Role of brain-derived neurotrophic factor during the regenerative response after traumatic brain injury in adult zebrafish
2018-06-21PietroCacialliAntonioPalladinoCarlaLucini
Pietro Cacialli, Antonio Palladino, Carla Lucini
Department of Veterinary Medicine, University of Naples Federico II, Naples, Italy
Introduction
Traumatic brain injury (TBI) is a dramatic event which annually involves million individuals, bringing neurological dysfunction or depriving patients of their self-sufficiency.Several mammalian animal models of TBI have been used,mostly rat and mouse. However, reparative mechanisms in mammalian brain are very limited, and newly formed neurons do not survive for long time, likely due to a non-suitable local environment. In contrast, the brain of adult fish has high regenerative properties after brain injury, with fast and complete recovery of damaged area. The striking differences in regenerative properties between mammalian and fish brain have been ascribed to remarkable different adult neurogenesis processes. In mammalian brain only two neurogenic regions are well described: the subventricular zone of the lateral ventricle and the subgranular zone of the dentate gyrus in the hippocampus. In fish, neurogenesis continues along the entire adult life in numerous stem cell niches found in various brain sub-divisions and mostly along ventricles, from which newly differentiated neurons start to migrate. Thus, brain regenerative mechanisms in fish have been the subject of numerous studies, due to their potential for development of new therapeutic approaches.
Brain-Derived Neurotrophic Factor (BDNF)and Its Receptors
BDNF is a member of neurotrophin (NT) family. NTs comprise, in addition to BDNF, the nerve growth factor (NGF),NT-3, NT-4/5, NT 6/7, the latter described only in fish. Regarding BDNF, three different forms derives from thebdnfgene: the precursor proBDNF, mature BDNF and the prodomain of BDNF (Hempstead, 2015). The preproBDNF carries a signal peptide that is cleaved off, forming proBDNF.This latter is further processed to generate the mature BDNF.Both pro and mature BDNF are secreted. Following secretion,proBDNF can be cleaved by extracellular proteases, leading to local formation of mature BDNF. Also the prodomain recently resulted a detectable protein and undergoes activity-dependent secretion from hippocampal neurons (Anastasia et al., 2013). The neurotrophins act by binding to two kinds of receptors, the tyrosine kinase receptors (Trk A, B and C) and the p75 receptor. Mature BDNF can bind to two TrkB receptor isoforms abundantly expressed in the brain: full-length TrkB and truncated TrkB, which lacks the intracellular kinase domain and thus cannot undergo autophosphorylation. The full length TrkB, activated by mature BDNF, dimerizes and,in the intracytoplasmic domain, phosphorylation at specific tyrosine residues creates docking sites for cytoplasmic adaptor proteins. Three major signaling pathway were recognized: 1)the phosphoinositide 3-kinase (PI3K), which promotes neuronal survival; 2) mitogen-activated protein kinases (MAPKs),which induces neuronal differentiation; 3) phospholipase Cγ1(PLCγ1), which promotes synaptic and neuronal structural plasticity (Longo and Massa, 2013).
Truncated TrkB binds to and internalizes BDNF, but it does not undergo autophosphorylation to function as a true tyrosine kinase receptor. Thus, it was defined as a dominant-negative receptor which indirectly inhibit BDNF function. However, recent studies indicate that truncated TrkB sequesters and translocates BDNF, induces neurite outgrowth, regulates cytoskeletal changes in astrocytes and glioma cells, regulates Rho GTPase activity, and may stimulate PLCγ and MAPK signaling (Fenner, 2012).
Also, mature BDNF binding to dimerized p75NTR, depending on the context, may enhance neurotrophin binding to TRK receptors, reinforce Trk signalling through AKT and MAPKs, and further promote survival through the nuclear factor-κB (NF-κB) pathway, or antagonize the actions of TRK through the activation of Jun N-terminal kinase (JNK)and RhoA pathways.
Pro-BDNF binding to p75NTRassociated to sortilin, induces apoptosis. The process requires the regulated shedding of the extracellular domain of p75NTRby the metalloproteinase ADAM17 and intramembrane proteolysis of the receptor by γ-secretase. Regulated intramembrane proteolysis results in the release of a p75NTRintracellular domain that mediates apoptotic cell deathviaJNK and caspase activation (Longo and Massa, 2013). p75NTRcan also form part of the Nogo receptor complex, which is activated by myelin proteins to inhibit axonal growth (Cagnolini, 2008). The pro-domain of pro-neurotrophins can concurrently bind to the vacuolar protein sorting 10 (VPS10) family member sortilin (also known as NTR3), which allows the formation of a ternary complex with p75NTR(Carter et al., 2010).
