Synthesis of butter fly-like BiVO4/RGO nanocomposites and their photocatalytic activities☆
2018-05-26LiangliangZhangAolanWangNanZhuBaochangSunYanLiangWeiWu
Liangliang Zhang ,Aolan Wang ,Nan Zhu ,Baochang Sun ,*,Yan Liang ,Wei Wu
1 State Key Laboratory of Organic-Inorganic Composites,Beijing University of Chemical Technology,Beijing 100029,China
2 Research Center of the Ministry of Education for High Gravity Engineering and Technology,Beijing University of Chemical Technology,Beijing 100029,China
3 Beijing Urban Drainage Monitoring Center Co.,Ltd.,Beijing 100061,China
1.Introduction
Water environment is not only essential to the balance of ecological environment,but also crucial to the health of human beings.With the ever-increasing emissions of pollutants,water pollution becomes more and more serious and arouses the concern of the whole society.Wastewater containing heavy metal ions or organic pollutants usually has great toxicity and carcinogenicity for environment and human being[1,2].Thus,water purification is of greatsignificance in mitigating the increasingly serious water resource crisis as well as tackling the increasing concern over the water pollution.A wide range of traditional approaches,such as adsorption[3],ion exchange[4],biological treatment[5]and membranes[6,7]have been widely used in the water pollutant treatment.However,these methods usually have been identified to be inefficient and cost-ineffective,which could also possibly lead to the production of secondary pollution.
Photocatalysis technology has by far drawn considerable concern on pollutant degradation and water purification during the past decades.Besides the advantages of low cost and green chemistry property,it offers an attractive route to meetthe globalchallenges associated with the energy and sustainability by utilizing of abundant solar resources[8–10].TiO2as the benchmark material for many photocatalytic reactions has been widely investigated.However,its application is restricted by its wide band gap(3.2 eV)which requires ultraviolet(UV)irradiation for photocatalytic activation.It's well known that the UV region only accounts for 4%of the entire solar radiation,while 45%of the energy belongs to visible light[11,12].In view of the efficient utilization of visible light,the major proportion of solar spectrum and artificial light sources,the development of visible-light-driven photocatalysts with high activity has already attracted great attention.
Many metaloxide semiconductors,such as Ag3VO4,ZnWO4,NaTaO3,BiVO4,and FeWO4,have been developed as photocatalysts with visible light activities[13–18].With a band gap energy of 2.4 eV,monoclinic bismuth vanadate(BiVO4)is a suitable candidate for the purification of polluted water under sunlight irradiation owing to its favorable properties such as non-toxicity,low cost and high stability against photocorrosion[19,20].Unfortunately,the photocatalytic activity of pure monoclinic BiVO4is seriously limited due to its poor charge transport property and weak surface absorption performance,which significantly restrict the practical applications of monoclinic BiVO4in photocatalysis.In order to solve these problems,efforts have been devoted to improve the photocatalytic activity and obtain enhanced application properties,which includes using nano-sized particles,obtaining hetero-junction structure,loading co-catalysts,as well as doping[21–27].
Graphene-related material(as well as RGO),as a two-dimensional(2D)crystalline material,has attracted a lot of scientific interest because of its excellent mechanical,electrical and thermal characters.Utilizing graphene as a supporting material to disperse and stabilize nanoparticles for potential application has achieved a lot of success in catalysis[28–30].In view of this,compositing BiVO4with graphene has been considered as a promising method to overcome the disadvantage of pure BiVO4.Firstly,graphene could enhance the transport of photo-generated electrons and holes in semiconductor particles,owing to the abundance of delocalized electrons from the conjugated sp2-bonded carbon network[31,32].For instance,Gong describes a construction of RGO/BiVO4composite with maximized interfacial coupling,which shows improved photocatalytic activity and enhanced charge separation efficiency[33].Secondly,graphene has large surface area,which can provide sufficient active sites and effectively inhibit the aggregation of these nanoparticles,thereby improving the adsorption capacity for pollutants.
Many methods have been developed for the preparation of graphene/BiVO4composites involving sol–gel method,photocatalytic reduction method and hydrothermal method.However,these methods usually call for complex process and strict synthesis condition,which greatly blocks their practical application[34–36].
In the present study,a simple and high efficient method via microwave assistant has been proposed for the synthesis of uniform 3D BiVO4/RGO nanocomposite photocatalyst with butter fly-like morphology.The obtained composites were well characterized with the aid of various techniques to study the morphology,structure,composition,optimal and electrical property.Photocatalytic performances of asprepared BiVO4/RGO composite have been evaluated by investigating the reduction of Cr(VI)ion-contained wastewater under simulated solar light irradiation.It is found that the BiVO4/RGO nanocomposite displays superior performance in catalyzing the degradation of Cr(VI)ion under visible-lightirradiation compared with the pure BiVO4.Moreover,a possible mechanism for the enhanced photocatalytic activity of BiVO4/RGO composite has been proposed here.
2.Experimental
2.1.Materials
Bismuth(III)nitrate pentahydrate(Bi(NO3)3·5H2O),ammonium metavanadate(NH4VO3),hydrochloric acid(HCl,36.0 wt%–38.0 wt%),hydrogen peroxide(H2O2,30 wt%),sulfuric acid(H2SO4,95 wt%),potassium permanganate(KMnO4),sodium hydroxide(NaOH),sodium nitrate(NaNO3),absolute ethanol(C2H6O),ethylene glycol(C2H4O),ammonia solution(NH3·H2O,25 wt%),carbon black(Super-P)and polytetra fluoroethylene(PTFE)were obtained from Beijing Chemical Plant,China.Pristine graphite powder was commercially obtained from Nanjing Xianfeng Nanomaterial Technology Co.Ltd.,China.Foamed nickel was supplied by Sinopharm Chemical Reagent Co.Ltd.(Beijing,China).All chemicals were analytical reagent grade and used as received without further purification.Deionized water used in the synthesis was obtained from a water purification system(RO-DI plus,Hitech,PRC).
2.2.Synthesis of graphite oxide(GO)
GO was synthesized from natural graphite powder by a modified Hummers method[37-39].Firstly,graphite powder(5 g,325 mesh)and NaNO3(2.5 g)were dispersed into a 0°C solution of concentrated H2SO4(120 ml),which was bathed by ice-water.Successively,KMnO4(15 g)was slowly added into the solution and the mixture was kept at 0 °C for 2 h.Afterward,the mixture was warmed to 35 °C and stirred for 2 h.Then 200 mL of distilled water was added while the temperature was kept at 98°C for 1.5 h.In this process,bubbles were continuously generated and the solution changed into brilliant yellow.The suspension was further diluted to approximate 1 L with distilled water.To reduce the residual permanganate and manganese dioxide,the suspension was next treated with 30 wt%of H2O2until to colorless.Finally,the suspension was filtered and the sediment was washed by diluted hydrochloric acid solution(5 wt%)and deionized water aiming to remove metal ions,until the pH value approached neutral.The GO was obtained after freeze drying.
