Diagnostic value of whole-body MRI with diffusion-weighted sequence for detection of peritoneal metastases in colorectal malignancy
2018-05-24HuanZhangWeixingDaiCaixiaFuXuYanAltoStemmerTongTongGuoxiangCai
Huan Zhang, Weixing Dai, Caixia Fu, Xu Yan, Alto Stemmer, Tong Tong, Guoxiang Cai
1Department of Radiology; 2Department of Colorectal Surgery, Fudan University Shanghai Cancer Center; Department of Oncology, Shanghai Medical College, Fudan University, Shanghai 200032, China; 3Siemens Shenzhen Magnetic Resonance Ltd, Shenzhen 518000, China; 4MR Collaboration NE Asia, Siemens Healthcare, Shanghai 210318, China; 5MR Applications Development, Siemens Healthcare, Erlangen 91052, Germany
Introduction
Peritoneal metastases (PM) refers to malignant metastasis involving the peritoneum in the abdominopelvic cavity with primary modes of spreading by hematogenous and implantation metastasis. For patients with gastrointestinal cancer, in addition to the result of full-thickness invasion of the bowel wall by an invasive cancer, preoperative seeding may also occur due to the rupture of structure (s) by a noninvasive tumor, such as less-invasive mucinous appendiceal cancers1. The presence of peritoneal disseminators has been historically associated with a very poor prognosis2.
The peritoneal cancer index (PCI), as reported by Jacquet and Sugarbaker3, quantitatively evaluates both cancer distribution and lesion size throughout the abdominopelvic region4,5. The PCI score is not only considered to be an independent prognostic factor for patients with PM who are recommended to undergo surgical cytoreduction (CRS) and heated intraperitoneal chemotherapy (HIPEC), but also an important influencing factor determining whether complete macroscopic cytoreduction can be achieved6. An accurate preoperative PCI score assessment would be useful for appropriate treatment strategy selection and prognosis prediction.
Multidetector computed tomography (CT) was previously the most common preoperative staging and follow-up method for patients with PM. However, due to the limited soft tissue resolution of CT, its sensitivity for detecting small lesions noticeably decreases7-9. Equipped with high soft tissue resolution, MRI can use different types of image contrast to more accurately describe the distribution and extent of peritoneal tumor10-15. Diffusion-weighted imaging (DWI)exploits the thermally driven motion of water molecules.Most tumors are characterized by the restricted diffusion of water molecules due to increased cellularity and disordered arrangement, and are highlighted on DWI as hyperintense signal.
We undertook the present study to evaluate the accuracy of WB-DWI for determining the extent of PM and its correlation with surgical and histopathological findings.
Materials and patients
Patients
Between September 2015 and December 2017, a total of 27 patients with colorectal malignancy, in whom PM were known or suspected, and who were candidates for surgical exploration, were recruited into the study. The patients (13 women, 14 men) ranged in age from 27 to 67 years (mean, 51 years), and were confirmed histopathologically to have primary tumors of the appendix (n = 15) and colorectum(n = 12). The study protocol was approved by the authors’institutional review board, and all patients provided informed written consent.
MR imaging
All MRI examinations were performed using a 3T MRI scanner (MAGNETOM Skyra, Siemens Healthcare, Erlangen,Germany) equipped with a 16-channel phase-array body coil.The MRI protocol included two-step T1-weighted and T2-weighted sequences, with coverage of the abdominal and pelvic regions and multiple-step EPI DWI (prototype, iShim whole body DWI) sequence with coverage from the head to the pelvic cavity. Other parameters of WB-DWI included:TR/TE = 5600/60 ms; FOV = 480 × 330 mm2; scan matrix =128 × 128; slice thickness = 5 mm; b = 50, 800 s/mm2;diffusion mode = 3D diagonal; slice-selective IR, with TI =240 ms for fat suppression; iPAT factor = 2; and scan time =2 mins, 14 sec/step.
