Phenolic compounds from Pithecellobium clypearia with tyrosinase inhibitory activity
2018-06-22HanZhangZhiyangYanYuxiWangXiaoxiaoHuang
Han Zhang, Zhiyang Yan, Yuxi Wang, Xiaoxiao Huang*
Department of Natural Products Chemistry, Shenyang Pharmaceutical University, Shenyang 110016, PR China
1 Introduction
Melanin is a heterogeneous polyphenol-like biopolymer with a complex structure and color varying from yellow to black [1], which is formed by a combination of enzymatically catalyzed and chemical reactions. It plays a crucial protective role against skin photocarcinogenesis, however,the production of abnormal melanin pigmentation is a serious esthetic problem for human beings [2].Tyrosinase, a copper-containing enzyme, exists widely in plants and animals, and is involved in the formation of melanin pigments [3,4]. The investigation of tyrosinase inhibitors may lead to the development of novel skin-whitening substances,medicinal agents and antibrowning constituents [5].These phenomena have encouraged researchers to seek new potent tyrosinase inhibitors for use in foods and cosmetics.
In recent years, a number of tyrosinase inhibitors from natural sources have been identified [6].Pithecellobiumclypearia(Jack)Benth., a member of Mimosaceae family, is a well-known and widely used traditional Chinese medicine [7]. It is mostly distributed in the south of China, such as Sichuan, Yunnan and Guangdong provinces. It has been used as a herbal medicine in the treatment of respiratory tract diseases in China for many years [8,9]. Previous studies on various active constituents ofP. clypeariashowed their effects on anti-inflammatory and anti-tumor [10].One Chinese patent medicine manufactured from the aqueous extract of the leaves and twigs ofP. clypeariahas been recorded in the Pharmacopeia of China, and used for the treatment of upper respiratory tract infections, pharyngitis, laryngitis,and acute tonsillitis [11]. However, little information is available on the tyrosinase inhibitory components fromP. clypearia. Hence, this led to us to investigate the tyrosinase inhibitory activity of constituents fromP. clypearia.
Phytochemistry study of the ethanol extract ofP. clypearialed to the isolation of seven compounds.All the isolates were tested for their tyrosinase inhibitory activity aiming to study their effects on melanogenesis. And then molecular docking was performed to explore the binding interactions between tyrosinase and isolates (Fig. 1).

Fig. 1 The structures of compounds 1-7
2 Results
2.1 Structure Elucidation
The structure of these compounds were defined through extensive NMR analyses as the known phenolic acids. Compound 1 was observed as a white crystal with the molecular formula of C8H8O5.1H NMR spectrum gave the signal of three hydroxyl signals atδH8.98 (1H, s), 9.26 (2H, s), two aromatic proton signals atδH6.97 (2H, s), one methoxyl atδH3.73 (3H, s).13C NMR spectrum revealed the existance of a benzene ring (δc166.5, 145.3, 124.9,109.6, 109.6, 45.3), one methoxyl (δc 52.3). By comparing the spectrum data with the reported literature [12], compound 1 was determined to be methyl 3,4,5-trihydroxybenzoate.
Compounds 2-7 were determined to be 3,5-dimethoxy-4-hydroxybenzoic acid [13],3,4,5-trimethoxy-benzoic acid [14], 3,4-dimethoxy-5-hydroxybenzoic acid [15], 3,5-dihydroxy-4-methoxybenzoic acid [16], 3-methoxy-4-hydroxybenzoic acid [17], 3-methoxy-4-hydroxybenzaldehyde [18], respectively.
2.2 Tyrosinase inhibitory activity
Compounds 1-7 were evaluated for their tyrosinase inhibitory activity and arbutin was used as positive control. The result is summarized in Fig. 2.Based on the experimental results, compounds 1 and 5 exhibited prominent inhibitory activity against mushroom tyrosinase with inhibitory ratios at 92.15% and 93.57%, compared with arbutin(57.35%). compounds 4 and 6 exhibited moderate inhibitory activity against mushroom tyrosinase(70.1% and 76.0% inhibition, respectively), while compounds 2, 3 and 7 exhibited weak inhibitory rates (34.86%, 25.62% and 12.54%, respectively).