The zebrafish BDNF gene is more compact than the mammalian gene. Moreover, it is as complex in its promoter structure and patterns of differential promoter expression as is its murine counterpart. At least five promoters and possibly as many as six concern the zebrafish BDNF gene.Two of the seven 5′ non-coding exons associated with promoters were found in the embryo before transcription starts and therefore must be parts of maternally transmitted transcripts. Of the adult organs examined, the heart expressed a single 5′ exon whereas the brain, liver and eyes expressed four of the seven 5′ exons (Heinrich and Pagtakhan, 2004).The DNA-deduced amino acid sequence of the processed mature BDNF in the teleost fishXiphophorus maculatumshowed 90% identity with the mouse sequence (Götz et al.,1992). Also, the primary amino acid sequences of zebrafish and human BDNF are 91% identical (Hashimoto and Heinrich, 1997; Heinrich and Lum, 2000; Lum et al., 2001;Heinrich and Pagtakhan, 2004). Regarding BDNF receptors, in zebrafish there are two genes encoding for TrkB(Martin et al., 1995) and TrkB proteins are > 90% identical with the mammalian counterpart (Sandell et al., 1994). The amino acid residues of the kinase domain involved in signal transduction are identical in zebrafish and mammalian Trks(Heinrich and Lum, 2000). Full-length zebrafishp75cDNA was cloned and is predicted to encode a protein with 61%similarity to both human and mouse p75. Four cysteine-rich domains (CRD1–4) were identified in the extracellular region, and a death domain at the C-terminal tail of the intracellular region of zebrafish p75 (Han et al., 2014).
BDNF is Involved in Brain Functions and Regenerative Mechanisms
BDNF acts as survival factor in the peripheral nervous system (Sendtner et al., 1992), whereas in the brain, it rather mediates region-specific effects on synaptic function and neuronal morphology (Sasi et al., 2017). BDNF is also involved in ageing (Silhol et al., 2005), depression (Molendijk et al., 2014), schizophrenia (Wysokiński, 2016) and Alzheimer’s disease (Tapia-Arancibia et al., 2008). Finally, BDNF plays an important role following TBI. Severe penetrating TBI caused an increase of BDNF mRNAs and protein levels in rat brain homogenates (Rostami et al., 2014). In contrast,mild severity level of TBI obtained by lateral percussion displayed unchanged BDNF mRNAs levels in hippocampal homogenates (Schober et al., 2012). Also increase of BDNF mRNAs expressing cells was reported in the contralateral side of lesion (Rostami et al., 2014). In injury side, studies have reported increase (Felderhoff-Mueser et al., 2002), and decrease (Rostami et al., 2014) of BDNF mRNAs expressing cells 24 hours post lesion. Finally, bilateral increase was described by Hicks et al. (1999).
Additionally, the mRNA expression of full length and truncated TrkB and p75 receptors increased after the acute phase of experimental injury (Rostami et al., 2014). After TBI, BDNF appears to mediate some beneficial treatments,such as: a) simvastatin administration, which induces BDNF upregulation, promotes neurogenesis in the dentate gyrus of the hippocampus, thereby leading to the restoration of cognitive function in rats (Wu et al., 2008); b) transcranial low-level laser light therapy to the brain, partly mediated by stimulation of BDNF in the dentate gyrus of the hippocampus and the subventricular zone, seems to encourage synaptogenesis (Xuan et al., 2015); c) exercise, which upregulates BDNF within the hippocampus, is associated with an enhancement of cognitive recovery both in human and rodents (Griesbach et al., 2009).
Therapeutic potential of BDNF for TBI is restricted due to its short half-life and inability to cross the blood-brain barrier. Recently however, the flavonoid 7,8-dihydroxy flavone(7,8-DHF), a small TrkB agonist that mimics BDNF function, has shown similar effects as BDNF in promoting neuronal survival and regeneration following TBI (Wurzelmann et al., 2017).