Review of MRI
The MR images were reviewed by a radiologist with 13 years’experience in abdominal MRI. The reviewer was blind to all patient clinical information. Plaque-like areas of hyperintensity in the subphrenic regions, and focal nodular or mass areas of hyperintensity involving the peritoneum,omentum, mesentery or bowel serosa, were recorded as tumors on DWI with b = 800 s/mm2. The abdominal and pelvic intraperitoneal spaces of each patient were divided into nine regions and the small bowel was divided into four regions6. The reviewer recorded the presence or absence of metastatic peritoneal tumors and scored each of the 13 anatomical sites as follows: 0, no visible tumor; 1, tumor <0.50 cm in greatest diameter; 2, tumor = 0.50–5.0 cm; and 3,tumor > 5.0 cm or confluence.
Following a blinded review of the MR examinations, the MRI results were compared with the results from the surgical and histopathological records. The sensitivity, specificity, and accuracy for identifying PM were calculated for each respective anatomical site. The PCI scores were categorized as follows: PCI = 0–10 (small-volume tumor); 10 < PCI < 20(moderate-volume tumor); and PCI ≥ 20 (large-volume tumor). WB-DWI PCI and surgical PCI were compared using the paired t-test in SPSS version 21 (IBM Corporation,Armonk, NY, USA).
Results
A total of 351 anatomical sites were reviewed in this study,203 of which were confirmed to comprise PM at surgery and histopathological analysis (47 lesions of adenocarcinoma in 9 patients, 140 lesions of mucinous adenocarcinoma in 15, and 16 lesions of signet ring cell carcinoma in 3). The lesion size was < 0.5 cm in 108 sites, 0.5–5.0 cm in 80 sites, and > 5.0 cm in the remaining 15 sites. WB-DWI correctly depicted tumors in 163 regions with 40 false-negative regions and 23 false-positive regions (Table 1, and Figures 1 and 2). The overall sensitivity, specificity, and accuracy of WB-DWI for the detection of peritoneal tumors were 80.3%, 84.5%, and 82.1%, respectively. For lesions < 0.5 cm in diameter, DWI demonstrated good sensitivity (69.4%) (Table 2).
The PCI on preoperative WB-DWI and the corresponding surgical PCI score for all 27 patients are shown in Figure 3.There was no statistical difference between the WB-DWI PCI and surgical PCI (P = 0.574). WB-DWI correctly predicted the PCI type in 24 of 27 patients with high accuracy (88.9%),including 10 of 10 patients with small-volume tumor, 12 of 14 with moderate volume tumor, and 2 of 3 with largevolume tumor (Table 3).
Discussion
Colorectal cancer patients with PM are traditionally believed to have poor prognosis and low-value prospectivesurgical treatment. However, with the development of multidisciplinary treatment, a growing number of recent clinical studies indicate that CRS and HIPEC may improve the survival time and the quality of life of patients with PM16-19.The PCI score, which represents the distribution of the tumor in the abdominopelvic regions and lesion size, is considered to be a prognostic factor for patients after CRS and HIPEC. Patients with low-volume peritoneal tumor could be more likely to benefit from CRS and HIPEC20. Some survival analyses have found that PCI score was closely related to patient survival21,22. Patients with small-volume tumor (PCI < 10) achieved a higher 5-year survival rate than patients with a PCI ranging from 10 to 20, or > 20 (P < 0.05).PCI < 20 is recommended as one criterion of the principle of CRS and HIPEC in patients with colorectal peritoneal metastatic lesions23,24. For patients with tumors that are too extensive and cannot be adequately cytoreduced, good preoperative imaging helps prevent unnecessary surgeries.This study aimed to contribute to careful patient selection criteria for CRS and HIPEC.