2.3 Molecular Docking
The program Accelrys Discovery Studio 4.3 and Molegro Virtual Docker 4.0 were applied for the molecular docking simulations between tyrosinase and compounds. The 3D structureof mushroom tyrosinase (PDB ID: 2Y9X) was retrieved from a protein data bank (PDB). The results obtained are shown in Fig. 3. The benzene ring of compound 1 formed π-alkyl interactions with Ala 50 (distance 4.38 Å) residues compared with the molecular docking models. And two hydrogen bond interactions were observed between the two hydroxide radicals and Asn 25 (distance 2.11 Å and 2.09 Å) residues. In addition, the ester carbonyl and methoxy groups formed three hydrogen bonds with Amn 8 (distance 1.77 Å), Asp 10 (distance 2.54 Å) and Gln 48 (distance 2.76 Å) residues,respectively. Two hydroxyls of compound 5 displayed two hydrogen bonds with neighboring amino acid residues (Asp 10 distance 2.26 Å, Gly 68 distance 2.27 Å). Similarly, ester carbonyls and ester hydroxyls formed hydrogen bonds with Gly 47 (distance 2.81 Å), Asn 25 (distance 2.13 Å),respectively. Comparison with compounds 1 and 5,however, the ester carbonyl group of compounds 6 did not form a hydrogen bond with the amino acid residue. This may be the reason that compounds 1, 5 have better inhibitory activity than 6.

Fig. 2 The tyrosinase inhibitory rate of compounds 1-7. All compounds were dissolved in 20% MeOH-H2O to 100 mM in the test. These data was expressed as mean ± SD (n = 3). The control group was treated with arbutin.(**) p<0.05, (***) p<0.01 are compared with the arbutin
3 Conclusion
Through long term investigation, we isolated and identified seven compounds from the ethanol extract ofP. clypeariaand tested the tyrosinase inhibitory activity of all the separated components.The result showed that compounds 1, 5 exhibited prominent inhibition against mushroom tyrosinase with 92.15% and 93.57% inhibitory ratios at 100mM, respectively, compared with arbutin(57.35%). Howover, compounds 4, 6 showed moderate inhibiting activity and compounds 2, 3, 7 exhibited weak inhibiting activity. A subsequentin silicodocking study demonstrated the interaction between the compounds and the enzyme. This syudy provided valuable experience for potential natural tyrosinase inhibitors for further analysis.
4 Materials and Methods
4.1 Reagents
40 kg branches and leaves was provided by Jiangxi by Prof. Jincai Lu, Department of Traditional Chinese Materia Medica, Shenyang Pharmaceutical University. A voucher specimen (No.20151213) has been deposited in the Nature Products Laboratory,Shenyang Pharmaceutical University, Liaoning, PR China. Column chromatography (CC) was carried out on D101 macroporous adsorption resin (Tianjin University Chemical Plant, Tianjin, PR China), ODS gel (60-80μm, Merck KGaA, Darmstadt, Germany),Sephadex LH-20 (80-120 mesh, Beijing Greenherbs Science and Technology Development Co., LTD,Beijing, PR China) and silica gel (200-300 mesh,Qingdao Marine Chemical Inc., Qingdao, PR China).All solvents for extraction and chromatography were commercially purchased and routinely distilled prior to use. Arbutin and L-tyrosine were purchased from Sigma-Aldrich (St. Louis, MO, USA).
