility that MS17 exerts antitumor effects on pathways linked to immune response,metabolism, proteolysis, apoptosis, unfolded protein response, cell adhesion, cytoskeletal organization, phosphatase activity, signal transduction and transcription regulator activity. Despite the differing biological properties of HPV-18 infected HeLa and HPV-16 infected CaSki cells, the common regulation of these genes indicates shared therapeutic targets and mechanism of action by MS17 in cervical cancer cells. Thus the findings of this study present an insight into the potential antitumor activity of MS17 in cervical cancer and identify key biological targets that serve as a basis for future investigation.
Conflict of interest statement
The authors declare no conflict of interest.
Acknowledgments
The authors are grateful to the Jeffrey Cheah School of Medicine and Health Sciences, Monash University Malaysia for providing the research facilities and support.
[1] Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, et al.Globocan 2012 v1.0, Cancer incidence and mortality worldwide: IARC cancerbase No. 11. Lyon, France: International Agency for Research on Cancer. [Online] Available from: http://globocan.iarc.fr. [Accessed on:September 1, 2016].
[2] Maher DM, Bell MC, O’Donnell EA, Kohaar I, Hedau S, Bharti AC, et al. Curcumin suppresses human papillomavirus oncoproteins, restores p53, Rb, and PTPN13 proteins and inhibits benzo[a]pyrene-induced upregulation of HPV E7.Mol Carcinog2011; 50: 47-57.
[3] Mishra A, Kumar R, Tyagi A, Kohaar I, Hedau S, Bharti AC, et al.Curcumin modulates cellular AP-1, NF-κB, and HPV16 E6 proteins in oral cancer.Ecancermedicalscience2015; 9: 525.
[4] Goodwin EC, DiMaio D. Repression of human papillomavirus oncogenes in HeLa cervical carcinoma cells causes the orderly reactivation of dormant tumor suppressor pathways.Proc Natl Acad Sci U S A2000; 97:12513-12518.
[5] Dang YP, Yuan XY, Tian R, Li DG, Liu W, et al. Curcumin improves the paclitaxel-induced apoptosis of HPV-positive human cervical cancer cells via the NF-κappaB-p53-caspase-3 pathway.Exp Ther Med2015; 9: 1470-1476.
[6] Singh M, Singh N. Molecular mechanism of curcumin induced cytotoxicity in human cervical carcinoma cells.Mol Cell Biochem2009;325: 107-119.
[7] Divya CS, Pillai MR. Antitumor action of curcumin in human papillomavirus associated cells involves downregulation of viral oncogenes, prevention of NFκB and AP-1 translocation, and modulation of apoptosis.Mol Carcinog2006; 45: 320-332.
[8] Ohori H, Yamakoshi H, Tomizawa M, Shibuya M, Kakudo Y, Takahashi A, et al. Synthesis and biological analysis of new curcumin analogues bearing an enhanced potential for the medicinal treatment of cancer.Mol Cancer Ther2006; 5: 2563-2571.
[9] Sato A, Kudo C, Yamakoshi H, Uehara Y, Ohori H, Ishioka C, et al. Curcumin analog GO-Y030 is a novel inhibitor of IKKbeta that suppresses NF-κappaB signaling and induces apoptosis.Cancer Sci2011;102: 1045-1051.
[10] Kudo C, Yamakoshi H, Sato H, Nanjo H, Ohori H, Ishioka C, et al.Synthesis of 86 species of 1,5-diaryl-3-oxo-1,4-pentadienes analogs of curcumin can yield a good leadin vivo.BMC Pharmacol2011; 11: 4.
[11] Selvendiran K, Kuppusamy ML, Bratasz A, Tong L, Rivera BK, Rink C,et al. Inhibition of vascular smooth-muscle cell proliferation and arterial restenosis by HO-3867, a novel synthetic curcuminoid, through upregulation of PTEN expression.J Pharmacol Exp Ther2009; 329: 959-966.
[12] Selvendiran K, Tong L, Vishwanath S, Bratasz A, Trigg NJ, Kutala VK,et al. EF24 induces G2/M arrest and apoptosis in cisplatin-resistant human ovarian cancer cells by increasing PTEN expression.J Biol Chem2007; 282: 28609-28618.
[13] Tan X, Sidell N, Mancini A, Huang RP, Wang S, Horowitz IR, et al.Multiple anticancer activities of EF24, a novel curcumin analog, on human ovarian carcinoma cells.Reprod Sci2010; 17: 931-940.