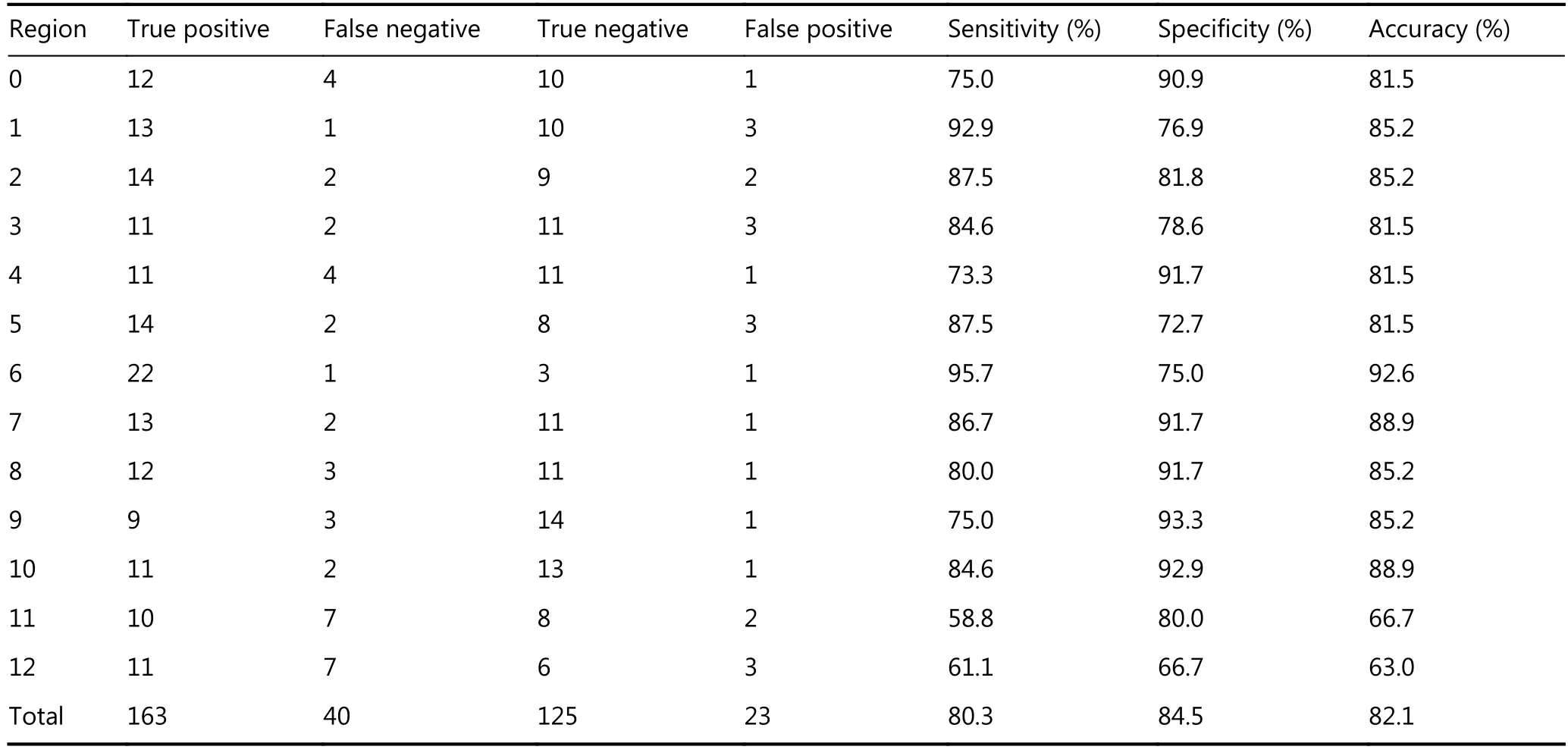
Table 1 Peritoneal tumor detection on WB-DWI at 13 anatomic sites compared with surgical and histopathologic findings

Figure 1 A 34-year-old man with a history of resection of adenocarcinoma of left colon. WB-DWI showed anterior abdominal wall with focal nodular of hyperintensity during postoperative follow-up, which was confirmed as PM in the subsequent cytoreductive surgery. The preoperative PCI of WBDWI was 5. The surgery PCI was 2. The PCI Types were matched.