2.3.Preparation of BiVO4/RGO nanocomposites
In a typical synthesis,60,90,120,and 180 mg of GO prepared by modified Hummor's method and 485 mg of Bi(NO3)3·5H2O were dispersed into 60 ml of ethanediol under ultrasonication for 60 min and 117 mg of NH4VO3was dispersed into 20 mL of distilled water under ultrasonication for 30 min.Then,the two solutions were mixed and the pH value of the mixed solution was adjusted to 10 with ammonia solution.After stirring for 40 min,the mixed solution was put into a microwave reactor for further reaction of 15 min at 700 W and 60 duty cycle.After the production was cooled to room temperature,the suspension was filtered,washed with absolute ethanol for five times to remove soluble by-products,and dried in a vacuumoven at60°C for 12 h.The synthesized samples were denoted as BiVO4/RGO20,BiVO4/RGO30,BiVO4/RGO40,BiVO4/RGO60.A simple diagram for the synthesis of BiVO4/RGO nanocomposites was displayed in Fig.1.

Fig.1.Schematic diagram for the synthesis of BiVO4/RGO nanocomposites.
For comparison,the same method was used to synthesize RGOwithout coupling BiVO4and pure BiVO4particle without adding RGO.
2.4.Photo-catalytic experiment
The photocatalytic activities of the as-obtained products were monitored through the photo-degradation of K2Cr2O7(Cr(VI))solution under the illumination of simulated solar light at ambient temperature.In all experiments,100 mg of the as-synthesized catalyst was dispersed in 100 mlofa 10 mg·L−1Cr(VI)aqueous solution underultrasonication for 3 min.And then the pH value of the solution was adjusted to 3 by 0.75 mol·L−1of HNO3.In order to achieve an adsorption–desorption equilibrium between the Cr(VI)ions and catalyst particles,the mixture was magnetically stirred in the dark for 30 min prior to irradiation.A solar simulator with a 500 WXe lamp was used as the lightsource.Photocatalytic reactions were carried out in a quartz cuvette and vertically placed at a distance of 200 mm from the light source.During each photocatalytic experiment,3 ml of the suspension was withdrawn and centrifuged to remove the dispersed catalyst powder at predesigned time interval.
The concentration of Cr(VI)was analyzed by measuring the absorption intensity atits maximum absorbance wavelength(553 nm)using a UV spectrophotometer(UV-3600,SHIMADU,Japan)with a 1 cm path length spectrometric quartz cell,and was calculated from the calibration curve,which gives a linear relationship between the absorbance and the concentration of Cr(VI).The degradation efficiency of the Cr(VI)wastewater was determined according to the following equation:

where D was the degradation efficiency of the Cr(VI),C0was the initial concentration of Cr(VI)and Ctwas the concentration of Cr(VI)at certain reaction time t(min).
2.5.Characterization methods
The morphologies ofthe as-prepared samples were characterized by transmission electron microscope(TEM,H-800)and scanning electron microscope(SEM).The crystal structure of the samples were characterized by X-ray diffraction(XRD)on a D/max 2500 VB2+/PC Advance diffractometer with Cu Kαradiation at a slow scan rate of 1.25(°)·min−1.The composition of the samples was characterized by Fourier transform infrared(FT-IR),Raman shift and X-ray photoelectron spectroscopy(XPS).FT-IR spectrum was measured on a Nicolet 6700 FT-IR spectrometer and the KBr was used to serve as a reference.Raman spectrum was collected on an INVIA Raman microprobe(Renishaw Instruments,England)with 718 nm laser excitation.XPS spectrum was measured on an Escalab 250 instrument(ThermoFisher Scientific,American).
The optical and electrical properties of the samples were characterized by UV–Vis diffuse re flectance spectra,Fluorescence spectra and electrochemical impedance spectroscopy(EIS).UV–Vis spectrum(UV–Vis)of the solid samples was recorded on a UV-3600 spectrophotometer,and the aqueous samples were recorded on a UV-5200PC spectrophotometer.The Fluorescence spectrum was characterized on an F-7000 PL spectrometer with 375 nm laser excitation.EIS measurements were carried out on a CHI660E workstation using a three-electrode system.
3.Results and Discussions
3.1.Morphology
The morphologies and microstructures of the BiVO4and BiVO4/RGO30 composites were characterized by TEM.Fig.2a shows a typical TEM image of BiVO4nanoparticles.The BiVO4particles are irregularly rodlike structure with average length of about 70–150 nm and lengthdiameter ratio of 2.5–3.0.
From Fig.2b itis can be seen thatafter reaction 5 min,BiVO4does not crystallize completely,forming irregular shape of small particles.With the reaction time increasing to 10 min,the BiVO4particles appear large X shape particles(Fig.2c),which shows that the crystallization of BiVO4is enhanced with the increase of reaction time and the morphology of the particle is also changed.When the reaction time extends to 15 min,BiVO4crystallizes completely into butter fly-like BiVO4morphology on the surface of RGO and well bonds with the RGO,as shown in Fig.2d.The changing of the BiVO4particles' morphology maybe due to the presence of graphite oxide in the synthesis process.The surface of graphite oxide contains a small amount of--COOH,which affects the nucleation and growth of BiVO4.Furthermore,the presence of RGO and microwave field seems in favor of the BiVO4particles' growth.It can also be seen that in the composite the RGO sheet is smooth and almost transparent,indicating that the restacking of RGO is restrained on a certain extent in the synthesis process.
In order to furtherclarify the specific morphology ofthe samples,the samples in Fig.2d is characterized by scanning electron microscopy(SEM),which are shown in Fig.3.It can be clearly seen that the butterfly-like BiVO4particles,which are assembled by irregular flakes,attached to the RGO sheet.The length and the width of the butter flylike BiVO4particle are about 1.5 μm,and the thickness of the flake is about 20 nm.

Fig.2.TEM image of(a).pure BiVO4 particles,(b).BiVO4/RGO30 composite of reaction 5 min,(c).BiVO4/RGO30 composite of reaction 10 min,(d).BiVO4/RGO30 composite of reaction 15 min.