Zebra fish is A Suitable Model for Studies on Regenerative Processes
Zebra fish (Danio rerio) is a teleost fish widely used as model for genetic developmental studies, possesses high regenerative properties of the central nervous system. Profound stab on encephalon revealed a series of regenerative processes,forming mature neurons with marker profile similar to the preexisting neurons (Kishimoto et al., 2012). The high regenerative properties of zebrafish brain can be correlated to the intense adult neurogenesis, mainly localized in 16 neurogenic niches (Grandel et al., 2006). The zebrafish dorsal telencephalon comprises the most studied neuronal stem cell niches (Grandel et al., 2006; Kishimoto et al., 2012) and its dorso-lateral zone is retained to be equivalent to the medial pallium (hippocampus) of mammals (Salas et al., 2003),which contains one of the two constitutive neurogenic niches of mammals: the subgranular zone of the dentate gyrus.
Does BDNF Exert A Particular Role in Telencephalic Regenerative Capacities of Zebrafish Brain?
BDNF was previously identified in the developing and adult brain of zebrafish (Cacialli et al., 2016; Gatta et al., 2016),as well as in other teleost fish, such asCichlasoma dimerus(Vissio et al., 2008), European eel (Dalton et al., 2009) and african turquoise killifish (D’Angelo et al., 2014). However,because the role of BDNF in the zebrafish telencephalic regenerative processes had not been previously investigated,our recent study (Cacialli et al., 2018) was aimed to this issue and the results achieved represent the first report concerning BDNF involvement in injured brain of non-mammalian vertebrates. The research was performed using stab wound in the dorso-lateral telencephalon of zebrafish, the teleost brain area equivalent to the hippocampus of mammals, in order to mimic the cellular phenomena of TBI during adulthood.During the experimentation we noticed:
1) BDNF mRNAs levels increased in the brain after injury.A remarkable increase of BDNF mRNAs levels in homogenates of the whole lesioned telencephalon occurred at 1 day post lesion (dpl), compared to control unlesioned animals.Then BDNF mRNA levels decreased with time after lesion.
2) BDNF mRNAs expressing cells were neurons and their number increased mostly at early time after injury. BDNF mRNAs expressing cells in the whole dorsal telencephalon clearly increased in number early, at 1 dpl, compared to control animals. The number of BDNF mRNA expressing cells decreased as time after lesion passed, but remained significantly higher than the control. In situ hybridization/immunohistochemistry analysis demonstrated that BDNF mRNAs was co-localized with acetylated-tubulin (a marker of mature neurons), very rarely with HuC/D (a marker of early differentiated neurons) and never with PCNA (a proliferative marker). Thus, the majority of BDNF mRNAs expressing cells in the lesioned telencephalon, for the entire examined post injury period, were retained as mature neuronal populations. The findings were consistent with our previous observations in the brain of juvenile zebrafish and adult unlesioned brain of zebrafish (Cacialli et al., 2016).This increasing number of BDNF-expressing cells following injury resulted from mature neurons, which triggered BDNF translation after lesion and not from newly generated neurons. The maximum increase of BDNF mRNAs expressing neurons occurred soon after the lesion: the time frame is too short for neuronal precursor cells migration, moving from ventricular zone to the injury site where they differentiate into mature neurons (Kishimoto et al., 2012).
3) BDNF mRNAs expressing neurons were more numerous in the lesioned side. BDNF mRNAs expressing neurons were more numerous in the injured side compared to the contralateral side at each time points (1, 4, 7, and 15 days).This difference was remarkable 1dpl after the lesion, and became less evident from 4 day onwards because a slight increase of BDNF mRNAs expressing neurons was seen from 4 dpl to 15 dpl in the contralateral side.
Are the Different Responses in Zebra fish and Rodent Brain Mediated through Different BDNF Receptors?
It is known thatbdnfundergoes post-translational mechanisms leading to proBDNF and mature BDNF, which exert opposite effects. The receptor TrkB binds with high affinity to mature BDNF, mediating survival of neurons, while the receptor p75, with sortilin, binds with high affinity to proBDNF, mediating apoptotic events. Thus, the post-injury loss of neurons in rodent could be ascribed to the induction of p75 receptor and to some extent to proBDNF (Rostami et al., 2014). As further evidence, p75 mutant mice as well as mice treated with the p75 antagonist or the TrkB agonist exhibited reduced post traumatic events, such as neuronal death and degeneration, and reduced astrocytosis (Alder et al., 2016). In contrast, adult gerbil CA1 neurons, which showed BDNF and TrkB colocalization, resulted resistant to damage during forebrain ischemia (Ferrer et al., 1997).