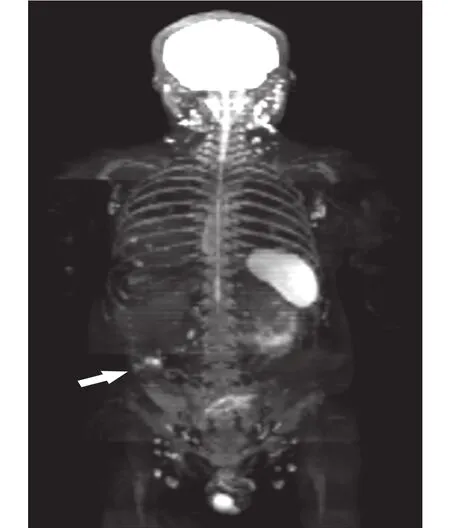
Figure 2 A 36-year-old man with signet cell cancer of the rectum was diagnosed as multiple peritoneal metastases on preoperative WB-DWI, while preoperative positron emission tomography-CT(PET-CT) was negative. PM was confirmed in the followed cytoreductive surgery along with the resection for primary lesion.The PCI of preoperative WB-DWI and surgery were 13 and 18 respectively, with a same PCI Type.
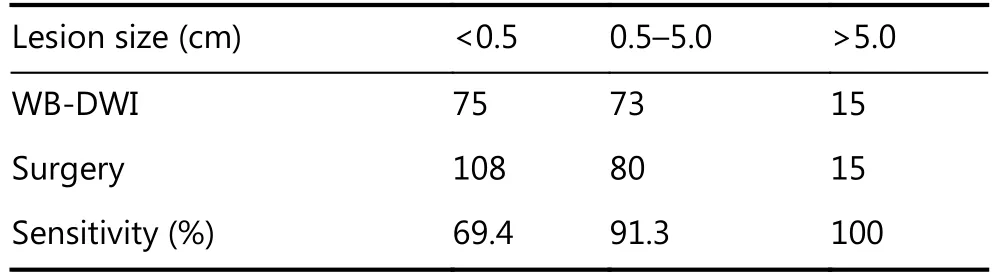
Table 2 Sensitivity of WB-DWI for the detection of peritoneal metastases of different size
CT used to be the most common preoperative staging method for patients with PM, but was limited by soft tissue resolution, and its sensitivity is not ideal when the size of the peritoneal lesion is < 1 cm25,26. Preoperative PCI was significantly underestimated on CT comparing with surgical findings (P < 0.001), and small PM (< 0.5 cm) were visualized on CT with a sensitivity of only 11 %–48 %4,9. The low detection veracity rate of CT for small peritoneal tumors may result in underestimation of the extent of PM and the preoperative PCI score. With a high sensitivity for depicting the increased cellularity that characterizes most solid tumors,DWI helps to overcome these limitations and increase the detectability of even small malignant deposits10,11,13. Low et al.27reported that the accuracy in depicting peritoneal lesions was 95% for DWI, compared with 55% for CT, and that MRI more accurately predicted the PCI category preoperatively with high accuracy (91%).
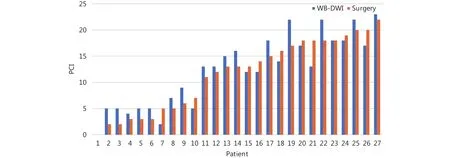
Figure 3 Comparison of PCI on preoperative WB-DWI and surgical PCI score.