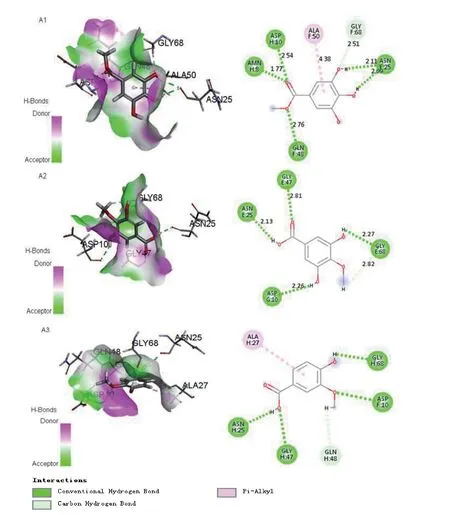
Fig. 3 Molecular docking simulation of compounds 1, 5, 6 to tyrosinase. (A1) H-bonds and hydrophobic interactions between 1 and the tyrosinase pocket in a 3D docking model; H-bonds and hydrophobic interactions between compound 1 and tyrosinase. (A2) H-bonds and hydrophobic interactions between 5 and the tyrosinase pocket in a 3D docking model; H-bonds and hydrophobic interactions between compound 5 and tyrosinase. (A3) H-bonds and hydrophobic interactions between 6 and the tyrosinase pocket in a 3D docking model; H-bonds and hydrophobic interactions between compound 6 and tyrosinase
4.2 Instrumentation
The NMR spectra were recorded on Bruker ARX-300 spectrometers (Bruker Corporation,Bremen, Germany) using trimethylchlorosilane(TMS) as the internal standard, with chemical shifts recorded as δ values. HRESIMS data were measured on a Bruker Micro Q-TOF spectrometer (Bruker Daltonics, Billerica, USA). The UV spectra were measured on a Shimadzu UV-1700 spectrometer(Shimadzu, Tokyo, Japan). CD spectra were recorded on a JASCO JP/J-715 detector from Bio-Logic (MOS 450) in the 200-400 nm wavelength range. An Agilent 1100 HPLC (detector at 210 nm)was used for isolation and purification with a preparative HPLC YMC ODS-A column (Shimadzu,5 μm, 250 × 10 mm, Japan) and a analytical HPLC YMC ODS-A column (Thermo, 5μm, 150 × 4.6 mm,USA).
4.3 Extraction and Isolation
40 kg of branches and leaves was extracted with 70% EtOH at 70 °C for 2 h for twice and then chromatographed over D101 macroporous resin(1.8 kg), using a gradient of EtOH/H2O (30:70→60:40→90:10) and yielded a 70% EtOH/H2O extract(1.8 kg). The extractive was then leached with n-Butyl alcohol and the air-dried extractive was then chromatographed over silica gel column (100-200 mesh), using a gradient of MeOH/CH2Cl2(1:10→2:1) as eluant to yield four fractions A to C. Fraction A (157 g) was then chromatographed over a silica gel column (200-300 mesh), eluted with a gradient of EtOH/H2O (0:100→30:70→60:40→90:10→acetone) to yield subfraction A1 to A2. Subfraction A1 (70 g) was again chromatographed over a HP-20 column, eluting with a gradient of EtOH/H2O (0:100→20:80→40:60→60:40→80:20→90:10) to yield subfraction A1-1 to A1-2, and A1-1 (45 g) was then chromatographed over a ODS column, eluted with a gradient of MeOH/H2O (30:70→100:0) to yield subfraction A1-1-1 to A1-1-4. And A1-1-1 (36 g)was chromatographed over preparative HPLC to yield compounds 1 (10 g), 3 (5 mg) and 4 (5 mg)respectively, while compound 2 (3 mg) and 6 (5 mg)was obtained from A1-1-2 and compounds 5 (9 mg)and 7 (8 mg) were purified from A1-1-3. The isolated compounds were identified by comparison of their experimental spectroscopic NMR data with literature values.
4.3.1 Methyl 3,4,5-trihydroxybenzoate(1)
HRESIMS at m/z 207.0372 [M + Na]+,1H NMR (400 MHz, DMSO-d6):δH9.26 (2H, s,3,5-OH), 8.98 (1H, s, 4-OH), 3.73 (3H, s, 7-OCH3),6.97 (2H, s, 2,6-H).13C NMR (100 MHz, DMSO-d6)δC: 169.2 (-COOH), 137.9 (C-1), 124.9 (C-4),109.6 (C-3, 5), 143.9 (C-2, 6), 166.6 (C-7), 53.3(OCH3).
4.3.2 3,5-dimethoxy-4-hydroxybenzoic acid(2)
1H NMR (400 MHz, DMSO-d6):δH7.21 (2H,s, 2,6-H), 3.81 (6H, s, 3,5-OCH3), 7.96 (1H, s,4-OH), 3.69 (3H, s, 7-OCH3).13C NMR (100 MHz,DMSO-d6):δC168.2 (COOH), 147.8 (C-3, 5), 141.1(C-4), 122.5 (C-1), 105.9 (C-2, 6), 56.6 (3,5-OCH3).
4.3.3 3,4,5-trimethoxy-benzoic acid(3)
1H NMR (400 MHz, DMSO-d6):δH6.05 (2H,s, 2,6-H), 3.69 (6H, s, 3,5-OCH3), 3.54 (3H, s,4-OCH3).13C NMR (100 MHz, DMSO-d6):δC171.7(COOH), 153.0 (C-3,5), 143.0 (C-4), 123.9 (C-1),107.8 (C-2,6), 60.75 (4-OCH3), 57.3 (3,5-OCH3).