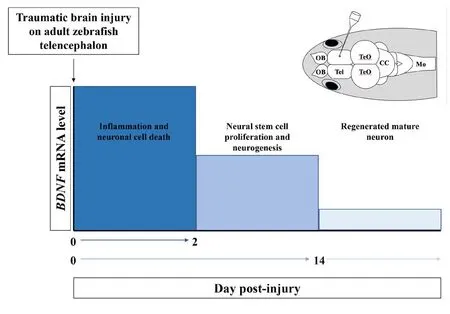
Figure 1 Brain-derived neurotrophic factor (BDNF) mRNA levels referred to time intervals characterized by cellular events from the lesion.
In zebrafish, preliminary results showed an increase of the receptor p75NTRwithin 24 hours after telencephalic injury.Instead, TrkB increased at 24–48 hours after injury and this increase was sustained during following 14 days (unpublished-data). It is tempting to consider p75 as proBDNF receptor mediating neuronal loss immediately after the injury and TrkB as mature BDNF receptor involved during the fast and complete recovery and neural repair of the telencephalic lesioned area, but further experimental studies are necessary(Figure 1).
Could BDNF Mediate the Acute Inflammation during the Regenerative Response after TBI in Adult Zebra fish?
It has been shown that BDNF is secreted by immune cells in multiple sclerosis whereas neurons and astrocytes express TrkB receptor, supporting the concept of neuroprotective immunity. (Kerschensteiner et al., 2003). In zebrafish, by using cerebro-ventricular microinjection of immunogenic particles and immunosuppression assays, it has been observed that inflammation is required and sufficient to induce the proliferation of neural progenitors and subsequent neurogenesis by activating injury-induced molecular programs that can be observed after 24–48 hours post-traumatic brain injury (Kyritsis et al., 2012). Because in our study (Cacialli et al., 2018), BDNF mRNA serum levels and expressing neurons highly and suddenly increased after the lesion, it could be plausible its involvement in inflammation process occurring after telencephalic injury.
Concluding Remarks
Our results demonstrated that: a) BDNF mRNAs induction was observed in mature neuronal populations; b) the peak of BDNF mRNAs expression was observed early after the lesion in the area and in neurons surrounding the lesion; c)BDNF mRNAs expression was slightly increased in the contralateral side.
Comparing our data with numerous studies in the literature, we can define BDNF increasing after TBI as an early response to damage in both zebrafish and rodents. However, remarkable difference exists: in zebrafish BDNF mRNA presence persists around the lesioned area, while in rodents BDNF expression, because of neuronal loss and glial scar formation in the injured area, persists only in the contralateral side. Considering the complete repair of the damaged area in fish, it could be tempting to consider BDNF as a factor contributing to create a permissive environment that enables the establishment of new neuronal population in damaged brain.
Author contributions:PC collected data, designed all figures, partecipated discussion and revised the manuscript; AP partecipated discussion and revised the manuscript; CL conceived and wrote the manuscript.
Conflicts of interest:The authors declare no conflicts of interest.
Financial support:None.
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer:Peng Luo, Fourth Military Medical University, China.
Alder J, Fujioka W, Giarratana A, Wissocki J, Thakkar K, Vuong P, Patel B,Chakraborty T, Elsabeh R, Parikh A, Girn HS, Crockett D, Thakker-Varia S(2016) Genetic and pharmacological intervention of the p75NTR pathway alters morphological and behavioural recovery following traumatic brain injury in mice. Brain Inj 30:48-65.
Anastasia A, Deinhardt K, Chao MV, Will NE, Irmady K, Lee FS, Hempstead BL, Bracken C (2013) Val66Met polymorphism of BDNF alters prodomain structure to induce neuronal growth cone retraction. Nat Commun 4:2490.
Cacialli P, Gueguen MM, Coumailleau P, D’Angelo L, Kah O, Lucini C, Pellegrini E (2016) BDNF expression in larval and adult zebrafish brain: distribution and cell identification. PLoS One 11:e0158057.
Cacialli P, D’Angelo L, Kah O, Coumailleau P, Gueguen MM, Pellegrini E, Lucini C (2018) Neuronal expression of brain derived neurotrophic factor in the injured telencephalon of adult zebrafish. J Comp Neurol 526:569-582.