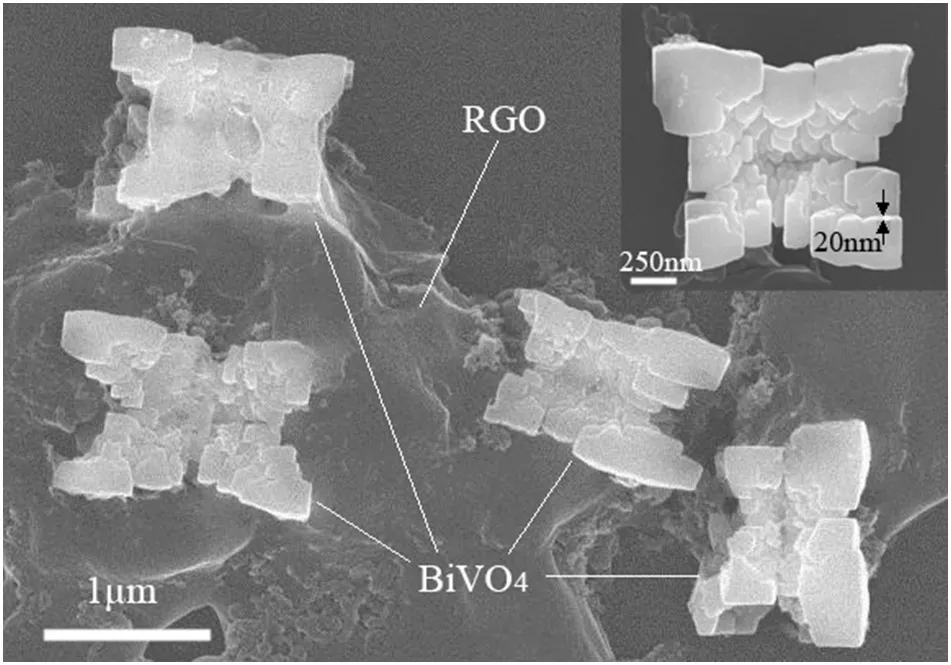
Fig.3.Typical SEM images of the as-prepared samples of BiVO4/RGO30 composites.
3.2.Crystal structure
Fig.4 shows the XRD patterns of pure BiVO4particles and BiVO4/RGO30 composites of different reaction times.It is obvious that better crystallization of the composites will be achieved if the reaction duration time is longer.When the reaction duration time reaches 15 min,the stable crystal is formed.The Bragg diffraction peaks of the samples in the 2θ range of 10°–65°are consistent with the monoclinic BiVO4(JCPDS No.14-0688).Three peaks centered at19.0°,35.2°,and 46.0°belonging to the monoclinic Scheelite structure of BiVO4can be clearly observed in the patterns of the BiVO4particles and BiVO4/RGO30 composite of15 min reaction[34].There is nearly no change in the crystal structure of BiVO4after RGO-coupling.It is evident that RGO coupling has a negligible effect on the crystal structure of BiVO4-RGO composites.Besides,no peak of RGO is observed in BiVO4/RGO30 composites.This is due to the fact that GO sheets are reduced under the microwave condition in the presence of alcohol,forming RGO,which usually appears no XRD peak[35].Based on the effect of reaction time on morphologies of composites,the reaction duration time is controlled to be 15 min.
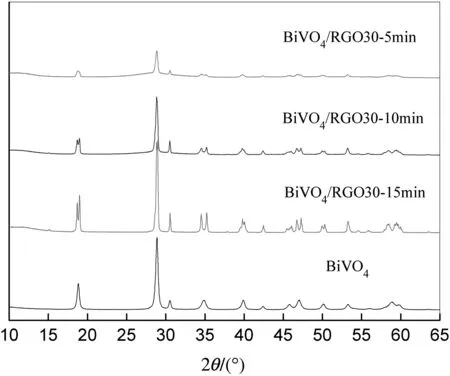
Fig.4.XRD patterns of pure BiVO4 particles and BiVO4/RGO30 composite.
Based on the Scherrer equation of D=Kλ/βcosθ(where the K,λ,β,θ,and D represent Scherrer constant,X-ray wavelength,the full width at half maximum,diffraction angle,and average grain,respectively),the values of crystallite average size of BiVO4-a(in Fig.2a),BiVO4-b(in Fig.2b),BiVO4-c(in Fig.2c)and BiVO4-d(in Fig.2d)calculated are 24.5 nm,20.8 nm,26.2 nm and 28.0 nm,respectively.Combining with the SEM and TEM results,it is shown that a number of small crystallite aggregates together to form the polymer particles of butter fly-like morphology in the synthesis process.The formation of the BiVO4particles' morphology may be due to the presence of graphite oxide.The chemical groups in graphite oxide surface are easy to combine with BiVO4which can affect the crystal growth and crystallographic orientation of BiVO4.Under the microwave field,continuous deposition and growth of BiVO4crystallite results in the formation of butter fly-like particles and RGO composites.
3.3.Composition
FT-IR spectrum can effectively determine the change of the composition by testing the functional group of material.FT-IR spectrum of GO and BiVO4/RGO composite is shown in Fig.5.The GO spectrum shows the presence of various oxygen-containing groups,including the absorption peaks C=O stretching vibrations of the COOH groups(1720 cm−1),O--H deformation vibrations of the COOH groups(1620 cm−1),O--H deformation vibrations of tertiary C--OH(1396 cm−1)and C--O stretching vibrations of the epoxy groups(1050 cm−1),while the adsorption around 1570 cm−1may be assigned to the stretching vibrations of the unoxidized carbon backbone[40,41].In the spectrum of GO,the broad band at 3410 cm−1belongs to O--H stretching vibrations of water molecules,which is adsorbed on GO surface.Compared to the spectrum of GO,part of the bands featuring oxygen-containing functional groups becomes weak and some almost vanishes in the spectrum of the BiVO4/RGO composite,indicating that the GO in the composite has been effectively reduced.

Fig.5.FT-IR spectra of Graphite oxide and BiVO4/RGO30 composite.
Raman spectroscopy is one of the most effective tools used for the characterization of carbon-based materials,especially fordistinguishing ordered and disordered crystal structures of carbon.The Raman spectrum of the composite is excited using a red(718 nm)laser,and Raman spectra of BiVO4,GO and BiVO4/RGO30 composite are shown in Fig.6.In Raman spectra,BiVO4exhibits typical vibrational bands at the location of around 120,210,324,366 and 826 cm−1.RGO has been reported to exhibit Raman bands at around 1600 and 1350 cm−1,corresponding to the G and D bands,respectively[42].The G band,which provides information on the in-plane vibration of sp2bonded carbon atoms,is usually used to semi-quantitatively determine the extent of reduction,and the D band corresponds to the presence of sp3defects in RGO[43].As shown in Fig.6,the as-prepared BiVO4/RGO30 composite presents the D and G bands at 1320 and 1610 cm−1respectively,which correspond to the characteristic peaks of RGO.A high intensity of the D band is observed in the composite,indicating that there are some surface defects present in BiVO4/RGO30 composite,whereas defects are a key influence factor for photo catalytic performance.As compared with GO(Gband at1590 cm−1)and RGO(G band at 1600 cm−1reported in the literature[42]),the slightly upshifted Gb and to 1610 cm−1can possibly be explained by the formation of heteroj unction in BiVO4/RGO30 where RGO was hybridized with an electron donor component.Besides,compared with pure RGO,lower D/G ratio is displayed for BiVO4/RGO30 composite,which suggests that RGOis effectively reduced during the synthesis process with the assistance of microwave field and BiVO4.