[14] Kasinski AL, Du Y, Thomas SL, Zhao J, Sun SY, Khuri FR, et al.Inhibition of IκappaB kinase-nuclear factor-κappaB signaling pathway by 3,5-bis(2-flurobenzylidene)piperidin-4-one (EF24), a novel monoketone analog of curcumin.Mol Pharmacol2008; 74: 654-661.
[15] Zhang X, Wang R, Chen G, Dejean L, Chen QH. The effects of curcumin-based compounds on proliferation and cell death in cervical cancer cells.Anticancer Res2015; 35: 5293-5298.
[16] Wang R, Chen C, Zhang X, Chen G, Sarabia C, Zhang Q, et al.Structure-activity relationship and pharmacokinetic studies of 1,5-diheteroarylpenta-1,4-dien-3-ones: a class of promising curcumin-based anticancer agents.J Med Chem2015; 58: 4713-4726.
[17] Zaman MS, Chauhan N, Yallapu MM, Gara RK, Maher DM, Kumari S,et al. Curcumin nanoformulation for cervical cancer treatment.Sci Rep2016; 6: 20051.
[18] Paulraj F, Abas F, Lajis NH, Othman I, Hassan SS, Naidu R. The curcumin analogue 1,5-bis(2-hydroxyphenyl)-1,4-pentadiene-3-one induces apoptosis and downregulates E6 and E7 oncogene expression in HPV16 and HPV18-infected cervical cancer cells.Molecules2015; 20(7):11830-11860.
[19] Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources.Nat Protoc2009; 4: 44-57.
[20] Uraki S, Sugimoto K, Shiraki K, Tameda M, Inagaki Y, Ogura S, et al. Human beta-defensin-3 inhibits migration of colon cancer cells via downregulation of metastasis-associated 1 family, member 2 expression.Int J Oncol2014; 45: 1059-1064.
[21] Haga Y, Yanagisawa K, Ohto-Ozaki H, Tominaga S, Masuzawa T,Iwahana H. The effect of ST2 gene product on anchorage-independent growth of a glioblastoma cell line, T98G.Eur J Biochem2003; 270: 163-170.
[22] O’Donnell C, Mahmoud A, Keane J, Murphy C, White D, Carey S, et al.An antitumorigenic role for the IL-33 receptor, ST2L, in colon cancer.Br J Cancer2016; 114: 37-43.
[23] Okamoto M, Furuichi S, Nishioka Y, Oshikawa T, Tano T, Ahmed SU, et al. Expression of toll-like receptor 4 on dendritic cells is significant for anticancer effect of dendritic cell-based immunotherapy in combination with an active component of OK-432, a streptococcal preparation.Cancer Res2004; 64: 5461-5470.
[24] Huang MC, Chen HY, Huang HC, Huang J, Liang JT, Shen TL, et al.C2GnT-M is downregulated in colorectal cancer and its re-expression causes growth inhibition of colon cancer cells.Oncogene2006; 25: 3267-3276.
[25] Nakamura H, Fujii Y, Ohuchi E, Yamamoto E, Okada Y. Activation of the precursor of human stromelysin 2 and its interactions with other matrix metalloproteinases.Eur J Biochem1998; 253: 67-75.
[26] Conca W, Willmroth F, Human T. Lymphocytes express a member of the Matrix Metalloproteinase gene family.Arthritis Rheum1994; 37: 951-956.
[27] McCawley LJ, Crawford HC, King Jr. LE, Mudgett J, Matrisian LM.A protective role for matrix metalloproteinase-3 in squamous cell carcinoma.Cancer Res2004; 64: 6965-6972.
[28] Ramachandran C, Rodriguez S, Ramachandran R, Nair PKR, Fonseca H, Khatib Z, et al. Expression profiles of apoptotic genes induced by curcumin in human breast cancer and mammary epithelial cell lines.Anticancer Res2005; 25: 3293-3302.
[29] Ying J, Srivastava G, Hsieh WS, Gao Z, Murray P, Liao SK, et al. The stress-responsive gene GADD45G is a functional tumor suppressor, with its response to environmental stresses frequently disrupted epigenetically in multiple tumors.Clin Cancer Res2005; 11: 6442-6449.
[30] Vairapandi M, Balliet AG, Hoffman B, Liebermann DA. GADD45b and GADD45g are cdc2/cyclinB1 kinase inhibitors with a role in S and G2/M cell cycle checkpoints induced by genotoxic stress.J Cell Physiol2002; 192: 327-338.
[31] Saha A, Kuzuhara T, Echigo N, Fujii A, Suganuma M, Fujiki H.Apoptosis of human lung cancer cells by curcumin mediated through upregulation of ‘growth arrest and DNA damage inducible genes 45 and 153’.Biol Pharm Bull2010; 33: 1291-1299.