Carter BD, Feng N, Paolocci N (2010) The p75 neurotrophin receptor, semaphorins, and sympathetic traffic in the heart. Am J Physiol Heart Circ Physiol 298:H1633-1636.
D’Angelo L, De Girolamo P, Lucini C, Terzibasi ET, Baumgart M, Castaldo L,Cellerino A (2014) Brain-derived neurotrophic factor: mRNA expression and protein distribution in the brain of the teleost Nothobranchius furzeri. J Comp Neurol 522:1004-1030.
Dalton VS, Roberts BL, Borich SM (2009) Brain derived neurotrophic factor and trk B mRNA expression in the brain of a brain stem-spinal cord regenerating model, the European eel, after spinal cord injury. Neurosci Lett 461:275-279.
Felderhoff-Mueser U, Sifringer M, Pesditschek S, Kuckuck H, Moysich A, Bittigau P, Ikonomidou C (2002) Pathways leading to apoptotic neurodegeneration following trauma to the developing rat brain. Neurobiol Dis 11:231-245.
Fenner BM (2012) Truncated TrkB: beyond a dominant negative receptor. Cytokine Growth Factor Rev 23:15-24.
Ferrer I, Ballabriga J, Marti E, Pozas E, Planas AM, Blasi J (1997) BDNF and TrkB co-localize in CA1 neurons resistant to transient forebrain ischemia in the adult gerbil. J Neuropathol Exp Neurol 56:790-797.
Götz R, Raulf F, Schartl M (1992) Brain-derived neurotrophic factor is more highly conserved in structure and function than nerve growth factor during vertebrate evolution. J Neurochem 59:432-442.
Gatta C, Altamura G, Avallone L, Castaldo L, Corteggio A, D’Angelo L, de Girolamo P, Lucini C (2016) Neurotrophins and their Trk-receptors in the cerebellum of zebrafish. J Morphol 277:725-736.
Grandel H, Kaslin J, Ganz J, Wenzel I, Brand M (2006) Neural stem cells and neurogenesis in the adult zebrafish brain: origin, proliferation dynamics,migration and cell fate. Dev Biol 295:263-277.
Griesbach GS, Hovda DA, Gomez-Pinilla F (2009) Exercise-induced improvement in cognitive performance after traumatic brain injury in rats is dependent on BDNF activation. Brain Res 1288:105-115.
Han HW, Chou CM, Chu CY, Cheng CH, Yang CH, Hung CC, Hwang PP,Lee SJ, Liao YF, Huang CJ (2014) The Nogo-C2/Nogo receptor complex regulates the morphogenesis of zebrafish lateral line primordium through modulating the expression of dkk1b, a Wnt signal inhibitor. PLoS One 9:e86345.
Hashimoto M, Heinrich G (1997) Brain-derived neurotrophic factor gene expression in the developing zebrafish. Int J Dev Neurosci 15:983-997.
Heinrich G, Lum T (2000) Fish neurotrophins and Trk receptors. Int J Dev Neurosci 18:1-27.
Heinrich G, Pagtakhan CJ (2004) Both 5′ and 3′ flanks regulate Zebrafish brain-derived neurotrophic factor gene expression. BMC Neurosci 5:19.
Hempstead BL (2015) Brain-derived neurotrophic factor: three ligands, many actions. Trans Am Clin Climatol Assoc 126:9-19.
Hicks RR, Li C, Zhang L, Dhillon HS, Prasad MR, Seroogy KB (1999) Alterations in BDNF and trkB mRNA levels in the cerebral cortex following experimental brain trauma in rats. J Neurotrauma 16:501-510.
Kerschensteiner M, Stadelmann C, Dechant G, Wekerle H, Hohlfeld R (2003)Neurotrophic cross-talk between the nervous and immune systems: implications for neurological diseases. Ann Neurol 53:292-304.
Kishimoto N, Shimizu K, Sawamoto K (2012) Neuronal regeneration in a zebrafish model of adult brain injury. Dis Model Mech 5:200-209.
Kyritsis N, Kizil C, Zocher S, Kroehne V, Kaslin J, Freudenreich D, Iltzsche A,Brand M (2012) Acute inflammation initiates the regenerative response in the adult zebrafish brain. Science 338:1353-1356.
Longo FM, Massa SM (2013) Small-molecule modulation of neurotrophin receptors: a strategy for the treatment of neurological disease. Nat Rev Drug Discov 12:507-525.