Fig.6.Representative Raman spectra of RGO and BiVO4/RGO30.
The efficient reduction of GO to RGO after the microwave treatment can be further verified by the comparison of C 1s XPS of GO and BiVO4/RGO30 composite,which has been displayed in Fig.7.For bare GO,C 1s XPS spectra in Fig.7a suggests that most carbons are in the form of sp2bonds(C--C),and the abundance of oxygenated functional groups(HO--C=O,C--O--C and C--OH)on GO surface.For BiVO4/RGO30 composite(Fig.7b),the intensity of oxygenated functional groups on carbon sheet in BiVO4/RGO30 is obviously decreased compared with that of GO[44],indicating the effective reduction of GO to RGO after coupling BiVO4with GO by a microwave treatment.
3.4.Optimal and electrical properties
It is apparent that pure BiVO4exhibits an absorption edge at around 525 nm,showing good visible light response.For the RGO/BiVO4composite,the absorption edge red-shifted to around 565 nm,and the light absorption ability in the range of 540–800 nm is also increased due to the absorption of RGO.
The optical properties of BiVO4particles and BiVO4/RGO30 composites were investigated by the UV–vis spectroscopy,as shown in Fig.8.As we allknow,the electronic structure of semiconductor has a great influence on the optical absorption behavior.According to the spectra,it is obvious that pure BiVO4exhibits an absorption edge at around 525 nm,showing good visible light response.For the RGO/BiVO4composite,the absorption edge red-shifted to around 565 nm,and the light absorption ability in the range of 540–800 nm is also increased due to the absorption of RGO.It is evident that RGO coupling had an enhanced effect on the visible light absorption property of BiVO4/RGO30 composites.Band gap energy can be estimated from a plot of(Ahν)2versus photon energy(hv),where A,h,and ν are the absorption coefficient,Planck's constant,and the frequency of the light,respectively.The intercept of the tangent to the lateral axis gives an approximation of the band gap energy for materials.The band gap energies are estimated to be 2.46 and 2.31 eV for bare BiVO4and BiVO4/RGO30 composite,respectively.Therefore,the result shows that the BiVO4/RGO composite is easily excited to produce much more electron–hole pairs than BiVO4under solar light,leading to higher photocatalytic activity.
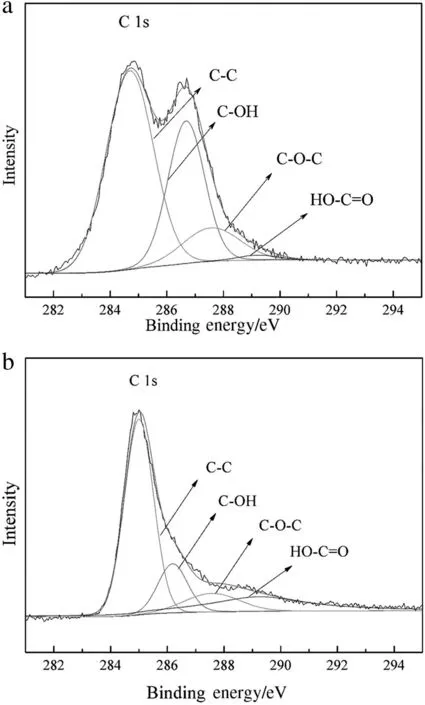
Fig.7.C 1s XPS of GO(a)and BiVO4/RGO30 composite(b).
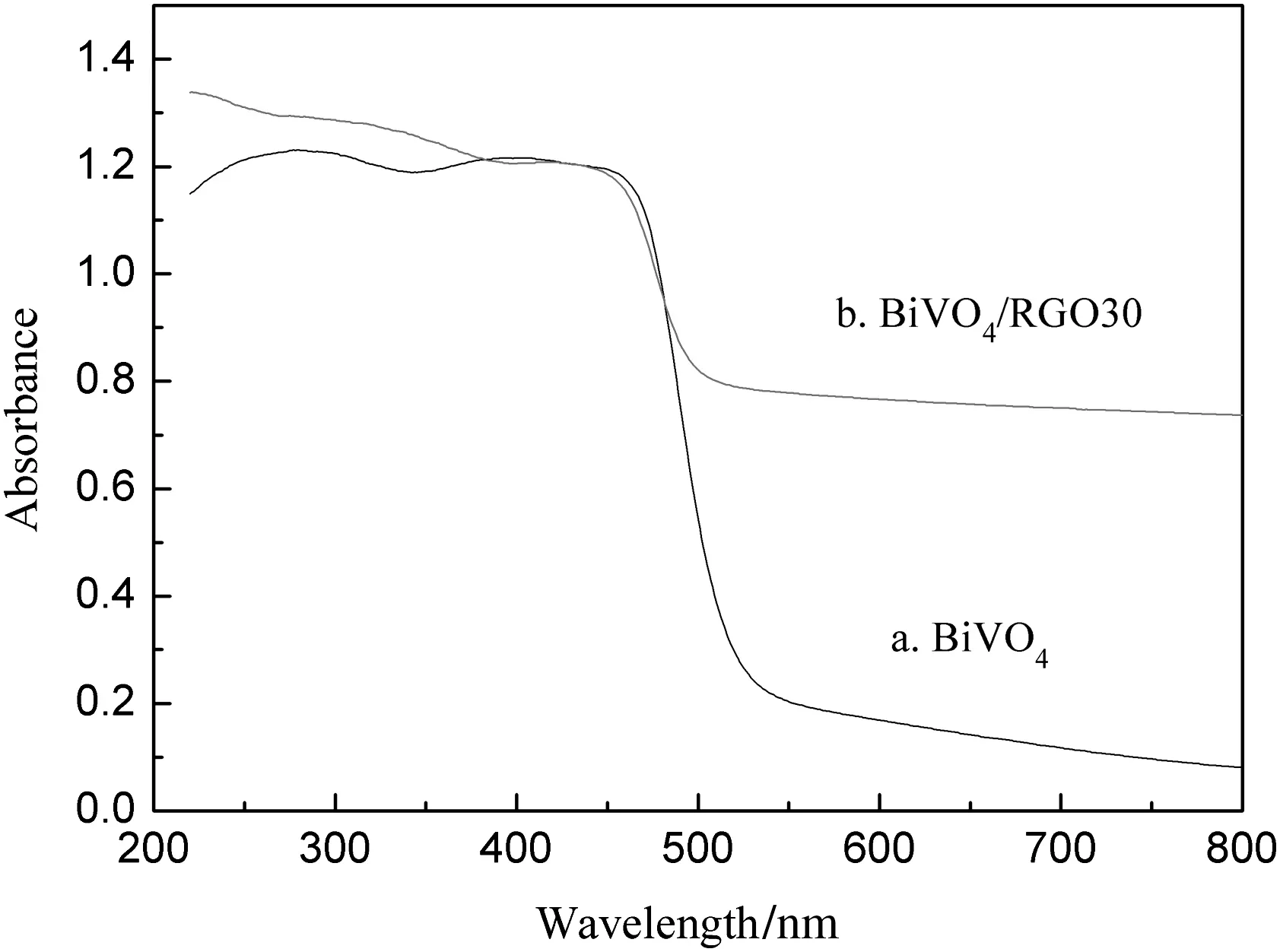
Fig.8.UV–vis diffuse reflectance spectra of BiVO4 and BiVO4/RGO30 composite.