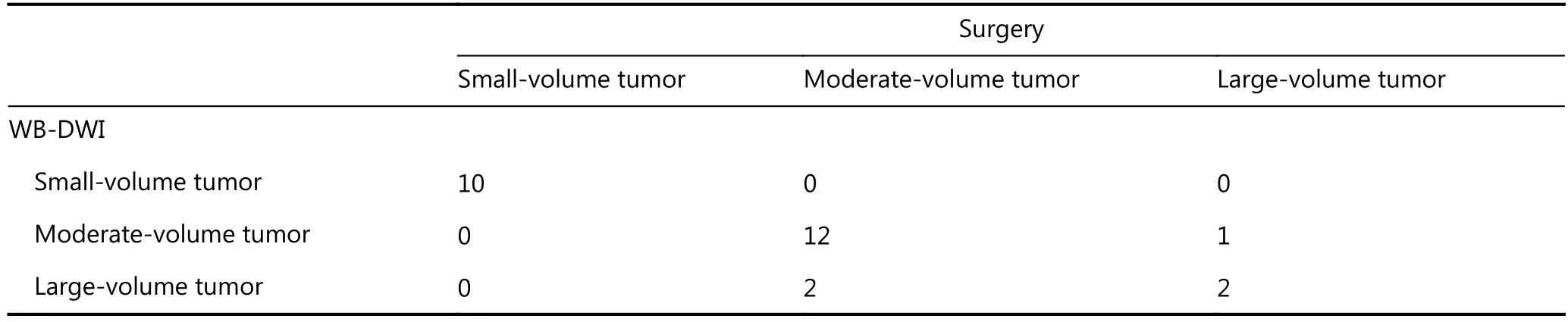
Table 3 PCI type of WB-DWI vs. surgery
These results are consistent with those of our study. We found that there was no statistical difference between the WB-DWI PCI and surgical PCI (P = 0.574). WB-DWI correctly predicted the PCI type in 24 of 27 patients with high accuracy [88.9% (including 2 of 3 patients with large-volume tumor who were found to have widespread PM during surgery that could not be completely removed)]. If considering PCI < 20 as a criterion in the principle of CRS and HIPEC in patients with colorectal peritoneal metastatic lesions, we can conclude that the overall sensitivity,specificity and accuracy of DWI for determining resectability was 91.7%, 66.7% and 88.9%, respectively. Therefore, this imaging mode may contribute to preventing unnecessary surgeries with careful patient selection. The three patients who were mistakenly estimated according to PCI types in our study had characteristic mucinous appendiceal neoplasm and massive ascites, which could easily lead to false assessment of peritoneal lesions, particularly in small bowel regions. For each anatomical region, compared with a previous study in which the MRI region sensitivity was 88% and accuracy 84%28, our study demonstrated a somewhat similar sensitivity (80.3%) and accuracy (82.1%). Moreover, for lesions < 0.5 cm in diameter, DWI demonstrated clearly better sensitivity (69.4%) vs. a sensitivity of 11% on CT4. No radiation exposure and superior diagnostic information in patients with peritoneal tumors are making DWI a better detection method for PM.
There were several potential limitations to our investigation, the first of which was its retrospective design.Second, due to the small number of enrolled patients, the applicability of our data need to be confirmed with a larger patient population. For the 10 patients with small-volume tumor, two had lesions > 5 cm, which may have somewhat overestimated the sensitivity of preoperative DWI in assessing patients with small-volume peritoneal tumor.Third, a direct comparison between DWI and CT was not undertaken in this study; we acknowledge that this would have been more conducive to confirming the superior performance of DWI in detecting peritoneal tumor.
Conclusions
Our results suggest that preoperative WB-DWI accurately predicts PCI in correlation with surgical and histopathological findings. The ability of preoperative WBDWI to accurately predict the PCI score may assist oncologic surgeons to select patients who may benefit more from CRS and HIPEC, and exclude those whose tumors are too extensive and unlikely to achieve complete macroscopic cytoreduction. However, larger scale and prospective studies are needed to establish the clinical application.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (Grant No. 81501437) and the Shanghai Municipal Planning Commission of Science and Research Fund (Grant No. JGGG1401).
Conflict of interest statement
No potential conflicts of interest are disclosed.
References
1.Koppe MJ, Boerman OC, Oyen WJG, Bleichrodt RP. Peritoneal carcinomatosis of colorectal origin: incidence and current treatment strategies. Ann Surg. 2006; 243: 212-22.