Lum T, Huynh G, Heinrich G (2001) Brain-derived neurotrophic factor and TrkB tyrosine kinase receptor gene expression in zebrafish embryo and larva. Int J Dev Neurosci 19:569-587.
Martin SC, Marazzi G, Sandell JH, Heinrich G (1995) Five Trk receptors in the zebrafish. Dev Biol 169:745-758.
Molendijk ML, Spinhoven P, Polak M, Bus BA, Penninx BW, Elzinga BM (2014)Serum BDNF concentrations as peripheral manifestations of depression:evidence from a systematic review and meta-analyses on 179 associations(N=9484). Mol Psychiatry 19:791-800.
Rostami E, Krueger F, Plantman S, Davidsson J, Agoston D, Grafman J, Risling M (2014) Alteration in BDNF and its receptors, full-length and truncated TrkB and p75NTR following penetrating traumatic brain injury. Brain Res 1542:195-205.
Salas C, Broglio C, Rodríguez F (2003) Evolution of forebrain and spatial cognition in vertebrates: conservation across diversity. Brain Behav Evol 62:72-82.
Sandell JH, Martin SC, Heinrich G (1994) The development of neurotrophin receptor Trk immunoreactivity in the retina of the zebrafish (Brachydanio rerio). Brain Res Dev Brain Res 81:192-200.
Sasi M, Vignoli B, Canossa M, Blum R (2017) Neurobiology of local and intercellular BDNF signaling. Pflugers Arch 469:593-610.
Schober ME, Block B, Requena DF, Hale MA, Lane RH (2012) Developmental traumatic brain injury decreased brain derived neurotrophic factor expression late after injury. Metab Brain Dis 27:167-173.
Sendtner M, Holtmann B, Kolbeck R, Thoenen H, Barde YA (1992) Brain-derived neurotrophic factor prevents the death of motoneurons in newborn rats after nerve section. Nature 360:757-759.
Silhol M, Bonnichon V, Rage F, Tapia-Arancibia L (2005) Age-related changes in brain-derived neurotrophic factor and tyrosine kinase receptor isoforms in the hippocampus and hypothalamus in male rats. Neuroscience 132:613-624.
Tapia-Arancibia L, Aliaga E, Silhol M, Arancibia S (2008) New insights into brain BDNF function in normal aging and Alzheimer disease. Brain Res Rev 59:201-220.
Vissio PG, Canepa MM, Maggese MC (2008) Brain-derived neurotrophic factor (BDNF)-like immunoreactivity localization in the retina and brain of Cichlasoma dimerus (Teleostei, Perciformes). Tissue Cell 40:261-270.
Wu H, Lu D, Jiang H, Xiong Y, Qu C, Li B, Mahmood A, Zhou D, Chopp M(2008) Simvastatin-mediated upregulation of VEGF and BDNF, activation of the PI3K/Akt pathway, and increase of neurogenesis are associated with therapeutic improvement after traumatic brain injury. J Neurotrauma 25:130-139.
Wurzelmann M, Romeika J, Sun D (2017) Therapeutic potential of brain-derived neurotrophic factor (BDNF) and a small molecular mimics of BDNF for traumatic brain injury. Neural Regen Res 12:7-12.
Wysokiński A (2016) Serum levels of brain-derived neurotrophic factor(BDNF) and neurotrophin-3 (NT-3) in depressed patients with schizophrenia. Nordic journal of psychiatry 70:267-271.
Xuan W, Agrawal T, Huang L, Gupta GK, Hamblin MR (2015) Low-level laser therapy for traumatic brain injury in mice increases brain derived neurotrophic factor (BDNF) and synaptogenesis. J Biophotonics 8:502-511.
杂志排行
中国神经再生研究(英文版)的其它文章
- Natural polyphenols effects on protein aggregates in Alzheimer’s and Parkinson’s prion-like diseases
- How random is the random forest ? Random forest algorithm on the service of structural imaging biomarkers for Alzheimer’s disease: from Alzheimer’s disease neuroimaging initiative (ADNI) database
- Protective effects of gonadal hormones on spinal motoneurons following spinal cord injury
- INVITED REVIEW
- Role of presynaptic calcium stores for neural network dysfunction in Alzheimer’s disease
- Potential utility of aldose reductasede ficient Schwann cells IKARS1 for the study of axonal degeneration and regeneration