Fluorescence spectrum is a reliable way to investigate the efficiency of charge carrier trapping,immigration and transfer.It also reflects the rate of photo-generated electron–hole pairs from semiconductor particles under lightirradiation[45,46].The recombination of electrons hole in semiconductor excites fluorescence emission.The faster the recombination rate of electron–hole is,the stronger the intensity of fluorescence will be.Fig.9 displays the fluorescence spectra of pure BiVO4and BiVO4/RGO30 composite.It is clearly that the fluorescence spectrum intensity of BiVO4sample is stronger than BiVO4/RGO30 composite,indicating that the recombination rate of electron–hole pair for BiVO4/RGO30 composite is lower.Therefore,more photo-generated electrons can be used to photo reduction,causing the enhancement of photo catalytic activity of composite.
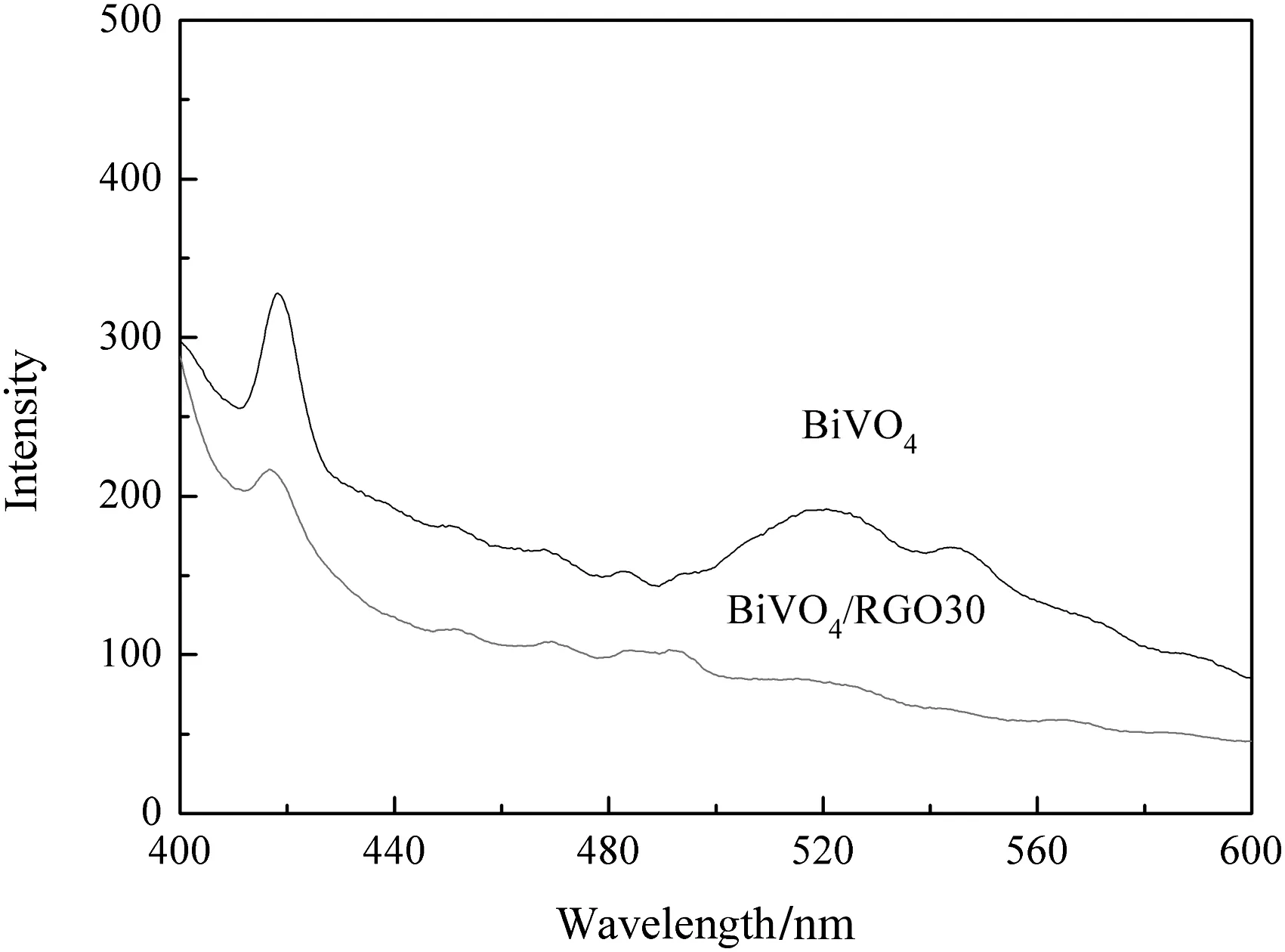
Fig.9.Fluorescence spectra of BiVO4 and BiVO4/RGO30 composites.
The electrochemical impedance technique has been used to characterize electrical conductivity.A typical EIS of BiVO4/RGO30 composite and pure BiVO4is shown in Fig.10.It is seen that compared with the BiVO4electrode,the impedance curve of BiVO4/RGO30 composite has an extremely small radius.The resistances of BiVO4and BiVO4/RGO30 composite are 36 Ω and 3 Ω respectively.With the presence of RGO,the solid state interface layer resistance and the charge transfer resistance on the surface significantly decreased.This is because RGO has a unique two-dimensional π-conjugation structure,in which the charge carriersbe have as massless fermions,leading to very high electrical conductivity.Thus,once photo-generated electrons are formed in BiVO4,they could rapidly transfer from the conduction band to RGO,which serves as an electrons acceptor.The recombination of the electron hole pairs is greatly restrained,resulting in more charge carriers to form reactive species,and therefore,photocatalytic activity is significantly promoted.