2.Franko J, Shi Q, Goldman CD, Pockaj BA, Nelson GD, Goldberg RM, et al. Treatment of colorectal peritoneal carcinomatosis with systemic chemotherapy: a pooled analysis of north central cancer treatment group phase III trials N9741 and N9841. J Clin Oncol.2012; 30: 263-7.
3.Jacquet P, Sugarbaker PH. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. In:Sugarbaker PH. Peritoneal Carcinomatosis: Principles of Management. Boston, MA: Springer. 1996; 82: 359-74.
4.Koh JL, Yan TD, Glenn D, Morris DL. Evaluation of preoperative computed tomography in estimating peritoneal cancer index in colorectal peritoneal carcinomatosis. Ann Surg Oncol. 2009; 16:327-33.
5.Glehen O, Gilly FN. Quantitative prognostic indicators of peritoneal surface malignancy: carcinomatosis, sarcomatosis, and peritoneal mesothelioma. Surg Oncol Clin N Am. 2003;: 649-71.
6.Harmon RL, Sugarbaker PH. Prognostic indicators in peritoneal carcinomatosis from gastrointestinal cancer. Int Semin Surg Oncol.2005; 2: 3
7.Baratti D, Kusamura S, Deraco M. The fifth international workshop on peritoneal surface malignancy (Milan, Italy, December 4-6,2006): methodology of disease-specific consensus. J Surg Oncol.2008; 98: 258-62.
8.Metser U, Jones C, Jacks LM, Bernardini MQ, Ferguson S.Identification and quantification of peritoneal metastases in patients with ovarian cancer with multidetector computed tomography: correlation with surgery and surgical outcome. Int J Gynecol Cancer. 2011; 21: 1391-8.
9.Esquivel J, Chua TC, Stojadinovic A, Melero JT, Levine EA,Gutman M, et al. Accuracy and clinical relevance of computed tomography scan interpretation of peritoneal cancer index in colorectal cancer peritoneal carcinomatosis: a multi-institutional study. J Surg Oncol. 2010; 102: 565-70.
10.Bozkurt M, Doganay S, Kantarci M, Yalcin Y, Eren S, Atamanalp SS, et al. Comparison of peritoneal tumor imaging using conventional MR imaging and diffusion-weighted MR imaging with different b values. Eur J Radiol. 2011; 80: 224-8.
11.Fehniger J, Thomas S, Lengyel E, Liao CH, Tenney M, Oto A, et al.A prospective study evaluating diffusion weighted magnetic resonance imaging (DW-MRI) in the detection of peritoneal carcinomatosis in suspected gynecologic malignancies. Gynecol Oncol. 2016; 142: 169-75.
12.Barral M, Eveno C, Hoeffel C, Boudiaf M, Bazeries P, Foucher R,et al. Diffusion-weighted magnetic resonance imaging in colorectal cancer. J Visc Surg. 2016; 153: 361-9.
13.Fujii S, Matsusue E, Kanasaki Y, Kanamori Y, Nakanishi J, Sugihara S, et al. Detection of peritoneal dissemination in gynecological malignancy: evaluation by diffusion-weighted MR imaging. Eur Radiol. 2008; 18: 18-23.
14.Klumpp BD, Aschoff P, Schwenzer N, Fenchel M, Koenigsrainer I,Falch C, et al. Peritoneal carcinomatosis: comparison of dynamic contrast-enhanced magnetic resonance imaging with surgical and histopathologic findings. Abdom Radiol. 2012; 37: 834-42.
15.Michielsen K, Dresen R, Vanslembrouck R, De Keyzer F, Amant F,Mussen E, et al. Diagnostic value of whole body diffusion-weighted MRI compared to computed tomography for pre-operative assessment of patients suspected for ovarian cancer. Eur J Cancer.2017; 83: 88-98.
16.Levine EA, Stewart IV JH, Russell GB, Geisinger KR, Loggie BL,Shen P. Cytoreductive surgery and intraperitoneal hyperthermic chemotherapy for peritoneal surface malignancy: experience with 501 procedures. J Am Coll Surg. 2007; 204: 943-53.