[32] Kirshner JR, He S, Balasubramanyam V, Kepros J, Yang CY, Zhang M,et al. Elesclomol induces cancer cell apoptosis through oxidative stress.Mol Cancer Ther2008; 7: 2319-2327.
[33] Dunsmore KE, Chen PG, Wong HR. Curcumin, a medicinal herbal compound capable of inducing the heat shock response.Crit Care Med2001; 29: 2199-2204.
[34] Teiten MH, Eifes S, Reuter S, Duvoix A, Dicato M, Diederich M, et al. Gene expression profiling related to anti-inflammatory properties of curcumin in K562 leukemia cells.Ann N Y AcadSci2009; 1171: 391-398.
[35] Rozzo C, Fanciulli M, Fraumene C, Corrias A, Cubeddu T, Sassu I, et al. Molecular changes induced by the curcumin analogue D6 in human melanoma cells.Mol Cancer2013; 12: 37.
[36] Beckmann J, Schubert R, Chiquet-Ehrismann R, Müller DJ. Deciphering teneurin domains that facilitate cellular recognition, cell-cell adhesion,and neurite outgrowth using atomic force microscopy-based single-cell force spectroscopy.Nano Lett2013; 13: 2937-2946.
[37] Buys TP, Chari R, Lee EH, Zhang M, MacAulay C, Lam S, et al. Genetic changes in the evolution of multidrug resistance for cultured human ovarian cancer cells.Genes Chromosome Cancer2007; 46: 1069-1079.
[38] Emerenciano M, Kowarz E, Karl K, de Almeida Lopes B, Scholz B,Bracharz S, et al. Functional analysis of the two reciprocal fusion genes MLL-NEBL and NEBL-MLL reveal their oncogenic potential.Cancer Lett2013; 332: 30-34.
[39] Zhang H, Chen X, Bollag WB, Bollag RJ, Sheehan DJ, Chew CS, et al.Lasp1 gene disruption is linked to enhanced cell migration and tumor formation.Physiol Genomics2009; 38: 372-385.
[40] Pannu V, Rida PC, Ogden A, Turaga RC, Donthamsetty S, Bowen NJ,et al. HSET overexpression fuels tumor progression via centrosome clustering-independent mechanisms in breast cancer patients.Oncotarget2015; 6: 6076-6091.
[41] Yeo M, Lee SK, Lee B, Ruiz EC, Pfaff SL, Gill GN. Small CTD phosphatases function in silencing neuronal gene expression.Science2005; 307: 596-600.
[42] Hou XJ, Zhang YZ, Liu X, Meng LH, Qiao YB. Expressions of IGFBP-5, cFLIP in cervical intraepithelial neoplasia, cervical carcinoma and their clinical significances: a molecular pathology.J Exp Clin Cancer Res2009; 28: 70.
[43] Perks CM, McCaig C, Holly JM. Differential insulin-like growth factor(IGF)-independent interactions of IGF binding protein-3 and IGF binding protein-5 on apoptosis in human breast cancer cells. Involvement of the mitochondria.J Cell Biochem2000; 80: 248-258.
[44] Tritschler I, Gramatzki D, Capper D, Mittelbronn M, Meyermann R,Saharinen J, et al. Modulation of TGF-beta activity by latent TGF-betabinding protein 1 in human malignant glioma cells.Int J Cancer2009;125: 530-540.
[45] Mariani L, McDonough WS, Hoelzinger DB, Beaudry C, Kaczmarek E,Coons SW, et al. Identification and validation of P311 as a glioblastoma invasion gene using laser capture microdissection.Cancer Res2001; 61:4190-4196.
[46] Barisone GA, Ngo T, Tran M, Cortes D, Shahi MH, Nguyen TV, et al.Role of MXD3 in proliferation of DAOY human medulloblastoma cells.PLoS One2012; 7: e38508.
杂志排行
Asian Pacific Journal of Tropical Biomedicine的其它文章
- A comprehensive review on anti-diabetic property of rice bran
- Ethnobotanical survey of antimalarial plants in Awash-Fentale District of Afar Region of Ethiopia and in vivo evaluation of selected ones against Plasmodium berghei
- Larvicidal activity of Neem oil and three plant essential oils from Senegal against Chrysodeixis chalcites (Esper, 1789)
- Protective effect of ashwagandha (Withania somnifera) against neurotoxicity induced by aluminum chloride in rats
- Oxidative stress mitigation, kinetics of carbohydrate-enzymes inhibition and cytotoxic effects of flavonoids-rich leaf extract of Gazania krebsiana (Less.): An in vitro evaluation
- Proximate composition, nutritional values and phytochemical screening of Piper retrofractum vahl. fruits