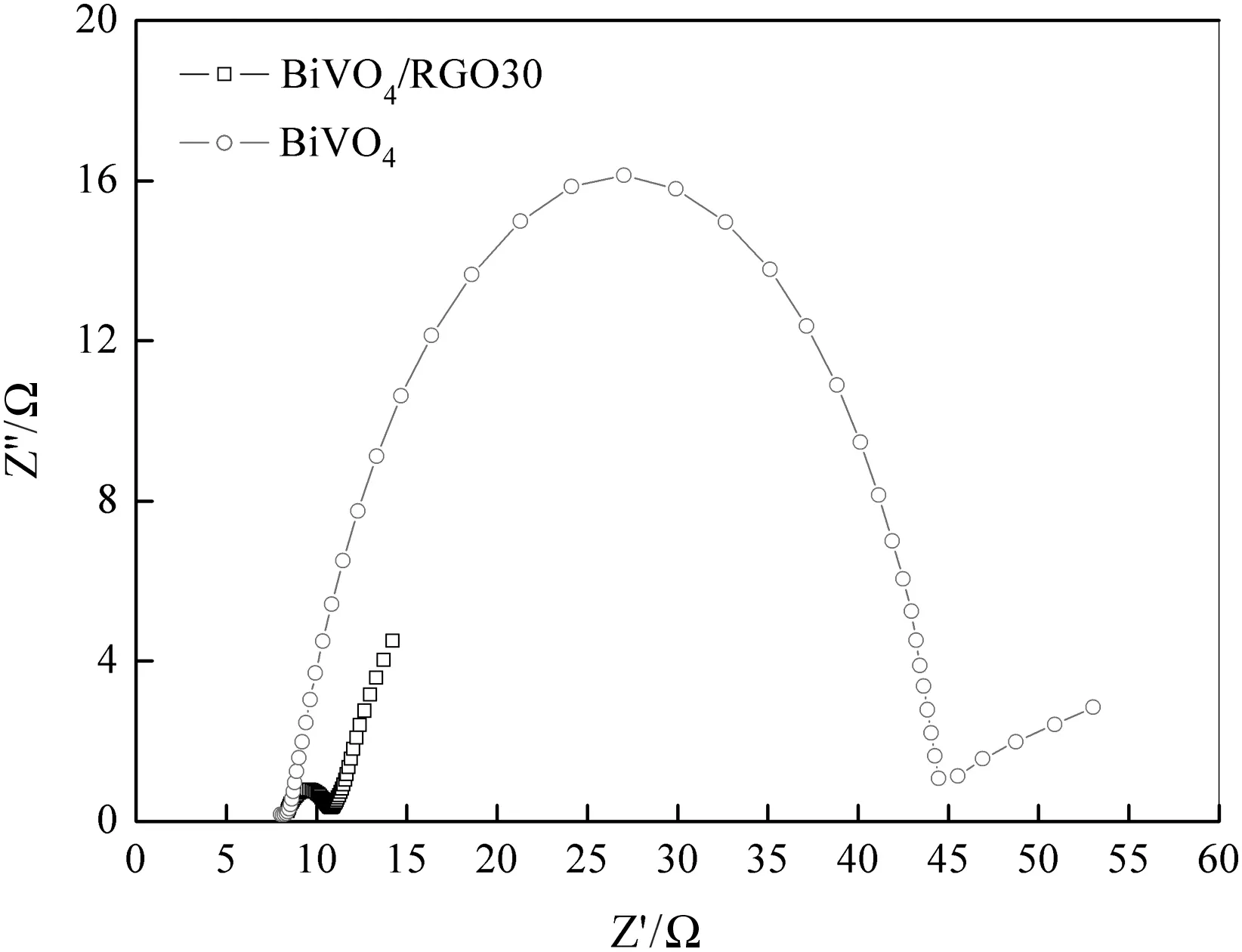
Fig.10.EIS of BiVO4 and BiVO4/RGO30 nanocomposites.
3.5.Photo-catalytic reduction activities
The photocatalytic activities of BiVO4/RGO composites and pure BiVO4are investigated by the reduction of Cr(VI)(10 mg·L−1,100 ml)under simulated solar radiation.The photo-reduction rates of BiVO4/RGO composite and pure BiVO4are shown in Fig.11a.It can be seen that with the increase of RGO content in BiVO4/RGO composite from 20%to 30%,the photocatalytic activity obviously increases.However,when the RGO contentis further increased,the photo-catalytic activity decreases.The ratio of 30%is regarded as the optimal content for the composites.In the photocatalytic reduction experiment,an approximate 90.3%of the Cr(VI)is reduced in the solution after irradiation for 180 min in the presence of BiVO4/RGO30 composite.In contrast,the photo-reduction ratio of Cr(VI)over pure BiVO4is 36.5%after irradiation for 180 min under the same conditions.It is fully demonstrated that BiVO4/RGO composites have much higher photocatalytic activities than bare BiVO4.
It is demonstrated that the photocatalytic reduction of Cr(VI)follows Langmuir-Hinshel wood first order reaction kinetics behavior.

Fig.11.(a)Photo-reduction rate of Cr(VI)using BiVO4/RGO composites and pure BiVO4 under simulated sunlight irradiation.(b)the pseudo- first-order rate constant(k)for the Photo-reduction rate of Cr(VI)using BiVO4/RGO composites and pure BiVO4under simulated sunlight irradiation.
According to Eq.(2).,the rate constant(k)can be deduced for the photocatalytic reduction of Cr(VI)under visible-light irradiation.

where C0and Ctare the concentration of Cr(VI)when reaction time is 0 and t,respectively.The slopes of lines in Fig.11b represent the values for the pseudo- first-order rate constants(k)for the photocatalytic reduction of Cr(VI)by BiVO4/RGO composites and pure BiVO4.Based on the linear fitting calculation(Table 1),the rate constant of the BiVO4/RGO30 composite system is 0.0132 min−1,which is about 4 times as high as that of the pure BiVO4(0.00311 min−1).Therefore,it is obvious that the combination of BiVO4and RGO displays a remarkable synergistic effect,which leads to high photo-catalytic activity.It can be found there is little deviation of photo-reduction rate from the firstorder reaction kinetics.This is because the adsorption of the metal ion plays a important role at the initial stage.Lot of metal ions are adsorbed at the surface of BiVO4/RGO composites owing to the large surface area provided by RGO,which leads to the reduction performance contains the effect of adsorption.

Table 1 Pseudo- first-order rate constants(k)for the BiVO4/RGO composite and pure BiVO4 catalysts
3.6.The mechanism analysis of photo-reduction Cr(VI)
A possible mechanism of photo-reduction Cr(VI),which is used to explain the photocatalytic activity enhancement for BiVO4/RGO composite and Cr(VI)system,is proposed as the following steps:

Under visible-lightir radiation,the electron–hole pairs are generated on the BiVO4surface.In the absence ofRGO,most of electrons and holes have no chance to escape and quickly recombine.Typically,only a small number of these charges are trapped and participate in photocatalytic reactions,resulting in low reactivity.When BiVO4is coupled with RGO,the photo-generated electrons instantly transfer to RGO sheets via a percolation mechanism(Reaction 4),allowing charges separation,stabilization,and restraining electron–hole pairs recombination.Meanwhile,the hole generated in the valence band reacts with H2O producing O2and H+(Reaction 5),which could effectively decrease the electron–hole recombination rate and improve the photo-catalytic performance.Finally,Cr2O72−is reduced to Cr3+via reaction with the electron and H+(Reaction 6).