17.Blackham AU, Russell GB, Stewart IV JH, Votanopoulos K, Levine EA, Shen P. Metastatic colorectal cancer: survival comparison of hepatic resection vs. cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol. 2014; 21: 2667-74.
18.Weber T, Roitman M, Link KH. Current status of cytoreductive surgery with hyperthermic intraperitoneal chemotherapy in patients with peritoneal carcinomatosis from colorectal cancer. Clin Colorectal Cancer. 2012; 11: 167-76.
19.Hornung M, Werner JM, Schlitt HJ. Applications of hyperthermic intraperitoneal chemotherapy for metastatic colorectal cancer.Expert Rev Anticancer Ther. 2017; 17: 841-50.
20.Yan TD, Sim J, Morris DL. Selection of patients with colorectal peritoneal carcinomatosis for cytoreductive surgery and perioperative intraperitoneal chemotherapy. Ann Surg Oncol. 2007;14: 1807-17.
21.Goéré D, Malka D, Tzanis D, Gava V, Gava V, Eveno C, et al. Is there a possibility of a cure in patients with colorectal peritoneal carcinomatosis amenable to complete cytoreductive surgery and intraperitoneal chemotherapy? Ann Surg. 2013; 257: 1065-71.
22.Yonemura Y, Tsukiyama G, Miyata R, Sako S, Endou Y, Hirano M,et al. Indication of peritonectomy for peritoneal dissemination.Gan to Kagaku Ryoho. 2010; 37: 2306-11.
23.Turaga K, Levine E, Barone R, Sticca R, Petrelli N, Lambert L, et al.Consensus guidelines from the American Society of Peritoneal Surface Malignancies on standardizing the delivery of hyperthermic intraperitoneal chemotherapy (HIPEC) in colorectal cancer patients in the United States. Ann Surg Oncol. 2014; 21: 1501-5.
24.Mo SB, Cai GX. Multidisciplinary treatment for colorectal peritoneal metastases: Review of the literature. Gastroenterol Res Pract. 2016; 2016: 1516259
25.De Bree E, Koops W, Kröger R, Van Ruth S, Witkamp AJ,Zoetmulder FAN. Peritoneal carcinomatosis from colorectal or appendiceal origin: correlation of preoperative CT with intraoperative findings and evaluation of interobserver agreement. J Surg Oncol. 2004; 86: 64-73.
26.Coakley FV, Choi PH, Gougoutas CA, Pothuri B, Venkatraman E,Chi D, et al. Peritoneal metastases: detection with spiral CT in patients with ovarian cancer. Radiology. 2002; 223: 495-9.
27.Low RN, Barone RM, Lucero J. Comparison of MRI and CT for predicting the peritoneal cancer index (PCI) preoperatively in patients being considered for cytoreductive surgical procedures.Ann Surg Oncol. 2015; 22: 1708-15.
28.Low RN, Barone RM. Combined diffusion-weighted and gadolinium-enhanced MRI can accurately predict the peritoneal cancer index preoperatively in patients being considered for cytoreductive surgical procedures. Ann Surg Oncol. 2012; 19:1394-401.
杂志排行
Cancer Biology & Medicine的其它文章
- Ovarian cancer presenting with hypercalcemia: two cases with similar manifestations but different mechanisms
- Primary resistance to crizotinib treatment in a non-small cell lung cancer patient with an EML4-ALK rearrangement: a case report
- Ultrasound features of extranodal extension in the metastatic cervical lymph nodes of papillary thyroid cancer: a case-control study
- Clinical significance of miRNA - 106a in non-small cell lung cancer patients who received cisplatin combined with gemcitabine chemotherapy
- Enterolactone modulates the ERK/NF-κB/Snail signaling pathway in triple-negative breast cancer cell line MDA-MB-231 to revert the TGF-β-induced epithelial–mesenchymal transition
- Progress in non-invasive detection of liver fibrosis