4.3.4 3,4-dimethoxy-5-hydroxybenzoic acid(4)
1H NMR (400 MHz, DMSO-d6):δH7.02 (1H,d,J=1.98, 2-H), 7.08 (1H, d,J=1.98, 6-H), 3.76(3H, s, 4-OCH3), 3.78 (3H, s, 5-OCH3).13C NMR(100 MHz, DMSO-d6):δC166.4 (COOH), 124.8(C-1), 111.7 (C-2), 151.1 (C-3), 142.1 (C-4), 152.5(C-5), 105.2 (C-6), 56.7 (3-OCH3), 60.6 (4-OCH3).
4.3.5 3,5-dihydroxy-4-methoxybenzoic acid(5)
1H NMR (400 MHz, DMSO-d6):δH6.98 (2H, s,2,6-H), 3.72 (3H, s, 4-OCH3).13C NMR (100 MHz,DMSO-d6):δC169.5 (COOH), 122.2 (C-1), 110.9(C-2,6), 148.6 (C-3,5), 140.8 (C-4), 53.6 (4-OCH3).
4.3.6 3-methoxy-4-hydroxybenzoic acid(6)
1H NMR (400 MHz, DMSO-d6):δH7.43 (1H,d,J=2.04, 2-H), 7.5 (1H, d,J=8.26,2.04, 6-H), 3.82(3H, s, 3-OCH3), 6.86 (1H, d,J=8.26, 5-H).13C NMR (100 MHz, DMSO-d6):δC170.1 (COOH),123.2 (C-1), 114.8 (C-2), 152.5 (C-3), 147.6 (C-4),112.8 (C-5), 123.6 (C-6), 56.4 (3-OCH3).
4.3.7 3-methoxy-4-hydroxybenzaldehyde(7)
1H NMR (400 MHz, DMSO-d6):δH7.29 (1H,d,J=1.92, 2-H), 7.2 (1H, d,J=8.14, 1.99, 6-H), 3.81(3H, s, 3-OCH3), 9.95 (1H, s, 4-OH), 6.88 (1H, d,J=8.15, 5-H).13C NMR (100 MHz, DMSO-d6):δC190.8 (-CHO), 153.3 (C-4), 148.6 (C-3), 127.7(C-1), 126.2 (C-6), 114.6 (C-5), 111.3 (C-2), 55.8(-OCH3).
4.4 Assay of Inhibitory Activity to Tyrosinase
Tyrosinase activity inhibition was assessed according to following procedure. A L-tyrosine solution (40 μL of 0.1 mg/mL) in phosphate-buffered saline (PBS) solution (25 mM, pH 6.8), 40 μL of sample (compounds 1-7 and arbutin) dissolved in 20% MeOH-H2O, and 80 μL of PBS solution(25 mM, pH 6.8) were sequentially added to a 96-well microplate and well mixed. In addition,instead of a sample in 20% MeOH solution, a 20%MeOH solution was added to a blank solution. The assay mixture was incubated at 37 °C for 30 min.Before and after incubation, the amount of dopachrome produced in the reaction mixture was measured at 492 nm in the microplate reader. The inhibitory percentage of tyrosinase was calculated by the following equation:
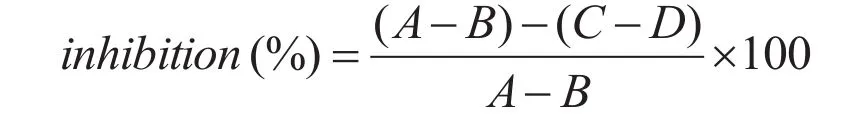
whileAis absorbance of blank solution after incubation,Bis absorbance of blank solution before incubation,Cis absorbance of sample solution after incubation, andDis absorbance of sample solution before incubation.
4.5 Molecular Docking
These structure optimizations and energy minimizations were performed with ChemBio3D Ultra 14.0. The crystalline structure of the mushroom tyrosinase (PDB: 2Y9X) was obtained from the RCSB protein data bank. The protein was edited using Discovery Studio 4.3 to remove all water molecules and all hydrogen atoms were added. The program Molegro Virtual Docker 4.0 was applied for the molecular docking simulations between tyrosinase and phenolic acids. Docking results were attributed to the different scoring functions. The best scoring was opted as the most rationalized binding mode between the compounds and tyrosinase.