The excellent photocatalytic performance of BiVO4/RGO nanocomposite can be explained by the unique structural characteristics and optimal properties of the composite:(1)RGO acts as a barrier to the growth of BiVO4nanoplate and thus present higher adsorption capacity than BiVO4particle;(2)the narrow band gap ofthe composite increases light absorption capacity and range;(3)RGO can act as an electron acceptor allowing for the photo-excited electrons of semiconductor in the composites to be quickly transferred to RGO,thereby reducing electron–hole pair recombination in the nanocomposite.
4.Conclusions
In this research,by adopting the microwave assistant method,using Bi(NO3)3·5H2O/GO and NH4VO3as precursors,a simple and high efficient method has been proposed for the synthesis of uniform 3D BiVO4/RGO nanocomposite as photocatalyst.In the as-obtained composites,BiVO4particle displayed monoclinic structure and the GOsheets were fully reduced and decorated with BiVO4lamellas during the synthesis process.Photocatalytic performances of as-prepared BiVO4/RGO composite and pure BiVO4particle have been evaluated by investigating the reduction ofCr(VI)ion-contained wastewater undersimulated solar light irradiation.It is found that the pseudo- first-order rate constants(k)for the photo-catalytic reduction of Cr(VI)by BiVO4/RGO composite was about 4 times as high as that of the pure BiVO4.It was obvious that the combination of BiVO4and RGO displayed a remarkable synergistic effect,which led to high photocatalytic activity.Moreover,a possible mechanism for the enhanced photocatalytic activity of BiVO4/RGO photocatalyst has been proposed here.
[1]N.M.Mubarak,J.N.Sahu,E.C.Abdullah,N.S.Jayakumar,Removal of heavy metals from wastewater using carbon nanotubes,Sep.Purif.Rev.43(2014)311–338.
[2]J.P.Zhao,W.C.Ren,H.M.Cheng,Graphene sponge for efficient and repeatable adsorption and desorption of water contaminations,J.Mater.Chem.22(2012)20197–20202.
[3]A.D.Martino,M.Iorio,B.S.Xing,R.Capassoa,Removal of 4-chloro-2-methylphenoxyacetic acid from water by sorption on carbon nanotubes and metal oxide nanoparticles,RSC Adv.2(2012)5693–5700.
[4]K.A.Landry,T.H.Boyer,Diclofenac removal in urine using strong-base anion exchange polymer resins,Water Res.47(2013)6432–6444.
[5]T.Nharingo,M.Moyo,Application of Opuntia ficus-indica in bioremediation of wastewaters.A critical review,J.Environ.Manag.166(2016)55–72.
[6]I.Musbah,D.Ciceron,F.Garcia,A.Saboni,S.Alexandrova,Nano filtration membranes for drinking water production-retention of nitrate ions,desalination and water treatment,Desalin.Water Treat.57(2016)16758–16769.
[7]Y.Zhang,C.Causserand,P.Aimar,J.P.Cravedi,Removal of bisphenol A by a nano filtration membrane in view of drinking water production,Water Res.40(2006)3793–3799.
[8]H.G.Yang,G.Liu,S.Z.Qiao,C.H.Sun,Y.G.Jin,S.C.Smith,J.Zou,H.M.Cheng,G.Q.Lu,Solvothermal synthesis and photoreactivity of anatase TiO2nanosheets with dominant 001 facets,J.Am.Chem.Soc.131(2009)4078–4083.
[9]S.Liu,J.Yu,M.Jaroniec,Tunable photocatalytic selectivity of hollow TiO2microspheres composed of anatase polyhedra with exposed 001 facets,J.Am.Chem.Soc.132(2010)11914–11916.
[10]R.Li,F.Zhang,D.Wang,J.Yang,M.Li,J.Zhu,X.Zhou,H.Han,C.Li,Spatial separation of photogenerated electrons and holes among 010 and 110 crystal facets of BiVO4,Nat.Commun.4(2013)1432–1439.
[11]D.Wodka,E.Bielanska,R.P.Socha,M.Elzbieciak-Wodka,J.Gurgul,P.Nowak,P.Warszynski,I.Kumakiri,Photocatalytic activity of titanium dioxide modified by silver nanoparticles,ACS Appl.Mater.Interfaces 27(2010)1945–1953.
[12]K.Kądziola,I.Piwonski,A.Kisielewska,D.Szczukocki,B.Krawczyk,J.Sielskic,The photoactivity of titanium dioxide coatings with silver nanoparticles prepared by sol-gel and reactive magnetron sputtering methods-comparative studies,Appl.Surf.Sci.288(2014)503–512.
[13]X.Bai,L.Wang,Y.Zhu,Visible photocatalytic activity enhancement of ZnWO4by graphene hybridization,ACS Catal.2(2012)2769–2778.
[14]Y.X.Zhou,H.B.Yao,Q.Zhang,J.Y.Gong,S.J.Liu,S.H.Yu,Hierarchical FeWO4microcrystals:Solvothermal synthesis and their photocatalytic and magneticproperties,Inorg.Chem.48(2009)1082–1090.
[15]S.Obregon,G.Colon,Heterostructured Er3+doped BiVO4with exceptional photocatalytic performance by cooperative electronic and luminescence sensitization mechanism,Appl.Catal.B Environ.158(2014)242–249.
[16]B.Inceesungvorn,T.Teeranunpong,J.Nunkaew,S.Suntalelat,D.Tantraviwat,Novel NiTiO3/Ag3VO4composite with enhanced photocatalytic performance under visible light,Catal.Commun.54(2014)35–38.
[17]T.Meyer,J.B.Priebe,R.O.da Silva,T.Peppel,H.Junge,M.Beller,A.Bruck-ner,S.Wohlrab,Advanced charge utilization from NaTaO3photocatalysts by multilayer reduced graphene oxide,Chem.Mater.26(2014)4705–4711.
[18]G.H.He,G.L.He,A.J.Li,X.Li,X.J.Wang,Y.P.Fang,Y.H.Xu,Synthesis and visible light photocatalytic behavior of WO3(core)/Bi2WO6(shell),J.Mol.Catal.A Chem.385(2014)106–111.
[19]A.P.Zhang,J.Z.Zhang,Effects of europium doping on the photocatalytic behavior of BiVO4,J.Hazard.Mater.173(2010)265–272.
[20]J.Q.Yu,A.Kudo,Effects of structural variation on the photocatalytic performance of hydrothermally synthesized BiVO4,Adv.Funct.Mater.16(2006)2163–2169.
[21]G.Nagabhushana,G.Nagaraju,G.Chandrappa,Synthesis of bismuth vanadate:its application in H2evolution and sunlight-driven photodegradation,J.Mater.Chem.A 1(2013)388–394.
[22]S.J.Hong,S.Lee,J.S.Jang,J.S.Lee,Heterojunction BiVO4/WO3electrodes for enhanced photoactivity of water oxidation,Energy Environ.Sci.4(2011)1781–1787.
[23]T.W.Kim,K.S.Choi,Nanoporous BiVO4photoanodes with dual-layer oxygen evolution catalysts for solar water splitting,Science 343(2014)990–994.
[24]S.K.Pilli,T.E.Furtak,L.D.Brown,T.G.Deutsch,J.A.Turner,A.M.Herring,Cobalt-phosphate(Co–Pi)catalyst modified Mo-doped BiVO4photoelectrodes for solar water oxidation,Energy Environ.Sci.4(2011)5028–5034.
[25]X.X.Chang,T.Wang,P.Zhang,J.J.Zhang,A.Li,J.L.Gong,Enhanced surface reaction kinetics and charge separation of p-n heterojunction Co3O4/BiVO4photoanodes,J.Am.Chem.Soc.137(2015)8356–8359.
[26]C.J.Li,S.P.Wang,T.Wang,Y.J.Wei,P.Zhang,J.L.Gong,Monoclinic porous BiVO4networks decorated by discrete g-C3N4nano-islands with tunable coverage for highly efficient photocatalysis,Small 10(2014)2783–2790.
[27]C.J.Li,P.Zhang,R.Lv,J.W.Lu,T.Wang,S.P.Wang,H.F.Wang,J.L.Gong,Photocatalysis:selective deposition of Ag3PO4on monoclinic BiVO4(040)for highly efficient photocatalysis,Small 9(2013)3951–3956.
[28]Y.Z.Liu,Y.Z.Zhu,X.B.Fan,S.B.Wang,Y.Li,F.B.Zhang,G.L.Zhang,W.C.Peng,(0D/3D)MoS2on porous graphene as catalysts for enhanced electrochemical hydrogen evolution,Carbon 121(2017)163–169.
[29]S.H.Yang,F.F.Zhang,C.L.Gao,J.F.Xia,L.Lu,Z.H.Wang,A sandwich-like PtCographene/carbon dots/graphene catalyst for efficient methanol oxidation,J.Electroanal.Chem.802(2017)27–32.
[30]G.L.He,M.J.Chen,Y.Q.Liu,X.Li,Y.J.Liu,Y.H.Xu,Hydrothermal synthesis of FeWO4-graphene composites and their photocatalytic activities under visible light,Appl.Surf.Sci.351(2015)474–479.
[31]Q.Xiang,J.Yu,Graphene-based photocatalysts for hydrogen generation,J.Phys.Chem.Lett.4(2013)753–759.
[32]M.Q.Yang,Y.J.Xu,Selective photoredox using graphene-based composite photocatalysts,Phys.Chem.Chem.Phys.15(2013)19102–19118.
[33]T.Wa,C.J.Li,J.Y.Ji,Y.J.Wei,P.Zhang,S.P.Wang,X.B.Fan,J.L.Gong,Reduced graphene oxide(rGO)/BiVO4composites with maximized interfacial coupling for visible light photocatalysis,ACS Sustain.Chem.Eng.2(2014)2253–2258.
[34]Y.H.Ng,A.Iwase,A.Kudo,R.Amal,Reducing graphene oxide on a visible-light BiVO4photocatalyst for an enhanced photoelectrochemical Water Splitting,J.Phys.Chem.Lett.1(2010)2607–2612.
[35]S.Yousefzadeh,M.Faraji,A.Z.Moshfegh,Constructing BiVO4/Graphene/TiO2nanocomposite photoanode for photoelectrochemical conversion applications,J.Electroanal.Chem.763(2016)1–9.
[36]A.L.Wang,S.Shen,Y.B.Zhao,W.Wu,Preparation and characterizations of BiVO4/reduced graphene oxide nanocomposites with higher visible light reduction activities,J.Colloid Interface Sci.445(2015)330–336.
[37]W.S.Hummers,R.E.Offeman,Preparation of graphitic oxide,J.Am.Chem.Soc.80(1958)1339.
[38]J.Yu,A.Kudo,Effects of structural variation of photocatalytic,hydrothermally synthesized BiVO4,Adv.Funct.Mater.16(2006)2163–2169.
[39]R.Muzyka,M.Kwoka,Ł.Smędowski,N.Díez,G.Gryglewicz,Oxidation of graphite by different modified Hummers methods,New Carbon Mater.32(2017)15–20.
[40]C.Nethravathi,T.Nisha,N.Ravishankar,C.Shivakumara,M.Rajamathi,Graphenenanocrystalline metal sulphide composites produced by a one-pot reaction starting from graphite oxide,Carbon 47(2009)2054–2059.
[41]H.K.Jeong,Y.P.Lee,R.J.W.E.Lahaye,M.H.Park,K.H.An,I.J.Kim,C.W.Yang,C.Y.Park,R.S.Ruoff,Y.H.Lee,Evidence of graphitic AB stacking order of graphite oxides,J.Am.Chem.Soc.130(2008)1362–1366.
[42]X.Pan,Y.Zhao,S.Liu,C.L.Korzeniewski,S.Wang,Z.Fan,Comparing graphene-TiO2nanowire and graphene-TiO2nanoparticle composite photocatalysts,ACS Appl.Mater.Interfaces 4(2012)3944–3950.
[43]C.Chen,W.Cai,M.Long,B.Zhou,Y.Wu,D.Wu,Y.Feng,Synthesis of visible-light responsive graphene oxide/TiO2composites with p/n heterojunction,ACS Nano 4(2010)6425–6432.
[44]C.Xu,X.Wang,J.Zhu,Graphene-metal particle nanocomposites,J.Phys.Chem.C 112(2008)19841–19845.
[45]L.Chen,S.F.Yin,R.Huang,Q.Zhang,S.L.Luo,C.T.Au,Hollow peanut-like m-BiVO4:Facile synthesis and solar-light-induced photocatalytic property,Cryst Eng Comm 14(2012)4217–4222.
[46]Y.L.Min,K.Zhang,Y.C.Chen,Y.G.Zhang,Enhanced photocatalytic performance of Bi2WO6by graphene supporter as charge transfer channel,Sep.Purif.Technol.86(2012)98–105.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Fabrication of chitosan microspheres for efficient adsorption of methyl orange☆
- The combined effects of lysozyme and ascorbic acid on microstructure and properties of zein-based films☆
- Effects of nitrogen doping on surface-enhanced Raman scattering(SERS)performance of bicrystalline TiO2 nano fibres☆
- Parametric study and effect of calcination and carbonation conditions on the CO2 capture performance of lithium orthosilicate sorbent
- Effect of heat flux and inlet temperature on the fouling characteristics of nanoparticles☆
- Variation of toxic pollutants emission during a feeding cycle from an updraft fixed bed gasifier for disposing rural solid waste☆