4.6 statistical analysis
Tests were conducted in triplicate, with data expressed as the means ± standard deviation (n= 3).The bar column was depicted by OriginLab. The significant level of differences in means was detected using a two-way ANOVA test. Statistics were analyzed using Graphpad Prism software.Statistic significances were defined at (**)p<0.05,(***)p<0.01.
[1] Prota G. Progress in the chemistry of melanins and related metabolites. Medicinal Research Reviews, 1988,8: 525-556.
[2] Priestley GC. Molecular aspects of dermatology. Cellular and Molecular Biology, 1993, 41:453-454.
[3] Mayer AM. Polyphenol oxidases in plants: recent progress. Phytochemistry, 1987, 26: 11-20.
[4] Pawelek JM, Körner AM. The biosynthesis of mammalian melanin. American Scientist, 1982, 70: 136-145.
[5] Lou LL, Liu S, Yan ZY, et al. Tetrahydro-â-Carboline alkaloids fromCarthamus tinctoriusL.with tyrosinase inhibitory activity. Phytochemistry Letters, 2017, 22:107-112.
[6] Saghaie L, Pourfarzam M, Fassihi A, et al. Synthesis and tyrosinase inhibitory properties of some novel derivatives of kojic acid. Research in pharmaceutical sciences, 2013, 8: 233-242.
[7] Li YL, Ooi LSM, Wang H, et al. Antiviral activities of medicinal herbs traditionally used in southern mainland China. Phytotherapy research, 2004, 18: 718-722.
[8] Xie CY, Lin LW. Study on the chemical constituents ofPithecellobium clypearia. Zhongyaocai, 2011, 34:1060-1062.
[9] Li YL, Li KM, Su MX, et al. Studies on antiviral constituents in stems and leaves ofPithecellibium clypearia. China journal of Chinese materia medica,2006, 31: 397-400.
[10] Liu CY, Lai YL, Lin CP, et al. Anti-tumor effect ofArchidendron lucidum(Benth.) against esophageal cancer, colorectal cancer and hepatoma. Journal of Medicinal Plants Research, 2011, 5: 5221-5229.
[11] Kang J, Liu C, Wang HQ. Studies on the bioactive flavonoids isolated fromPithecellobium clypeariaBenth. Molecules, 2014, 19: 4479-4490.
[12] Kujumgiev A, Tsvetkova I, Serkedjieva Y, et al.Antibacterial, anti-fungal and antiviral activity of propolis of different geographic origin. Journal of ethnopharmacology, 1999, 64: 235-240.
[13] Machida K and Kikuchi M. Norisoprenoids fromViburnum dilatatum. Phytochemistry, 1996, 41: 1333-1336.
[14] Chen H, Wang Q, Wang GR, et al. Chemical constituents ofIncarvillea delavayi. Natural Product Research and Development, 2012, 24: 1743-1746.
[15] Wang ZY, Wang LN, Qiu L, et al. Isolation and Identification of Phenolic Constituents ofSanguisorbaeRadix. Chinese Journal of Experimental Traditional Medical Formulae, 2017, 23: 82-85.
[16] Farag MA, Al-Mahdy DA, Salah El DR, et al.Structure- activity relationships of antimicrobial gallic acid derivatives fromPomegranateandAcaciaFruit Extracts against Potato Bacterial Wilt Pathogen.Chemistry & Biodiversity, 2015, 12: 955-962.
[17] Zheng RR, Ya J, Wang WJ, et al. Chemical studies on roots ofFicus hirta. Zhong guo Zhong yao Zazhi, 2013,38: 3696-3701.
[18] Polat M, Guclu SF, Okatan V, et al. Determination of phenolic compounds inAronia melanocarpagenotypes grown in Turkey. Oxidation Communications, 2017, 40:131-137.
杂志排行
Asian Journal of Traditional Medicines的其它文章
- Phytochemical and chemotaxonomic studies on the Trillium tschonoskii Maxim.
- Network pharmacology-based virtual screening of natural products from the samara of Ailanthus altissima (Mill.)Swingle for identification of anticancer therapeutics
- Prediction of the active ingredients and potential targets of Chinese herb Juglans mandshurica Maxim. against liver cancer based on network pharmacology
- Contribution Regulations for Asian Journal of Traditional Medicines
